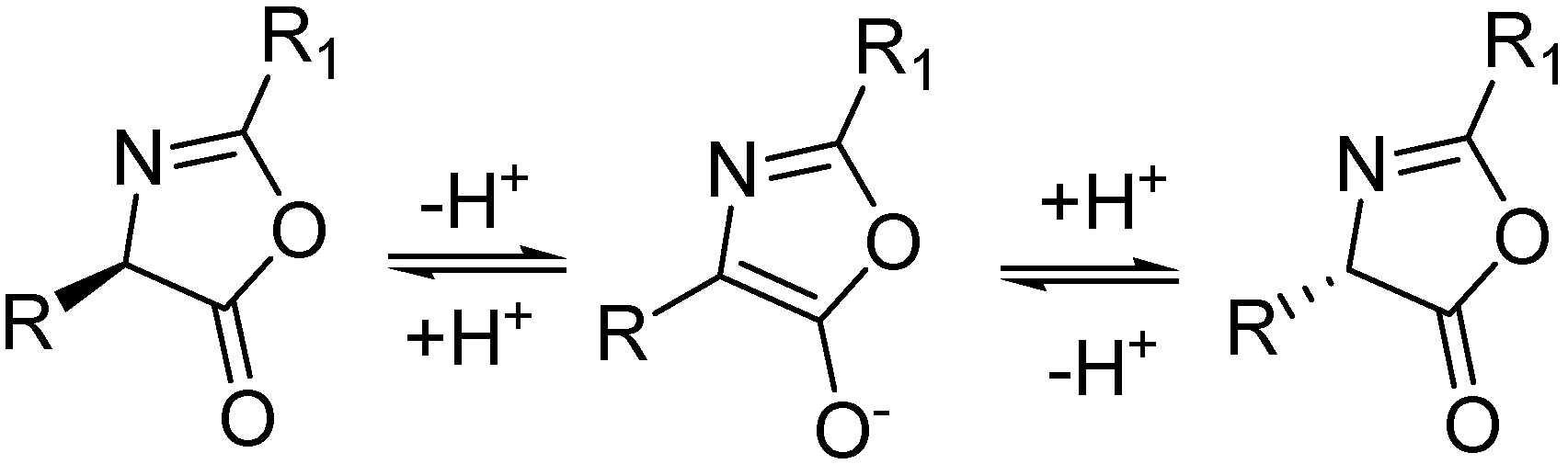
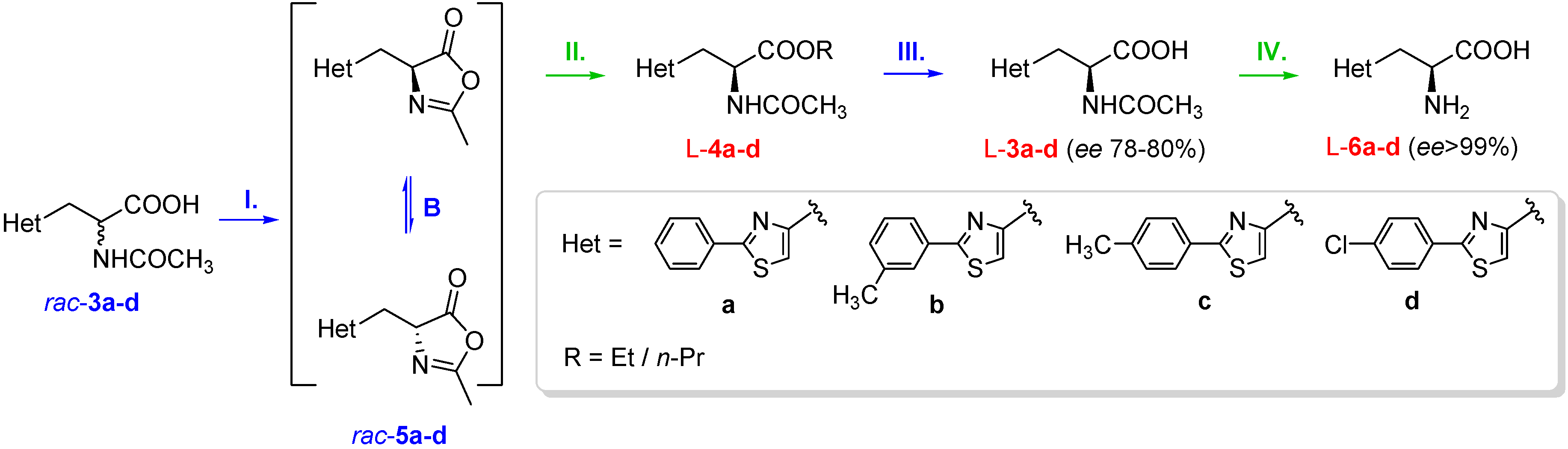
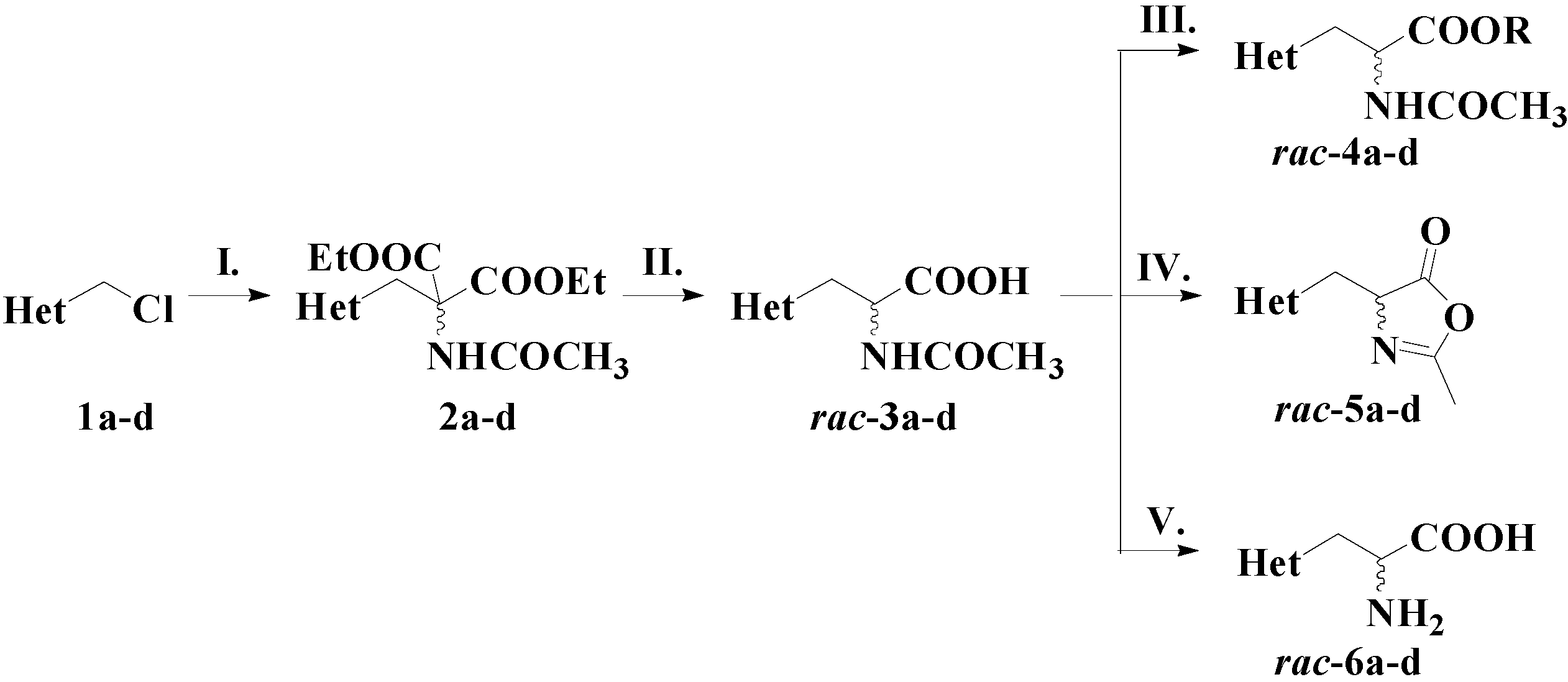3.3. Chemical Synthesis of Racemic 2-Arylthiazol-4-yl Alanines and Their Derivatives
3.3.1. Synthesis of Racemic 2-Acetamido-3-(2-arylthiazol-4-yl)propanoic Acids rac-3a–d
A dispersion of 60% NaH in mineral oil (0.84 g, 21 mmol) was suspended in dry N,N-dimethylformamide (12 mL) and stirred under argon at room temperature. After 30 min, diethyl acetamidomalonate (4.34 g, 20 mmol) was added, the mixture was stirred for 30 min and cooled, followed by the dropwise addition of the halogenated thiazole derivative 1a–d (22 mmol) dissolved in dry N,N-dimethylformamide (5 mL). The reaction mixture was stirred for 3 h at room temperature, and for the next 4 h at 60 °C. The solution was cooled and poured on a water–ice mixture. The formed precipitate was filtered off, dried, and suspended in an aqueous solution of 10% KOH (4–5 mL). The reaction mixture was refluxed for 4 h, in order to hydrolyse the ester groups. The resulting solution was cooled and the pH was adjusted to 1 with concentrated HCl. The formed precipitate was filtered, dried, suspended in toluene (10 mL), and refluxed for 2 h, until complete decarboxylation. The formed white crystals of 2-acetamido-3-(2-arylthiazol-4-yl)propanoic acids were isolated by filtration and dried.
2-Acetamido-3-(2-phenylthiazol-4-yl)propanoic acid (rac-3a): Yield: 64%; white solid; m.p. 175–176 °C; 1H-NMR (600 MHz, DMSO) δ 8.25 (1H, NH), 7.91 (dd, J = 7.9, 1.4 Hz, 2H), 7.52–7.46 (m, 3H), 7.38 (s, 1H), 4.60 (td, J = 8.7, 5.0 Hz, 1H), 3.14 (ddd, J = 23.7, 14.6, 7.0 Hz, 2H), 1.81 (s, 3H). 13C-NMR (151 MHz, DMSO) δ 172.99, 169.29, 166.38, 153.50, 133.11, 130.14, 129.21, 126.06, 116.24, 51.82, 32.89, 22.40; ESI-MS: 291.0800 (calculated: 291.0798, for C14H14N2O3S [M + H]+); m/z (%): 313 (24, [M + Na]+), 293 (4.5, [M + 3H]+), 292 (15.2, [M + 2H]+), 291 (100, [M + H]+), 284 (2.8), 279 (1.3), 273 (1.2).
2-Acetamido-3-(2-m-tolylthiazol-4-yl)propanoic acid (rac-3b): Yield: 65%; white solid; m.p. 160–161 °C; 1H-NMR (600 MHz, DMSO) δ 7.97 (1H, NH), 7.73 (s, 1H), 7.68 (d, J = 7.7 Hz, 1H), 7.36 (t, J = 7.6 Hz, 1H), 7.30 (s, 1H), 7.26 (d, J = 7.4 Hz, 1H), 4.43 (m, 1H), 3.13 (ddd, J = 23.2, 14.6, 6.6 Hz, 2H), 2.37 (s, 3H), 1.79 (s, 3H). 13C-NMR (151 MHz, DMSO) δ 173.32, 168.80, 165.97, 154.70, 138.51, 133.24, 130.66, 129.08, 126.42, 123.28, 115.31, 52.99, 33.75, 22.61, 20.91; ESI+-MS: 305.0960 (calculated: 305.0954 for C15H16N2O3S [M + H]+); m/z (%): 327 (85.3, [M + Na]+), 307 (4.4, [M + 3H]+), 306 (16.5, [M + 2H]+), 305 (100, [M + H]+), 292 (3.0), 291 (21.2), 288 (4.2), 284 (1.7), 263 (30.4), 251 (21.8), 210 (4.8).
2-Acetamido-3-(2-p-tolylthiazol-4-yl)propanoic acid (rac-3c): Yield: 67%; white solid; m.p. 181–182 °C; 1H-NMR (600 MHz, DMSO) δ 8.17 (1H, NH), 7.80 (d, J = 8.1 Hz, 2H), 7.31 (s, 1H), 7.30 (d, J = 8.0 Hz, 2H), 4.56 (dd, J = 13.2, 8.3 Hz, 1H), 3.12 (ddd, J = 23.6, 14.6, 6.9 Hz, 2H), 2.35 (s, 3H), 1.80 (s, 3H). 13C-NMR (151 MHz, DMSO) δ 173.03, 169.17, 166.41, 153.52, 139.88, 130.57, 129.72, 125.99, 115.50, 51.98, 33.03, 22.44, 20.93; ESI+-MS: 305.0967 (calculated: 305.0954, for C15H16N2O3S [M + H]+); m/z (%): 343 (100, [M + K]+), 327 (25.4, [M + Na]+), 307 (0.8, [M + 3H]+), 306 (3.2, [M + 2H]+), 305 (18.5, [M + H]+), 291 (0.9), 284 (1.8), 263 (1.5), 251 (1.3), 210 (0.2).
2-Acetamido-3-(2-p-clorophenylthiazol-4-yl)propanoic acid (rac-3d): Yield: 64%; white solid; m.p. 199–200 °C; 1H-NMR (600 MHz, DMSO) δ 8.20 (1H, NH), 7.92 (d, J = 7.2 Hz, 2H), 7.55 (d, J = 6.8 Hz, 2H), 7.40 (s, 1H), 4.57 (dd, J = 11.9, 8.9 Hz, 1H), 3.13 (ddd, J = 23.6, 14.6, 7.0 Hz, 2H), 1.80 (s, 3H). 13C-NMR (151 MHz, DMSO) δ 173.03, 169.29, 164.98, 153.89, 134.63, 131.98, 129.28, 127.77, 116.70, 51.99, 33.01, 22.46; ESI+-MS: 325.0408 (calculated: 325.0408, for C14H13ClN2O3S [M + H]+); m/z (%): 347 (12.5, [M + Na]+), 328 (11.1, [M + 2H]+, 37Cl), 327 (70.1, [M + H]+, 37Cl), 326 (3.2, [M + 2H]+, 35Cl), 325 (21.4, [M + H]+, 35Cl), 313 (9.5), 305 (32.6), 251 (18.7), 210 (3.7).
3.3.2. Synthesis of Racemic 2-Acetamido-3-(2-arylthiazol-4-yl)propanoic Esters rac-4a–d
To a solution of racemic 2-acetamido-3-(2-arylthiazol-4-yl)propanoic acid rac-3a–d (0.5 mmol) and carbonyl diimidazole (90 mg, 0.55 mmol) in anhydrous THF (2 mL), ethanol (321 μL, 5.5 mmol) was added. The reaction mixture was stirred at room temperature overnight. The solvent was removed in vacuo and the crude product was purified with column chromatography on silica gel using dichloromethane:acetone 9:1 (v/v) as eluent. Methyl, n-propyl and n-butyl 2-acetamido-3-(2-arylthiazol-4-yl)propanoates were obtained by the same procedure, using methanol, propanol or butanol instead of ethanol.
Ethyl 2-acetamido-3-(2-phenylthiazol-4-yl)propanoate (rac-4a): Yield: 64%; white solid; m.p. 116–117 °C; 1H-NMR (600 MHz, CDCl3) δ 7.94 (d, J = 4.0 Hz, 2H), 7.45–7.46 (m, 3H), 7.02 (s, 1H), 4.91–4.94 (m, 1H), 4.19 (q, J = 7.1 Hz, 2H), 3.37 (ddd, J = 17.4, 14.4, 3.7 Hz, 2H), 2.04 (s, 3H), 1.24 (t, J = 7.1 Hz, 3H). 13C-NMR (151 MHz, CDCl3) δ 171.32, 170.19, 168.65, 152.31, 133.18, 130.70, 129.25, 126.68, 115.93, 61.67, 52.16, 32.84, 23.42, 14.33; ESI+-MS: 319.1121 (calculated: 319.1111 for C16H18N2O3S [M + H]+); m/z (%): 357 (44.3, [M + K]+), 341 (16.5, [M + Na]+), 320 (18.8, [M + 2H]+), 319 (100, [M + H]+), 305 (10), 277 (14).
Ethyl 2-acetamido-3-(2-m-tolylthiazol-4-yl)propanoate (rac-4b): Yield: 63%; yellow solid; m.p. 81 °C; 1H-NMR (600 MHz, CDCl3) δ 7.72 (s, 1H), 7.70 (d, J = 7.8 Hz, 1H), 7.32 (t, J = 7.6 Hz, 1H), 7.24 (d, J = 7.5 Hz, 1H), 6.97 (s, 1H), 4.92 (dd, J = 12.9, 5.2 Hz, 1H), 4.18 (q, J = 7.1 Hz, 2H), 3.32 (ddd, J = 46.0, 14.8, 5.2 Hz, 2H), 2.41 (s, 3H), 2.03 (s, 3H), 1.22 (t, J = 7.1 Hz, 3H). 13C-NMR (151 MHz, CDCl3) δ 171.40, 170.05, 168.60, 152.47, 138.88, 133.22, 131.18, 129.03, 127.12, 123.70, 115.61, 61.56, 52.12, 33.03, 23.36, 21.49, 14.27. ESI+-MS: 333.1270 (calculated: 333.1267 for C17H20N2O3S [M + H]+); m/z (%): 371 (61.0, [M + K]+), 355 (36.9, [M + Na]+), 334 (19.8, [M + 2H]+), 333 (100.0, [M + H]+), 319 (2.7), 305 (1), 291 (6.2).
Ethyl 2-acetamido-3-(2-p-tolylthiazol-4-yl)propanoate (rac-4c): Yield: 65%; white solid; m.p. 98 °C; 1H-NMR (600 MHz, CDCl3) δ 7.78 (d, J = 8.0 Hz, 2H), 7.24 (d, J = 7.9 Hz, 2H), 6.94 (s, 1H), 4.91 (dt, J = 7.5, 5.1 Hz, 1H), 4.18 (q, J = 7.1 Hz, 2H), 3.31 (ddd, J = 50.6, 14.8, 5.0 Hz, 2H), 2.40 (s, 3H), 2.04 (s, 3H), 1.22 (t, J = 7.1 Hz, 3H). 13C-NMR (151 MHz, CDCl3) δ 171.45, 170.07, 168.56, 152.54, 140.60, 130.96, 129.81, 126.42, 115.22, 61.58, 52.15, 33.11, 23.43, 21.56, 14.31; ESI+-MS: 333.1265 (calculated: 333.1267 for C17H20N2O3S [M + H]+); m/z (%): 371 (12.1, [M + K]+), 355 (47.6, [M + Na]+), 334 (21.5, [M + 2H]+), 333 (100.0, [M + H]+), 319 (35), 305 (17.5), 291 (5), 259 (12.0), 253 (22.9), 217 (24.9).
n-Propyl 2-acetamido-3-(2-p-chlorophenylthiazol-4-yl)propanoate (rac-4d): Yield: 64%; white solid; 1H-NMR (600 MHz, CDCl3) δ 7.86 (d, J = 8.5 Hz, 2H), 7.40 (d, J = 8.5 Hz, 2H), 6.97 (s, 1H), 4.94 (dt, J = 7.7, 5.1 Hz, 1H), 4.08 (t, J = 6.7 Hz, 2H), 2.02 (s, 3H), 1.73–1.65 (m, 2H), 0.89 (t, J = 7.4 Hz, 3H). 13C-NMR (151 MHz, CDCl3) δ 171.55, 170.34, 168.02, 153.77, 148.43, 131.36, 129.35, 127.67, 115.97, 67.24, 52.07, 34.06, 23.40, 21.70, 10.46; ESI+-MS: 367.0879 (calculated: 367.0878 for C17H19ClN2O3S [M + H]+); m/z (%): 405 ([M + K]+), 389 ([M + Na]+), 370 (6.6, [M + 2H]+, 37Cl), 369 (37.2, [M + H]+, 37Cl), 368 (19.5, [M + 2H]+, 35Cl), 367 (100, [M + H]+, 35Cl), 333 (10), 319 (1.8), 305 (1.7), 287 (1.5), 244 (1.8).
3.3.3. Synthesis of Racemic 4-((2-Arylthiazol-4-yl)methyl)-2-methyloxazol-5(4H)-ones rac-5a–d
To a solution of racemic 2-acetamido-3-(2-arylthiazol-4-yl)propanoic acid rac-3a–d (1 mmol) in anhydrous dichloromethane (5 mL), a solution of N,N′-dicyclohexyl-carbodiimide (247.2 mg, 1.2 mmol) in anhydrous dichloromethane (2 mL) was added dropwise at 0 °C. The reaction mixture was stirred for 1 h at 0 °C. After the completion of the reaction (verified by TLC, eluent dichloromethane:acetone 9:1), the formed precipitate of dicyclohexyl urea was removed by filtration. The solvent was distilled off at reduced pressure, obtaining without further purifications the pure oxazol-5(4H)-ones rac-5a–d, which were directly used in the enzymatic reactions.
3.3.4. Synthesis of Racemic 2-Arylthiazole-4-yl Alanines rac-6a–d
A suspension of racemic 2-acetamido-3-(2-arylthiazol-4-yl)propanoic acid rac-3a–d (50 mg) in 18% HCl (6 mL) was refluxed for 4 h. The solvent was removed by distillation at reduced pressure, affording the corresponding 2-arylthiazole-4-yl alanine rac-6a–d as hydrochloride salt, which was dried and washed several times with diethyl ether.
2-Amino-3-(2-phenylthiazol-4-yl)propanoic acid (rac-6a): Yield: 91%; white powder; m.p. 241–248 °C for the hydrochloride salt, respectively m.p. over 300 °C with decomposition for the free amino acid; 1H-NMR (600 MHz, D2O) δ 7.82–7.79 (m, 2H), 7.62–7.38 (m, 4H), 4.44 (t, J = 6.5 Hz, 1H), 3.45 (ddd, J = 22.5, 15.6, 6.4 Hz, 2H). 13C-NMR (151 MHz, D2O) δ 172.4, 170.98, 170.67, 146.59, 132.00, 129.50, 126.90, 119.69, 52.24, 29.84; ESI+-MS: 249.0699 (calculated: 249.0692 for C12H12N2O2S [M + H]+); m/z (%): 263 (100), 249 (2.9, [M + H]+), 203 (1.5).
2-Amino-3-(2-m-tolylthiazol-4-yl)propanoic acid (rac-6b): Yield: 88%; white powder; m.p. 225–238 °C for the hydrochloride salt, respectively m.p. 227–242 °C with decomposition for the free amino acid; 1H-NMR (600 MHz, D2O) δ 7.54 (m, 3H), 7.32–7.31 (m, 2H), 4.42 (t, J = 6.6 Hz, 1H), 3.43 (ddd, J = 35.2, 15.6, 6.7 Hz, 2H), 2.28 (s, 3H). 13C-NMR (151 MHz, D2O) δ 173.86, 171.21, 170.58, 139.88, 133.06, 133.02, 129.42, 127.34, 123.93, 119.69, 52.13, 29.60, 20.35; ESI+-MS: 263.0857 (calculated: 263.0849 for C13H14N2O2S [M + H]+); m/z (%): 277 (100), 263 (4.6, [M + H]+), 217 (0.8).
2-Amino-3-(2-p-tolylthiazol-4-yl)propanoic acid (rac-6c): Yield: 92%; white powder; m.p. 180–190 °C for the hydrochloride salt, respectively m.p. 280–286 °C with decomposition for the free amino acid; 1H-NMR (600 MHz, D2O) δ 7.72 (d, J = 8.1 Hz, 2H), 7.69 (s, 1H), 7.34 (d, J = 8.1 Hz, 2H), 4.45 (t, J = 6.9 Hz, 1H), 3.50 (ddd, J = 22.8, 16.5, 6.9 Hz, 2H), 2.32 (s, 3H). 13C-NMR (151 MHz, D2O) δ 172.29, 170.18, 145.07, 143.56, 130.38, 127.38, 124.49, 120.25, 51.82, 28.80, 20.78; ESI+-MS: 263.0855 (calculated: 263.0849 for C13H14N2O2S [M + H]+); m/z (%): 277 (100), 263 (3.0, [M + H]+), 217 (0.9).
2-Amino-3-(2-(4-chlorophenyl)thiazol-4-yl)propanoic acid (rac-6d): Yield: 93%; white powder; m.p. 250–260 °C for the hydrochloride salt, respectively m.p. over 300 °C with decomposition for the free amino acid; 1H-NMR (600 MHz, Methanol-d4) δ 7.99 (d, J = 8.5 Hz, 2H), 7.50 (d, J = 8.5 Hz, 2H), 7.46 (s, 1H), 4.45 (t, J = 5.8 Hz, 1H), 3.46 (ddd, J = 22.9, 15.5, 5.9 Hz, 2H). 13C-NMR (151 MHz, Methanol-d4) δ 170.97, 169.18, 151.97, 137.46, 133.08, 130.34, 129.08, 118.91, 53.62, 32.26; ESI+-MS: 283.0306 (calculated: 283.0303 for C12H11ClN2O2S [M + H]+); m/z (%): 307 (4.1, [M + Na]+, 37Cl), 305 (44.6, [M + Na]+, 35Cl), 286 (4.6, [M + 2H]+, 37Cl), 285 (36.4, [M + H]+, 37Cl), 284 (12.9, [M + 2H]+, 35Cl), 283 (100, [M + H]+, 35Cl), 277 (47.6), 263 (6.6), 256 (4.3).