Culture Condition Optimization and Pilot Scale Production of the M12 Metalloprotease Myroilysin Produced by the Deep-Sea Bacterium Myroides profundi D25
Abstract
:1. Introduction
2. Results and Discussion
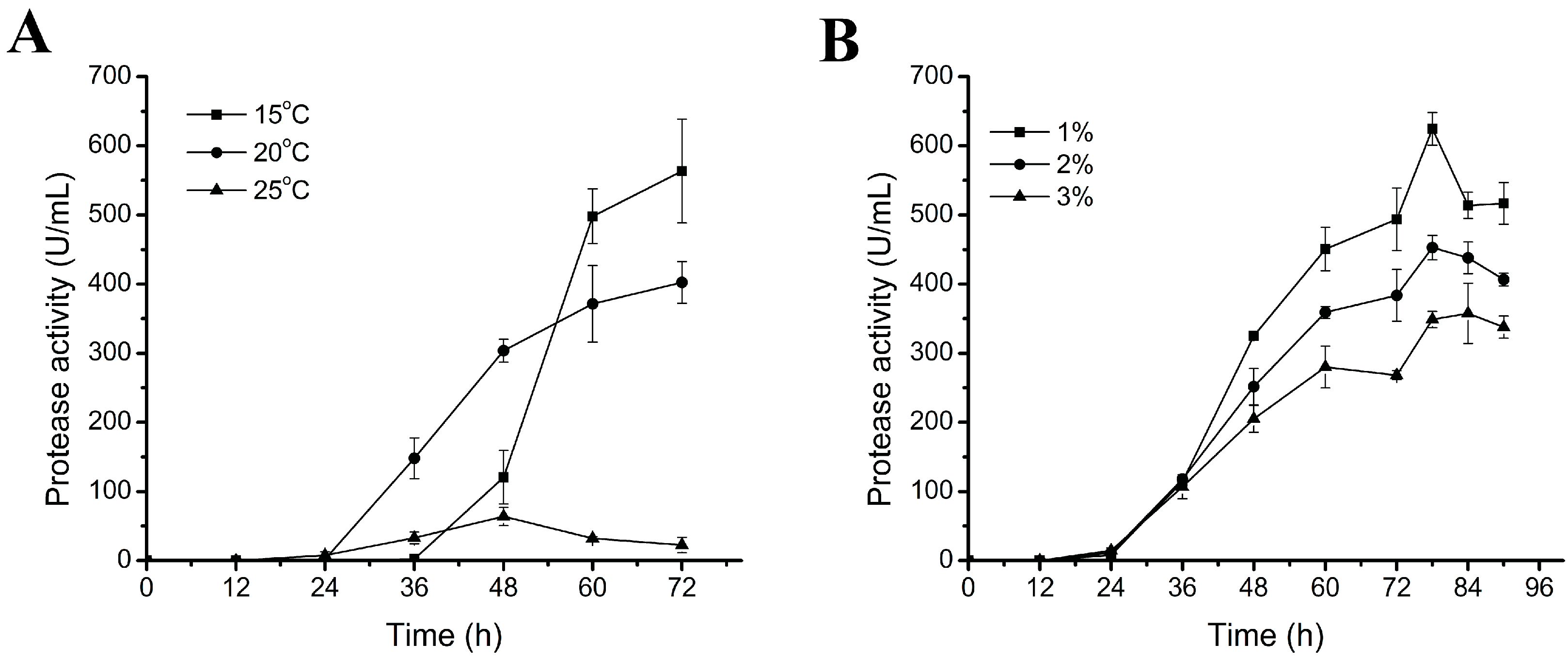
2.1. Effects of Culture Temperature and Inoculation Amount on the Production of Myroilysin
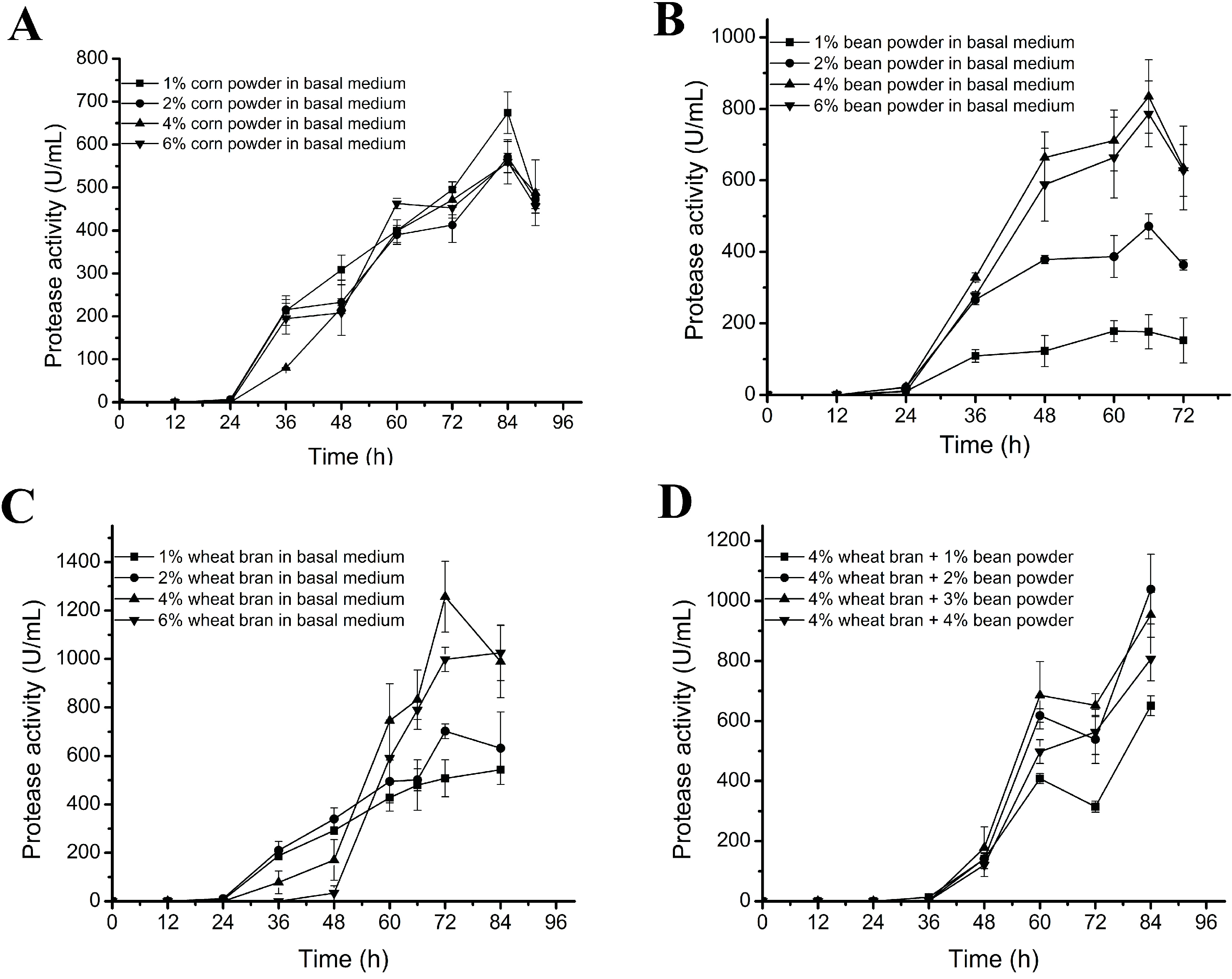
2.2. Optimization of the Contents of Carbon Source and Nitrogen Source in the Medium
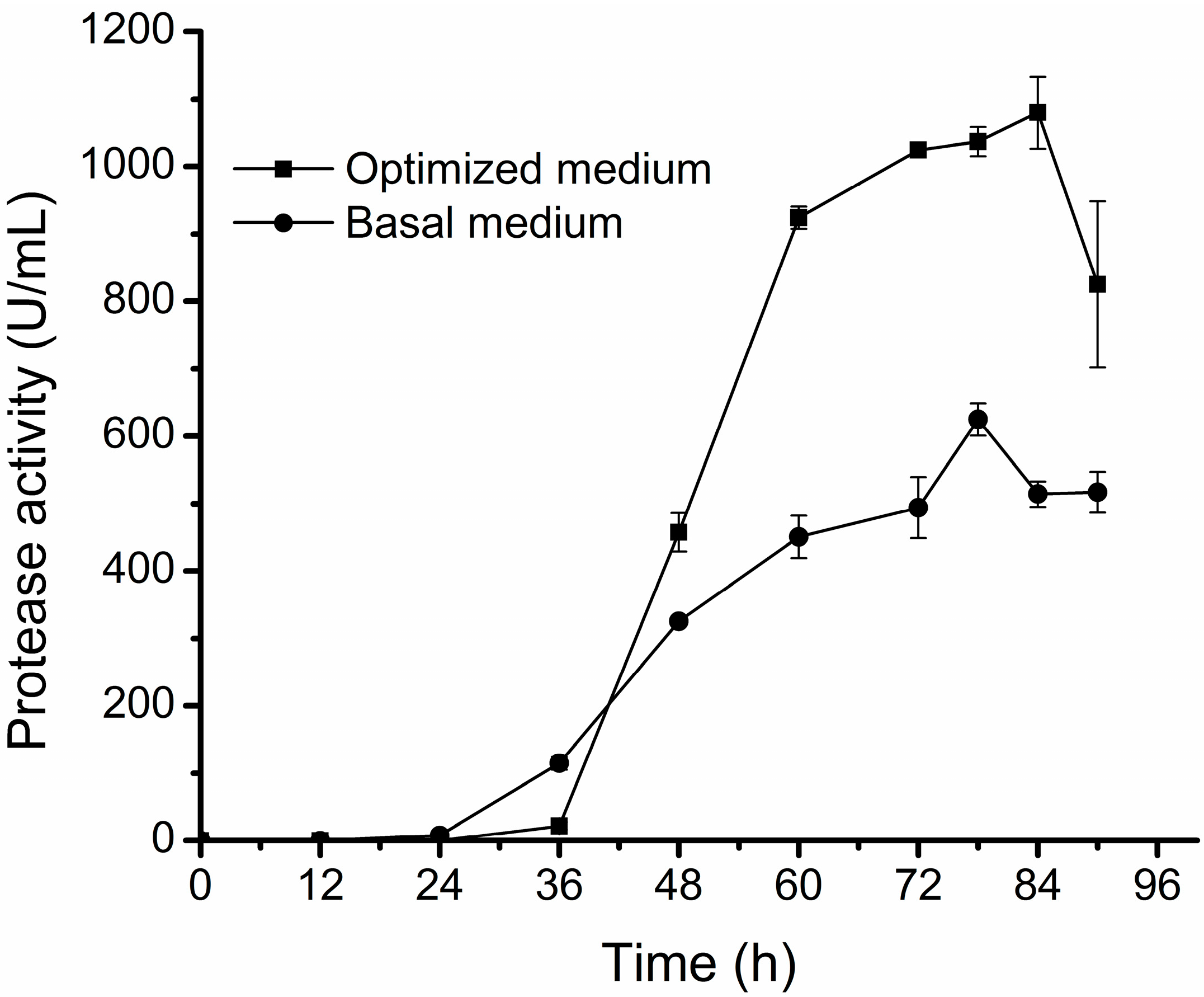
2.3. Protease Production of D25 under the Optimal Conditions
2.4. Small Scale Fermentation of D25
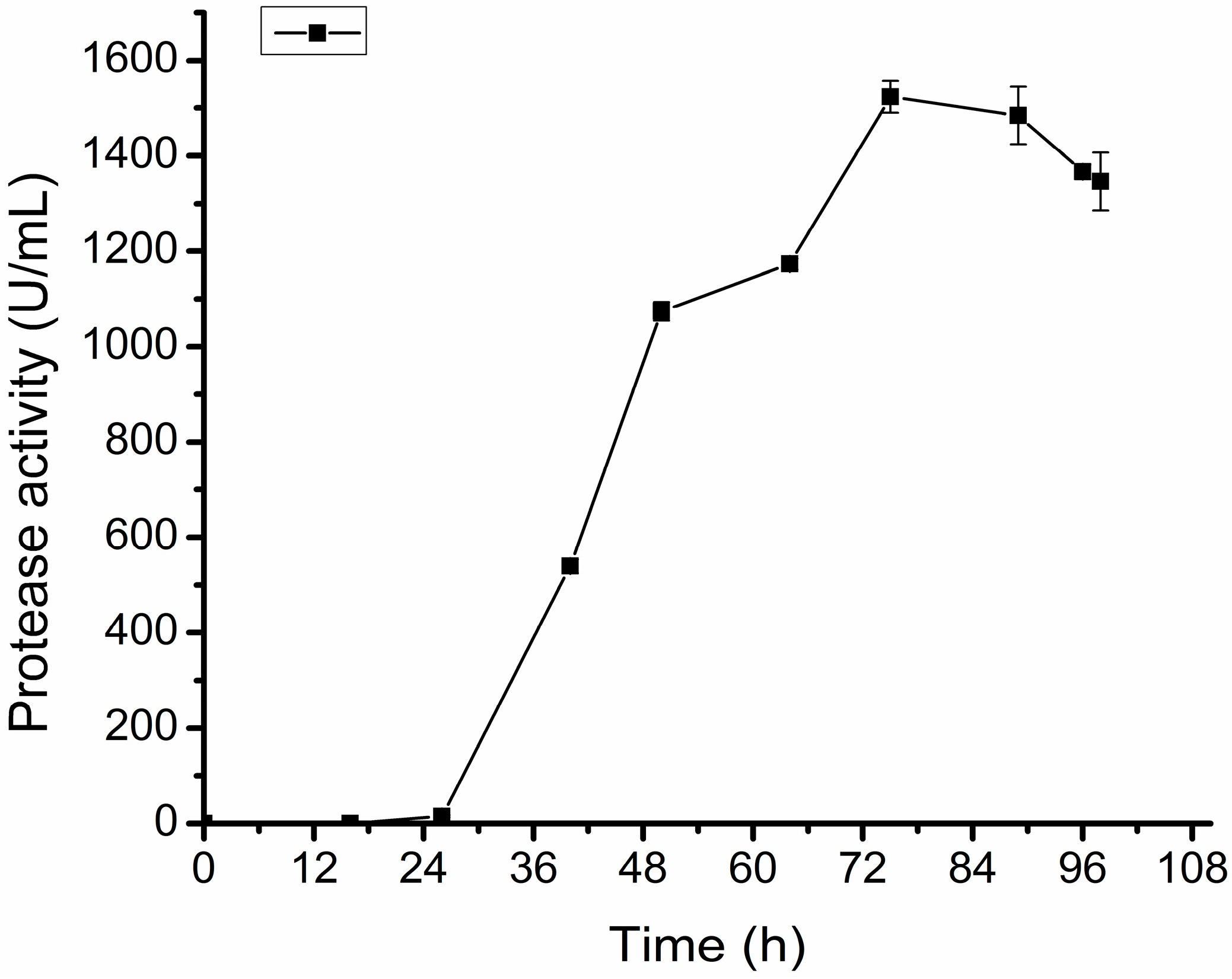
2.5. Pilot Scale Fermentation of D25

3. Experimental Section
3.1. Strain and Media
3.2. Inoculum Preparation and Flask Fermentation
3.3. Assay of the Protease Activity
3.4. Optimization by Single Factor Experiments
3.5. Small Scale Fermentation and Pilot Scale Fermentation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Talbot, V.; Bianchi, M. Bacterial proteolytic activity in sediments of the Subantarctic Indian Ocean sector. Deep Sea Res. Part II Top. Stud. Oceanogr. 1997, 44, 1069–1084. [Google Scholar] [CrossRef]
- Olivera, N.L.; Sequeiros, C.; Nievas, M.L. Diversity and enzyme properties of protease-producing bacteria isolated from sub-Antarctic sediments of Isla de Los Estados, Argentina. Extremophiles 2007, 11, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.Y.; Chen, X.L.; Zhao, H.L.; Dang, H.Y.; Luan, X.W.; Zhang, X.Y.; He, H.L.; Zhou, B.C.; Zhang, Y.Z. Diversity of both the cultivable protease-producing bacteria and their extracellular proteases in the sediments of the South China sea. Microb. Ecol. 2009, 58, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.Y.; Wang, G.L.; Li, D.; Zhao, D.L.; Qin, Q.L.; Chen, X.L.; Chen, B.; Zhou, B.C.; Zhang, X.Y.; Zhang, Y.Z. Diversity of both the cultivable protease-producing bacteria and bacterial extracellular proteases in the coastal sediments of King George Island, Antarctica. PLoS ONE 2013, 8, e79668. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Zhang, Y.Z.; Gao, P.J.; Luan, X.W. Two different proteases produced by a deep-sea psychrotrophic bacterial strain, Pseudoaltermonas sp. SM9913. Mar. Biol. 2003, 143, 989–993. [Google Scholar] [CrossRef]
- Chen, X.L.; Xie, B.B.; Lu, J.T.; He, H.L.; Zhang, Y. A novel type of subtilase from the psychrotolerant bacterium Pseudoalteromonas sp. SM9913: Catalytic and structural properties of deseasin MCP-01. Microbiology 2007, 153, 2116–2125. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.Y.; Chen, X.L.; Zhao, H.L.; Xie, B.B.; Zhou, B.C.; Zhang, Y.Z. Hydrolysis of insoluble collagen by deseasin MCP-01 from deep-sea Pseudoalteromonas sp. SM9913: Collagenolytic characters, collagen-binding ability of C-terminal polycystic kidney disease domain, and implication for its novel role in deep-sea sedimentary particulate organic nitrogen degradation. J. Biol. Chem. 2008, 283, 36100–36107. [Google Scholar] [PubMed]
- Xie, B.B.; Bian, F.; Chen, X.L.; He, H.L.; Guo, J.; Gao, X.; Zeng, Y.X.; Chen, B.; Zhou, B.C.; Zhang, Y.Z. Cold adaptation of zinc metalloproteases in the thermolysin family from deep sea and arctic sea ice bacteria revealed by catalytic and structural properties and molecular dynamics: New insights into relationship between conformational flexibility and hydrogen bonding. J. Biol. Chem. 2009, 284, 9257–9269. [Google Scholar] [PubMed]
- Yan, B.Q.; Chen, X.L.; Hou, X.Y.; He, H.; Zhou, B.C.; Zhang, Y.Z. Molecular analysis of the gene encoding a cold-adapted halophilic subtilase from deep-sea psychrotolerant bacterium Pseudoalteromonas sp. SM9913: Cloning, expression, characterization and function analysis of the C-terminal PPC domains. Extremophiles 2009, 13, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Zhang, R.; Zhao, J.; Lin, N. Cold-active serine alkaline protease from the psychrophilic bacterium Pseudomonas strain DY-A: Enzyme purification and characterization. Extremophiles 2003, 7, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.L.; Chen, X.L.; Xie, B.B.; Zhou, M.Y.; Gao, X.; Zhang, X.Y.; Zhou, B.C.; Weiss, A.S.; Zhang, Y.Z. Elastolytic mechanism of a novel M23 metalloprotease pseudoalterin from deep-sea Pseudoalteromonas sp. CF6-2: Cleaving not only glycyl bonds in the hydrophobic regions but also peptide bonds in the hydrophilic regions involved in cross-linking. J. Biol. Chem. 2012, 287, 39710–39720. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P. Marine enzymes and food industry: Insight on existing and potential interactions. Front. Mar. Sci. 2014, 1. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhang, Y.J.; Chen, X.L.; Qin, Q.L.; Zhao, D.L.; Li, T.G.; Dang, H.Y.; Zhang, Y.Z. Myroides profundi sp. nov., isolated from deep-sea sediment of the southern Okinawa Trough. FEMS Microbiol. Lett. 2008, 287, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Xie, B.B.; Bian, F.; Zhao, G.Y.; Zhao, H.L.; He, H.L.; Zhou, B.C.; Zhang, Y.Z. Ecological function of myroilysin, a novel bacterial M12 metalloprotease with elastinolytic activity and a synergistic role in collagen hydrolysis, in biodegradation of deep-sea high-molecular-weight organic nitrogen. Appl. Environ. Microbiol. 2009, 75, 1838–1844. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Gao, C.; Mao, Z.; Zhou, J.; Shen, J.; Hu, X.; Han, C. Collagen/chitosan porous scaffolds with improved biostability for skin tissue engineering. Biomaterials 2003, 24, 4833–4841. [Google Scholar] [CrossRef]
- Glowacki, J.; Mizuno, S. Collagen scaffolds for tissue engineering. Biopolymers 2008, 89, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Buttafoco, L.; Kolkman, N.G.; Engbers-Buijtenhuijs, P.; Poot, A.A.; Dijkstra, P.J.; Vermes, I.; Feijen, J. Electrospinning of collagen and elastin for tissue engineering applications. Biomaterials 2006, 27, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.D.; Salkin, L.M.; Freedman, A.L.; Glushko, V. Collagen sponge as a topical hemostatic agent in mucogingival surgery. J. Periodontol. 1985, 56, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Babbitt, P.C.; Vasquez, J.R.; West, B.L.; Kenyon, G.L. Cloning and expression of functional rabbit muscle creatine kinase in Escherichia coli. Addressing the problem of microheterogeneity. J. Biol. Chem. 1991, 266, 12053–12057. [Google Scholar] [PubMed]
- Jiang, X.Y.; Wang, C.F.; Wang, C.F.; Zhang, P.J.; He, Z.Y. Cloning and expression of Mycobacterium bovis secreted protein MPB83 in Escherichia coli. J. Biochem. Mol. Biol. 2006, 39, 22–25. [Google Scholar] [PubMed]
- Rawlings, N.D.; Barrett, A.J.; Bateman, A. MEROPS: The peptidase database. Nucleic Acids Res. 2010, 38, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.L.; Yang, J.; Chen, X.L.; Su, H.N.; Zhang, X.Y.; Huang, F.; Zhou, B.C.; Xie, B.B. Optimization of fermentation conditions for the production of the M23 protease Pseudoalterin by deep-sea Pseudoalteromonas sp. CF6-2 with artery powder as an inducer. Molecules 2014, 19, 4779–4790. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compound myroilysin are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, X.; Ran, L.-Y.; Liu, C.; Chen, X.-L.; Zhang, X.-Y.; Qin, Q.-L.; Zhou, B.-C.; Zhang, Y.-Z. Culture Condition Optimization and Pilot Scale Production of the M12 Metalloprotease Myroilysin Produced by the Deep-Sea Bacterium Myroides profundi D25. Molecules 2015, 20, 11891-11901. https://doi.org/10.3390/molecules200711891
Shao X, Ran L-Y, Liu C, Chen X-L, Zhang X-Y, Qin Q-L, Zhou B-C, Zhang Y-Z. Culture Condition Optimization and Pilot Scale Production of the M12 Metalloprotease Myroilysin Produced by the Deep-Sea Bacterium Myroides profundi D25. Molecules. 2015; 20(7):11891-11901. https://doi.org/10.3390/molecules200711891
Chicago/Turabian StyleShao, Xuan, Li-Yuan Ran, Chang Liu, Xiu-Lan Chen, Xi-Ying Zhang, Qi-Long Qin, Bai-Cheng Zhou, and Yu-Zhong Zhang. 2015. "Culture Condition Optimization and Pilot Scale Production of the M12 Metalloprotease Myroilysin Produced by the Deep-Sea Bacterium Myroides profundi D25" Molecules 20, no. 7: 11891-11901. https://doi.org/10.3390/molecules200711891
APA StyleShao, X., Ran, L.-Y., Liu, C., Chen, X.-L., Zhang, X.-Y., Qin, Q.-L., Zhou, B.-C., & Zhang, Y.-Z. (2015). Culture Condition Optimization and Pilot Scale Production of the M12 Metalloprotease Myroilysin Produced by the Deep-Sea Bacterium Myroides profundi D25. Molecules, 20(7), 11891-11901. https://doi.org/10.3390/molecules200711891





