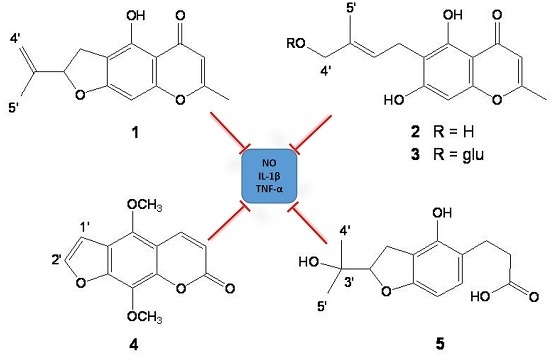
Anti-Inflammatory Activity of Heterocarpin from the Salt Marsh Plant Corydalis heterocarpa in LPS-Induced RAW 264.7 Macrophage Cells
Abstract
:1. Introduction
2. Results and Discussion
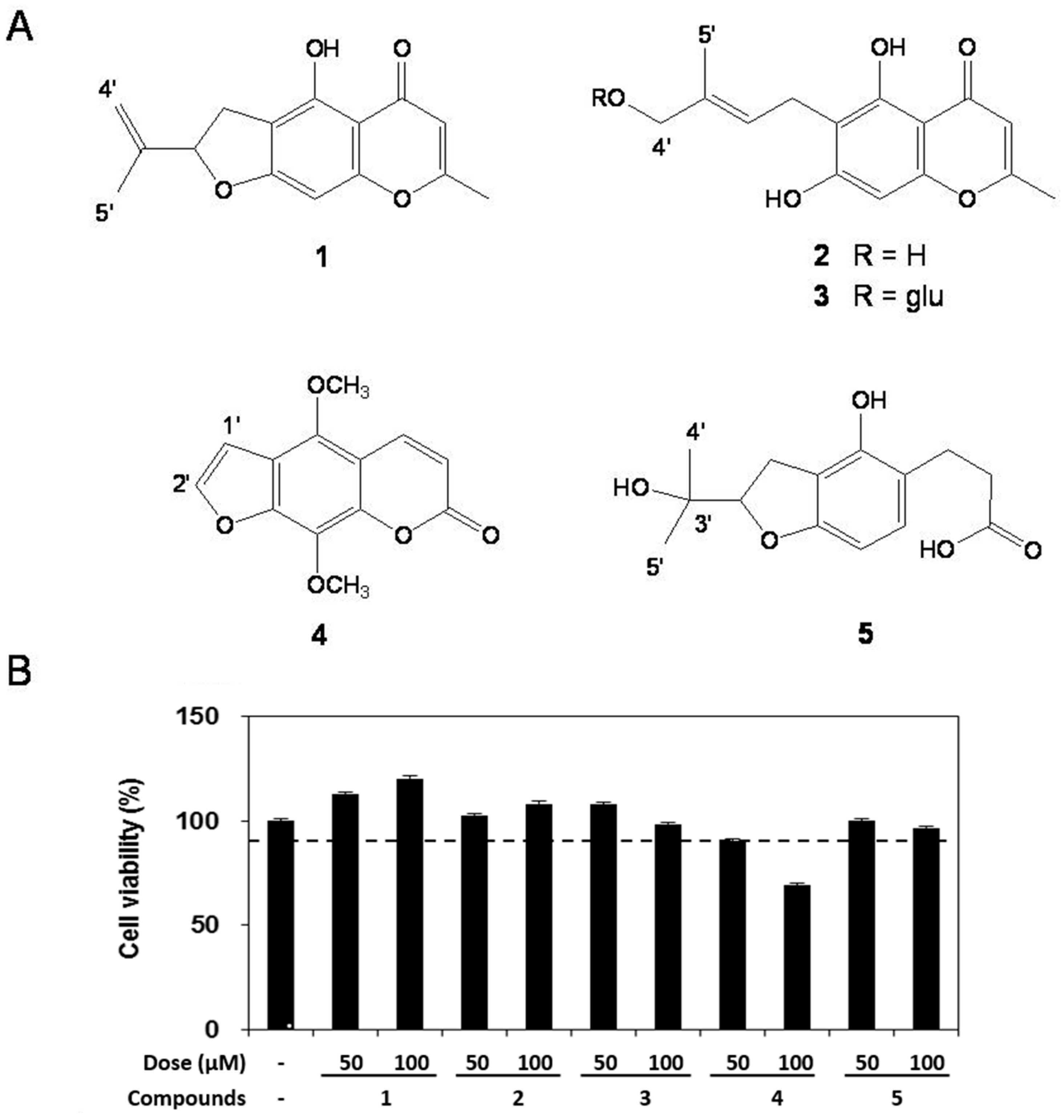
2.1. Isolation and Structure Determination of Compounds 1–5
2.2. Cell Viability
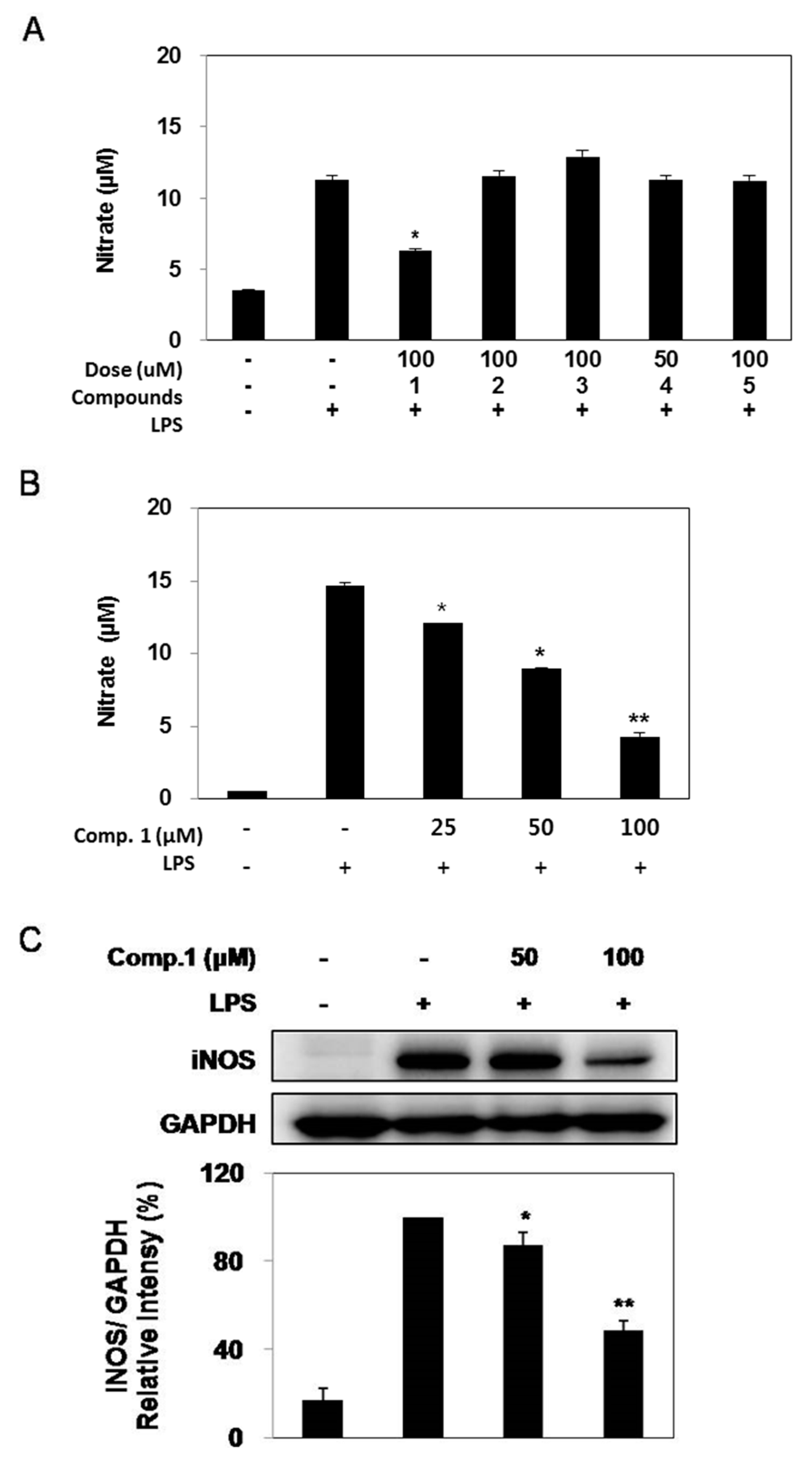
2.3. Effect of Compounds 1–5 on LPS-induced NO Production and iNOS Expression
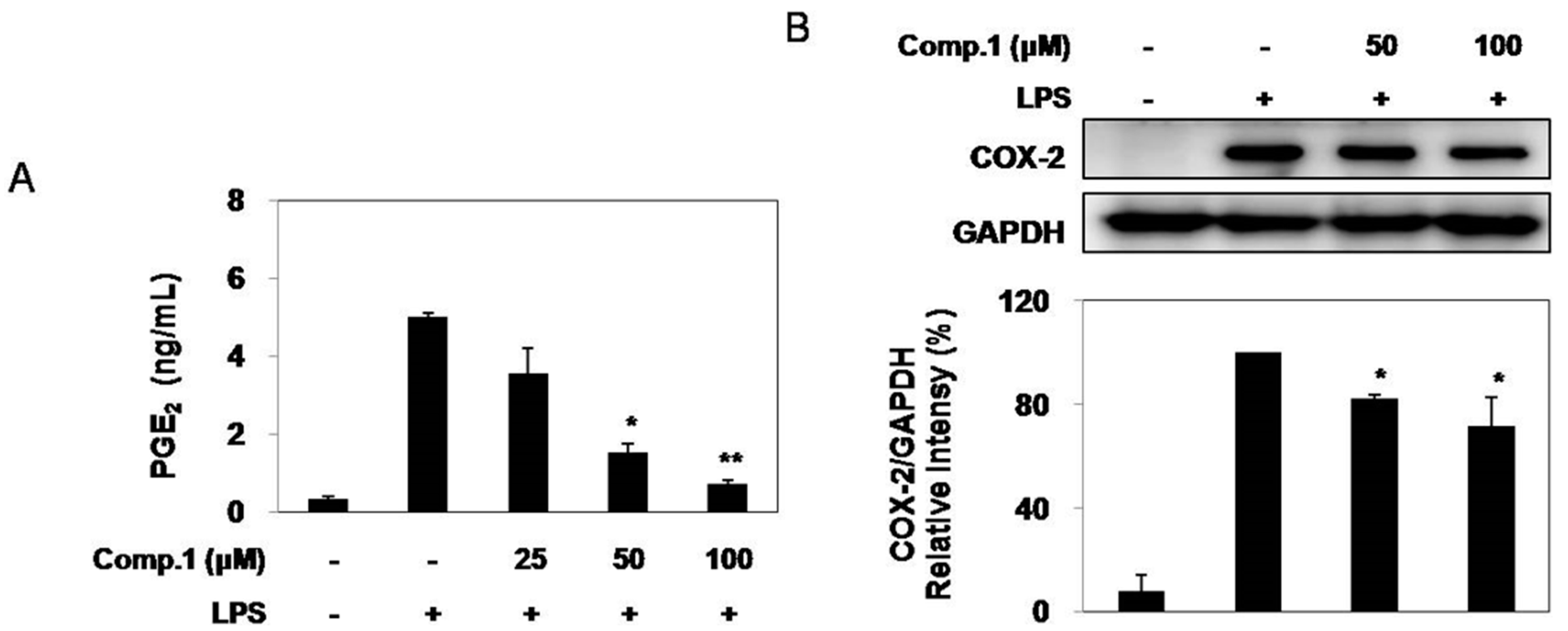
2.4. Effect of Compound 1 on LPS-Induced PGE2 Production and COX-2 Expression
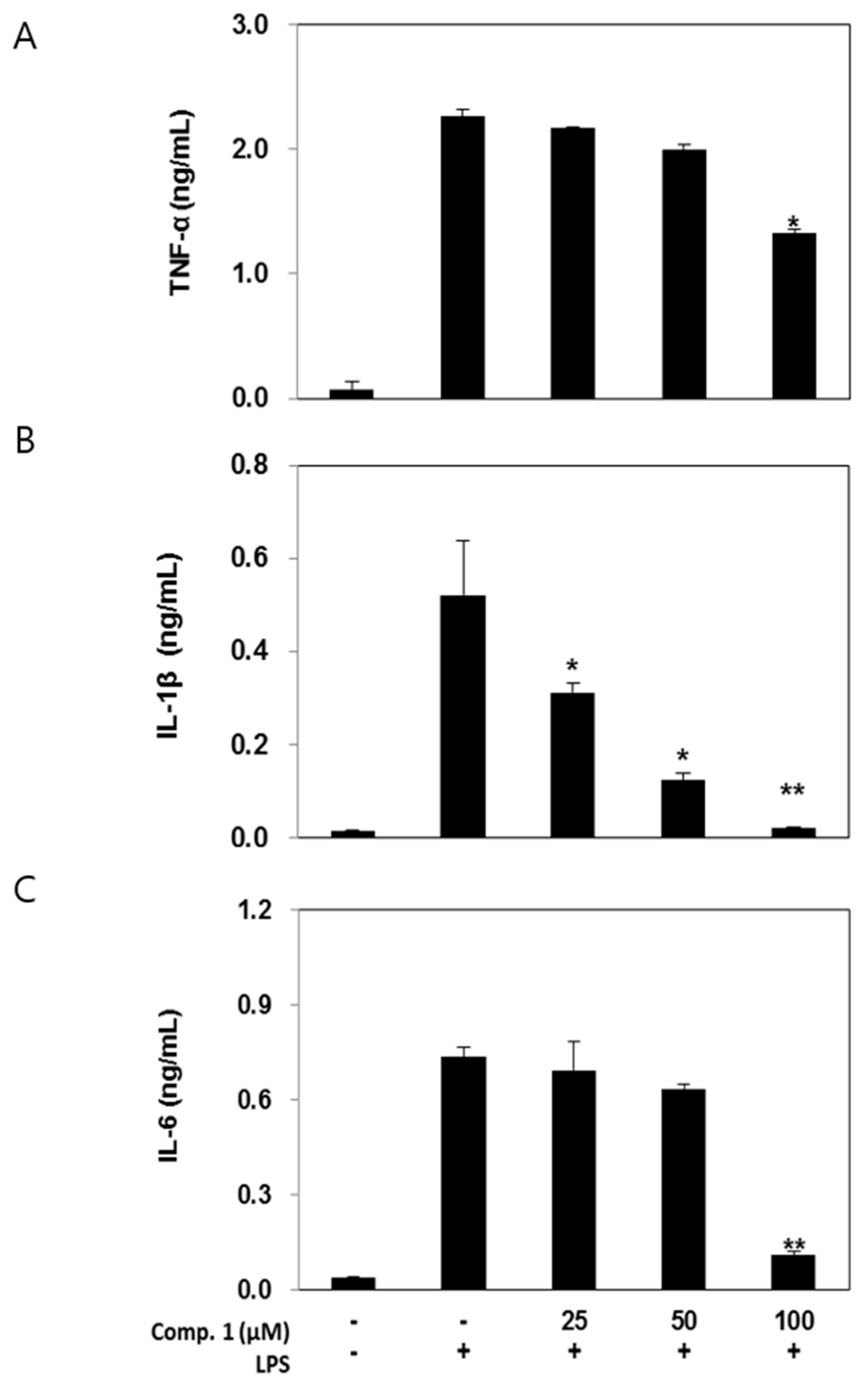
2.5. Effects of Compound 1 on Pro-Inflammatory Cytokine Production
2.6. Discussion
3. Experimental Section
3.1. General Procedures
3.2. Reagents
3.3. Plant Material
3.4. Extraction and Isolation
3.5. Cell Culture
3.6. Measurement of Cell Viability
3.7. Measurement of Nitrite and PGE2
3.8. Enzyme-Linked Immunosorbent Assay (ELISA)
3.9. Western Blot Analysis
3.10. Statistical Analysis
Acknowledgement
Author Contributions
Conflicts of Interest
References
- Kim, Y.A.; Kong, C.S.; Yea, S.S.; Seo, Y. Constituents of Corydalis heterocarpa and their anti-proliferative effects on human cancer cells. Food Chem. Toxicol. 2010, 48, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.N.; Kim, J.A.; Kong, C.S.; Seo, Y.; Kim, S.K. Protective effect of (20S)-columbianetin from Corydalis heterocarpa on UVB-induced keratinocyte damage. J. Photochem. Photobiol. B 2012, 109, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Kang, T.H.; Seo, J.U.; Na, H.J.; Kim, S.J.; Moon, P.D.; Kim, N.H.; Choi, I.Y.; Myung, N.Y.; Hong, S.H.; et al. Libanoridin inhibits the mast cell-mediated allergic inflammatory reaction. Immunopharmacol. Immunotoxicol. 2010, 32, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.H.; Kong, C.S.; Seo, Y.; Kim, M.M.; Kim, S.K. Anti-inflammatory effect of coumarins isolated from Corydalis heterocarpa in HT-29 human colon carcinoma cells. Food Chem. Toxicol. 2009, 47, 2129–2134. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Na, H.J.; Kim, S.J.; Rim, H.K.; Myung, N.Y.; Moon, P.D.; Han, N.R.; Seo, J.U.; Kang, T.H.; Kim, J.J.; et al. Anti-inflammatory effect of columbianetin on activated human mast cells. Biol. Pharm. Bull. 2009, 32, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Kozawa, M.; Baba, K. Coumarins from chinese crude drug “She Huangzi,” the fruits of Cnidium sp. and from Cnidium japonicum Mrq. Yakugaku Zasshi 1972, 92, 1289–1294. [Google Scholar] [PubMed]
- Morikawa, T.; Matsuda, H.; Nishida, N.; Qugushi, T.; Yoshikawa, M. Structures of new aromatics glycosides from a Japanese folk medicine, the roots of Angelica furcijuga. Chem. Pharm. Bull. 2004, 52, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Baba, K.; Kawanishi, H.; Taniguchi, M.; Kozawa, M. Chromones from Cnidium monnieri. Phytochemistry 1992, 31, 1367–1370. [Google Scholar] [CrossRef]
- Baba, K.; Kawanishi, H.; Taniguchi, M.; Kozawa, M. Chromone glucosides from Cnidium japonicum. Phytochemistry 1994, 35, 221–225. [Google Scholar] [CrossRef]
- Ma, W.G.; Fukushi, Y.; Tahara, S. Fungitoxic alkaloids from Hokkaido Corydalis species. Fitoterapia 1999, 70, 258–265. [Google Scholar] [CrossRef]
- Tatsuzawa, F.; Mikanagi, Y.; Saito, N.; Shinoda, K.; Shigihara, A.; Honda, T. Cyanidin glycosides in flowers of genus Corydalis (Fumariaceae). Biochem. Syst. Ecol. 2005, 33, 789–798. [Google Scholar] [CrossRef]
- Choi, S.U.; Baek, N.I.; Kim, S.H.; Yang, J.H.; Eun, J.S.; Shin, T.Y.; Lim, J.P.; Lee, J.H.; Jeon, H.; Yun, M.Y.; et al. Cytotoxic isoquinoline alkaloids from the aerial parts of Corydalis incise. Arch. Pharm. Res. 2007, 30, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Mongan, L.C.; Jones, T.; Patrick, G. Cytokine and free radical responses of alveolar macrophages in vitro to asbestos fibres. Cytokine 2000, 12, 1243–1247. [Google Scholar] [CrossRef] [PubMed]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell. Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Choi, J.H.; Park, Y.N.; Li, Y.; Jin, M.H.; Lee, J.; Lee, Y.; Son, J.K.; Chang, H.W.; Lee, E. Flowers of Inula japonica attenuate inflammatory responses. Immune Netw. 2010, 10, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiong, H.; Liu, L. Effects of taraxasterol on inflammatory responses in lipopolysaccharide-induced RAW 264.7 macrophages. J. Ethnopharmacol. 2012, 141, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.; Fernandes, E.; Lima, J. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Bioph. Meth. 2005, 65, 45–80. [Google Scholar] [CrossRef] [PubMed]
- Mangialasche, F.; Polidori, M.C.; Monastero, R.; Ercolani, S.; Camarda, C.; Cecchetti, R.; Mecocci, P. Biomarkers of oxidative and nitrosative damage in Alzheimer’s disease and mild cognitive impairment. Ageing Res. Rev. 2009, 8, 285–305. [Google Scholar] [CrossRef] [PubMed]
- Gross, S.S.; Wolin, M.S. Nitric oxide: Pathophysiological mechanisms. Annu. Rev. Physiol. 1995, 57, 737–769. [Google Scholar] [CrossRef] [PubMed]
- Marcinkiewicz, J.; Grabowska, A.; Chain, B. Nitric oxide upregulates the release of inflamatory mediators by mouse macrophages. Eur. J. Immunol. 1995, 25, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.L.; Martin, E.; Turko, I.V.; Murad, F. Novel effects of nitric oxide. Annu Rev. Pharmacol. Toxicol. 2001, 41, 203–236. [Google Scholar] [CrossRef] [PubMed]
- Marnett, L.J. The COXIB experience: A look in the rearview mirror. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 265–290. [Google Scholar] [CrossRef] [PubMed]
- Turini, M.E.; DuBois, R.N. Cyclooxygenase-2: A therapeutic target. Annu. Rev. Med. 2002, 53, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Prescott, S.M.; Fitzpatrick, F.A. Cyclooxygenase-2 and carcinogenesis. Biochim. Biophys. Acta 2000, 1470, 69–78. [Google Scholar] [CrossRef]
- Hinz, B.; Brune, K. Cyclooxygenase-2–10 years later. J. Pharmacol. Exp. Ther. 2012, 300, 367–375. [Google Scholar] [CrossRef]
- Glauser, M.P. The inflammatory cytokines. New developments in the pathophysiology and treatment of septic shock. Drugs 1996, 52, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Mannel, D.N.; Echtenacher, B. TNF in the inflammatory response. Chem. Immunol. 2000, 74, 141–161. [Google Scholar] [PubMed]
- Aggarwal, B.B.; Natarajan, K. Tumor Necrosis Factors: Developments during the last decade. Eur. Cytokine Netw. 1996, 7, 93–124. [Google Scholar] [PubMed]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar] [PubMed]
- Molloy, R.G.; Mannick, J.A.; Rodrick, M.L. Cytokines, sepsis and immunomodulation. Brit. J. Surg. 1993, 80, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Kasama, T.; Miwa, Y.; Isozaki, T.; Odai, T.; Adachi, M.; Kunkel, S.L. Neutrophil-derived cytokines: Potential therapeutic targets in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the all tested compounds are available from the corresponding author.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.A.; Kong, C.-S.; Park, H.H.; Lee, E.; Jang, M.-S.; Nam, K.-H.; Seo, Y. Anti-Inflammatory Activity of Heterocarpin from the Salt Marsh Plant Corydalis heterocarpa in LPS-Induced RAW 264.7 Macrophage Cells. Molecules 2015, 20, 14474-14486. https://doi.org/10.3390/molecules200814474
Kim YA, Kong C-S, Park HH, Lee E, Jang M-S, Nam K-H, Seo Y. Anti-Inflammatory Activity of Heterocarpin from the Salt Marsh Plant Corydalis heterocarpa in LPS-Induced RAW 264.7 Macrophage Cells. Molecules. 2015; 20(8):14474-14486. https://doi.org/10.3390/molecules200814474
Chicago/Turabian StyleKim, You Ah, Chang-Suk Kong, Hyo Hyun Park, Eunkyung Lee, Mi-Soon Jang, Ki-Ho Nam, and Youngwan Seo. 2015. "Anti-Inflammatory Activity of Heterocarpin from the Salt Marsh Plant Corydalis heterocarpa in LPS-Induced RAW 264.7 Macrophage Cells" Molecules 20, no. 8: 14474-14486. https://doi.org/10.3390/molecules200814474
APA StyleKim, Y. A., Kong, C.-S., Park, H. H., Lee, E., Jang, M.-S., Nam, K.-H., & Seo, Y. (2015). Anti-Inflammatory Activity of Heterocarpin from the Salt Marsh Plant Corydalis heterocarpa in LPS-Induced RAW 264.7 Macrophage Cells. Molecules, 20(8), 14474-14486. https://doi.org/10.3390/molecules200814474