Volatile Profile of Cashew Apple Juice Fibers from Different Production Steps
Abstract
:1. Introduction
2. Results and Discussion
| Peak | KI | Compound | Juice | Area Counts × 106 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Co-Products | |||||||||
| Fiber | Wash_1 | Wash_2 | Wash_3 | Wash_4 | Wash_5 | ||||
| 1 | <800 | 3-methylbutanal | 0.50 ± 0.0 | 0.63 ± 0.0 | 1.02 ± 0.2 | nd | nd | nd | nd |
| 2 | <800 | acetic acid | 13.55 ± 2.7 | 0.07 ± 0.3 | 13.42 ± 1.9 | nd | nd | nd | nd |
| 3 | 857 | ethyl (E)-2-butenoate | 15.14 ± 0.9 | 1.28 ± 0.2 | nd | nd | nd | nd | nd |
| 4 | 860 | ethyl 2-methyl butanoate | 10.62 ± 1.4 | 1.90 ± 0.2 | nd | nd | nd | nd | nd |
| 5 | 866 | ethyl 3-methyl butanoate | 130.05 ± 30.7 | 21.20 ± 4.5 | 6.77 ± 1.5 | 1.70 ± 0.3 | nd | nd | nd |
| 6 | 958 | ethyl (E)-2-methyl-2-butenoate | 6.92 ± 1.2 | nd | 0.73 ± 0.2 | nd | nd | nd | nd |
| 7 | 976 | ethyl 3-methyl pentanoate | 6.47 ± 1.1 | 0.41 ± 0.1 | 2.40 ± 0.4 | 1.04 ± 0.1 | nd | nd | nd |
| 8 | 981 | ethyl 4-methyl pentanoate | 1.89 ± 0.3 | nd | 0.52 ± 0.0 | nd | nd | nd | nd |
| 9 | 1004 | ethyl hexanoate | 27.33 ± 3.8 | 15.51 ± 2.0 | 9.23 ± 1.2 | 1.92 ± 0.3 | 1.56 ± 0.2 | 0.97 ± 0.1 | nd |
| 10 | 1012 | octanal | 2.65 ± 0.2 | nd | 0.95 ± 0.0 | nd | nd | nd | nd |
| 11 | 1034 | limonene | nd | nd | 0.96 ± 0.2 | 0.41 ± 0.1 | nd | nd | nd |
| 12 | 1057 | 3-methylbutyl butanoate | 1.24 ± 0.1 | 1.22 ± 0.1 | 2.03 ± 0.2 | 0.89 ± 0.1 | 0.39 ± 0.0 | 0.31 ± 0.0 | 0.11 ± 0.0 |
| 13 | 1065 | amyl butanoate | nd | nd | 0.90 ± 0.2 | nd | nd | nd | nd |
| 14 | 1081 | 1-octanol | 2.95 ± 0.1 | nd | 2.80 ± 0.2 | nd | nd | nd | nd |
| 15 | 1088 | terpinolene | nd | nd | 1.15 ± 0.1 | nd | nd | nd | nd |
| 16 | 1102 | ethyl heptanoate | nd | 0.93 ± 0.1 | 2.63 ± 0.2 | 0.81 ± 0.1 | 0.64 ± 0.0 | 0.46 ± 0.0 | 0.39 ± 0.0 |
| 17 | 1111 | (E)-2-nonen-1-ol | 8.97 ± 0.5 | 8.17 ± 0.4 | 12.12 ± 0.7 | 10.57 ± 0.6 | 9.13 ± 0.5 | 8.90 ± 0.5 | 8.77 ± 0.5 |
| 18 | 1152 | amyl 3-methyl butanoate | nd | 0.23 ± 0.0 | 0.96 ± 0.0 | 0.23 ± 0.0 | 0.19 ± 0.0 | nd | nd |
| 19 | 1181 | 1-nonanol | 2.05 ± 0.3 | nd | 2.06 ± 0.3 | nd | nd | nd | nd |
| 20 | 1194 | ethyl 7-octenoate | nd | 0.80 ± 0.2 | 1.57 ± 0.2 | 0.60 ± 0.0 | 0.55 ± 0.0 | 0.42 ± 0.0 | 0.40 ± 0.0 |
| 21 | 1200 | ethyl octanoate | 2.91 ± 0.3 | 38.86 ± 4.2 | 31.65 ± 3.5 | 39.37 ± 4.0 | 32.42 ± 3.6 | 25.45 ± 2.8 | 24.24 ± 2.5 |
| 22 | 1214 | decanal | 1.39 ± 0.1 | 0.42 ± 0.0 | 1.45 ± 0.2 | 0.68 ± 0.0 | 0.81 ± 0.0 | 0.72 ± 0.0 | 0.99 ± 0.1 |
| 23 | 1244 | (Z)-3-hexenyl isovalerate | nd | 0.20 ± 0.0 | 4.50 ± 0.5 | 0.20 ± 0.0 | 0.16 ± 0.0 | nd | nd |
| 24 | 1248 | hexyl 3-methyl butanoate | nd | 0.28 ± 0.0 | nd | 0.37 ± 0.0 | 0.24 ± 0.0 | 0.32 ± 0.0 | 0.32 ± 0.0 |
| 25 | 1254 | 3-methylbutyl hexanoate | nd | 2.63 ± 0.5 | 6.93 ± 0.9 | 3.24 ± 0.5 | 2.47 ± 0.4 | 2.26 ± 0.4 | 2.00 ± 0.3 |
| 26 | 1277 | 2-butyl-1-octanol | nd | nd | 0.89 ± 0.1 | nd | nd | nd | nd |
| 27 | 1274 | (E)-2-decenal | nd | 1.17 ± 0.2 | 5.09 ± 0.7 | 0.92 ± 0.2 | 0.81 ± 0.2 | 1.09 ± 0.2 | 1.08 ± 0.2 |
| 28 | 1290 | pentyl hexanoate | nd | nd | 0.90 ± 0.1 | 0.10 ± 0.0 | nd | nd | nd |
| 29 | 1297 | ethyl nonanoate | 0.30 ± 0.0 | 1.78 ± 0.2 | 2.32 ± 0.2 | 1.18 ± 0.1 | 0.96 ± 0.1 | 0.89 ± 0.1 | 1.13 ± 0.1 |
| 30 | 1302 | tridecane | nd | 0.34 ± 0.0 | 1.04 ± 0.2 | 0.21 ± 0.0 | nd | nd | nd |
| 31 | 1312 | undecanal | nd | nd | 0.44 ± 0.0 | nd | nd | nd | nd |
| 32 | 1318 | 6-methyltridecane | nd | nd | 0.39 ± 0.0 | nd | nd | nd | nd |
| 33 | 1345 | NI | nd | 0.26 ± 0.0 | 0.39 ± 0.0 | nd | nd | nd | nd |
| 34 | 1357 | α-cubebene | 9.21 ± 0.2 | 8.11 ± 0.2 | 3.04 ± 0.1 | 2.67 ± 0.1 | 2.36 ± 0.1 | nd | nd |
| 35 | 1372 | 2-methyltridecane | nd | 0.61 ± 0.0 | nd | nd | 0.31 ± 0.0 | 0.24 ± 0.0 | 0.60 ± 0.0 |
| 36 | 1377 | 2,6,10-trimethyldodecane | nd | nd | 4.69 ± 0.5 | 0.87 ± 0.1 | 0.39 ± 0.0 | 0.22 ± 0.0 | 0.83 ± 0.1 |
| 37 | 1382 | NI | 0.67 ± 0.0 | 1.33 ± 0.0 | 1.89 ± 0.1 | 0.57 ± 0.0 | 0.52 ± 0.0 | nd | nd |
| 38 | 1383 | octyl butanoate | nd | 0.99 ± 0.0 | nd | nd | nd | 0.42 ± 0.0 | 0.70 ± 0.0 |
| 39 | 1391 | copaene | 0.28 ± 0.0 | 3.63 ± 0.5 | 4.11 ± 0.5 | 2.42 ± 0.4 | 2.48 ± 0.4 | 1.73 ± 0.2 | 2.22 ± 0.3 |
| 40 | 1398 | ethyl decanoate | 0.50 ± 0.1 | 6.06 ± 0.3 | 7.52 ± 0.3 | 4.97 ± 0.2 | 4.37 ± 0.1 | 3.73 ± 0.1 | 4.03 ± 0.1 |
| 41 | 1402 | tetradecane | 0.27 ± 0.0 | 6.35 ± 0.3 | 11.41 ± 0.8 | 5.23 ± 0.2 | 3.55 ± 0.2 | 2.93 ± 0.2 | 4.13 ± 0.2 |
| 42 | 1412 | NI | nd | nd | 2.97 ± 0.1 | nd | nd | nd | nd |
| 43 | 1416 | NI | nd | nd | 2.00 ± 0.1 | nd | nd | nd | nd |
| 44 | 1424 | cedrene | nd | 3.22 ± 0.2 | 2.22 ± 0.2 | 1.53 ± 0.1 | 1.46 ± 0.1 | 1.00 ± 0.1 | 1.95 ± 0.1 |
| 45 | 1433 | α-santalene | nd | 0.60 ± 0.0 | 1.73 ± 0.3 | nd | nd | nd | nd |
| 46 | 1439 | caryophyllene | 0.56 ± 0.1 | 2.81 ± 0.5 | 2.67 ± 0.4 | 1.70 ± 0.3 | 1.55 ± 0.3 | 1.07 ± 0.2 | 1.67 ± 0.2 |
| 47 | 1445 | (E)-α-bergamotene | 0.36 ± 0.0 | 2.54 ± 0.3 | 3.35 ± 0.4 | 1.29 ± 0.2 | 1.11 ± 0.1 | 0.75 ± 0.0 | 1.19 ± 0.2 |
| 48 | 1451 | 3-methylbutyl octanoate | nd | 1.50 ± 0.2 | 2.74 ± 0.2 | 0.90 ± 0.1 | 0.86 ± 0.1 | 0.73 ± 0.0 | 0.92 ± 0.1 |
| 49 | 1454 | (Z)-geranylacetone | nd | nd | 2.19 ± 0.3 | 0.95 ± 0.2 | 0.92 ± 0.2 | 0.76 ± 0.0 | 0.98 ± 0.1 |
| 50 | 1461 | NI | 1.04 ± 0.2 | 2.74 ± 0.5 | 4.43 ± 0.6 | nd | nd | nd | nd |
| 51 | 1467 | 2-methyltetradecane | 0.36 ± 0.0 | 0.69 ± 0.0 | 1.87 ± 0.1 | 0.69 ± 0.0 | 0.48 ± 0.0 | nd | 0.42 ± 0.0 |
| 52 | 1475 | 2,6-di-tert-butylbenzoquinone | 0.48 ± 0.1 | 1.11 ± 0.1 | 5.82 ± 0.4 | 0.61 ± 0.1 | 0.47 ± 0.0 | 0.69 ± 0.0 | 0.65 ± 0.0 |
| 53 | 1484 | (E)-ethyl cinnamate | 1.74 ± 0.3 | 1.84 ± 0.3 | 16.99 ± 2.6 | 1.71 ± 0.2 | 1.60 ± 0.2 | 1.43 ± 0.2 | 1.56 ± 0.2 |
| 54 | 1491 | selinene | nd | 2.20 ± 0.2 | 6.06 ± 0.5 | 1.01 ± 0.1 | 0.99 ± 0.1 | 0.88 ± 0.1 | 0.98 ± 0.1 |
| 55 | 1501 | γ-muurolene | nd | 6.89 ± 0.4 | 12.78 ± 1.8 | 5.71 ± 0.6 | 5.46 ± 0.6 | 4.69 ± 0.5 | 5.57 ± 0.7 |
| 56 | 1506 | pentadecane | 0.85 ± 0.2 | nd | 1.63 ± 0.3 | nd | nd | nd | nd |
| 57 | 1513 | β-germacrene | nd | 0.71 ± 0.0 | 5.60 ± 0.2 | 2.77 ± 0.1 | 2.68 ± 0.1 | 2.52 ± 0.1 | 2.86 ± 0.1 |
| 58 | 1517 | γ-elemene | nd | 2.41 ± 0.3 | nd | nd | nd | nd | nd |
| 59 | 1522 | β-bisabolene | 1.06 ± 0.3 | 1.36 ± 0.2 | 0.81 ± 0.1 | nd | nd | nd | nd |
| 60 | 1534 | δ-cadinene | 0.42 ± 0.0 | 3.40 ± 0.3 | 3.71 ± 0.3 | 1.84 ± 0.2 | 1.77 ± 0.2 | 1.65 ± 0.1 | 1.81 ± 0.2 |
| 61 | 1551 | cadine-1,4-diene | nd | 0.61 ± 0.0 | 0.33 ± 0.0 | nd | nd | nd | nd |
| 62 | 1562 | dodecanoic acid | nd | nd | 1.26 ± 0.2 | nd | nd | nd | nd |
| 63 | 1574 | NI | nd | 0.72 ± 0.1 | 1.15 ± 0.1 | 0.42 ± 0.0 | 0.51 ± 0.0 | 0.43 ± 0.0 | 0.49 ± 0.0 |
| 64 | 1594 | ethyl dodecanoate | nd | 1.05 ± 0.2 | 2.18 ± 0.2 | 0.94 ± 0.1 | 0.76 ± 0.1 | 1.01 ± 0.1 | 0.85 ± 0.1 |
| 65 | 1603 | hexadecane | 0.36 ± 0.0 | 1.79 ± 0.2 | 1.12 ± 0.1 | 2.66 ± 0.2 | 1.05 ± 0.1 | 1.26 ± 0.01 | 1.00 ± 0.0 |
| 66 | 1618 | tetradecanal | 0.44 ± 0.1 | 0.49 ± 0.0 | 0.69 ± 0.0 | 0.54 ± 0.0 | 0.46 ± 0.0 | 0.67 ± 0.0 | 0.95 ± 0.1 |
| 67 | 1645 | 2,6,10-trimethylpentadecane | 0.92 ± 0.2 | 0.39 ± 0.0 | 1.59 ± 0.2 | 0.32 ± 0.0 | nd | nd | nd |
| 68 | 1680 | tetradecanol | 0.32 ± 0.0 | nd | 4.13 ± 0.2 | 0.45 ± 0.0 | 0.28 ± 0.0 | 0.47 ± 0.0 | 0.45 ± 0.0 |
| 69 | 1693 | γ-dodecalactone | 2.09 ± 0.6 | 2.87 ± 0.3 | 4.70 ± 0.3 | 3.31 ± 0.2 | 3.84 ± 0.4 | 4.40 ± 0.5 | 3.13 ± 0.2 |
| 70 | 1701 | heptadecane | nd | 0.80 ± 0.1 | 0.84 ± 0.1 | 0.77 ± 0.0 | 0.58 ± 0.0 | 0.56 ± 0.0 | nd |
| 71 | 1720 | pentadecanal | nd | 0.89 ± 0.1 | 2.87 ± 0.3 | 0.79 ± 0.0 | 1.37 ± 0.1 | 1.15 ± 0.1 | 1.57 ± 0.1 |
| 72 | 1758 | tetradecanoic acid | nd | nd | 3.83 ± 0.5 | 0.54 ± 0.0 | nd | nd | nd |
| 73 | 1765 | NI | 0.47 ± 0.1 | 1.97 ± 0.5 | 3.34 ± 0.7 | 1.17 ± 0.4 | 1.07 ± 0.2 | 1.12 ± 0.2 | 1.31 ± 0.2 |
| 74 | 1801 | octadecane | nd | 0.14 ± 0.0 | 0.46 ± 0.0 | 0.41 ± 0.0 | nd | nd | nd |
| 75 | 1822 | hexadecanal | nd | 1.75 ± 0.2 | 2.76 ± 0.2 | 0.77 ± 0.0 | 0.69 ± 0.0 | 0.61 ± 0.0 | 0.83 ± 0.1 |
| 76 | 1828 | pentadecanoic acid | nd | nd | 0.34 ± 0.0 | nd | nd | nd | nd |
| 77 | 1857 | NI | nd | nd | 1.39 ± 0.2 | nd | nd | nd | nd |
| 78 | 1884 | 1-hexadecanol | nd | nd | 4.10 ± 0.2 | 0.98 ± 0.0 | nd | nd | 1.70 ± 0.1 |
| 79 | 1901 | NI | nd | nd | 1.29 ± 0.1 | nd | 0.63 ± 0.0 | 6.26 ± 0.3 | nd |
| 80 | 1938 | 9-hexadecenoic acid | nd | nd | 2.87 ± 0.2 | nd | nd | nd | nd |
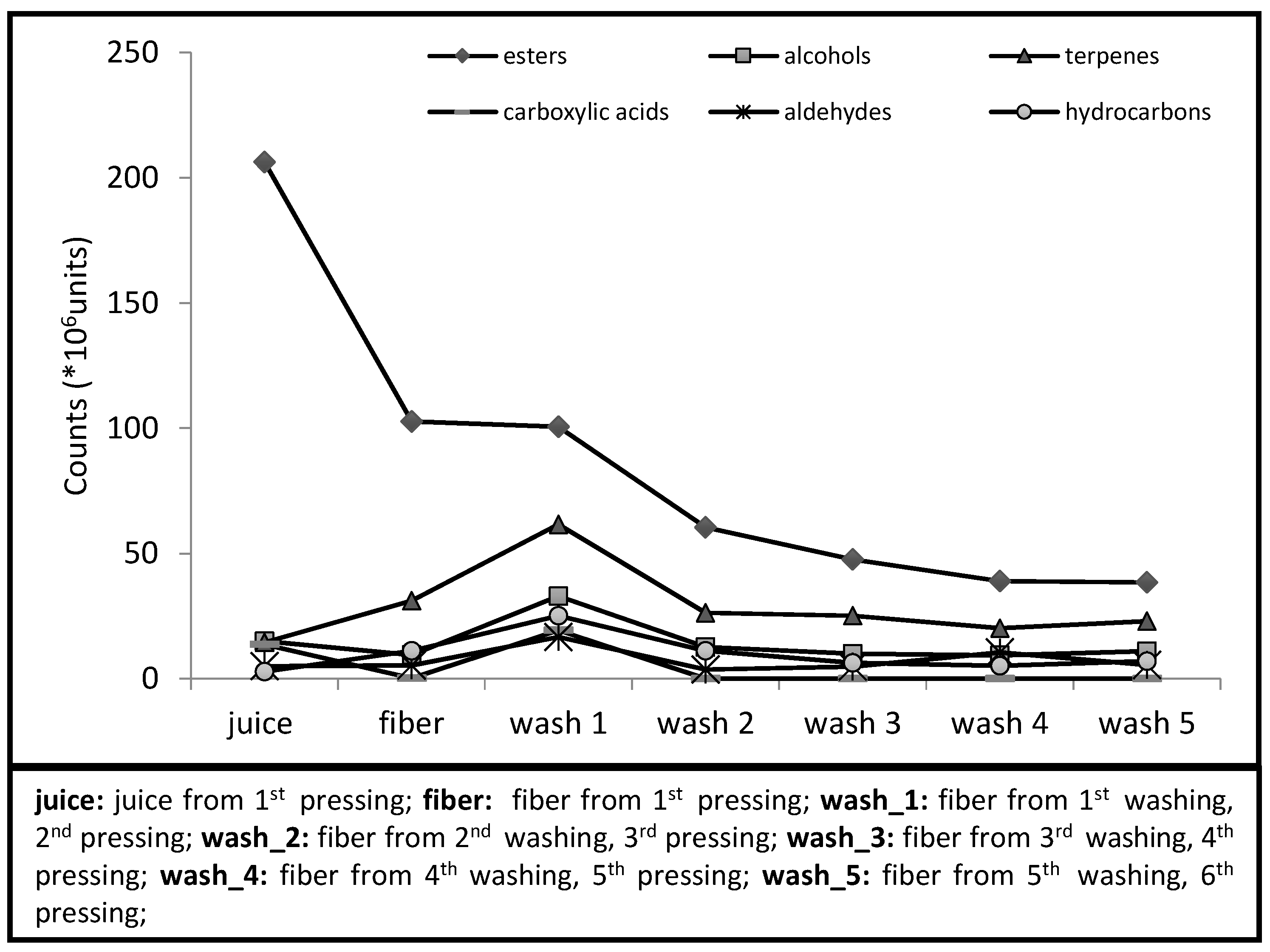
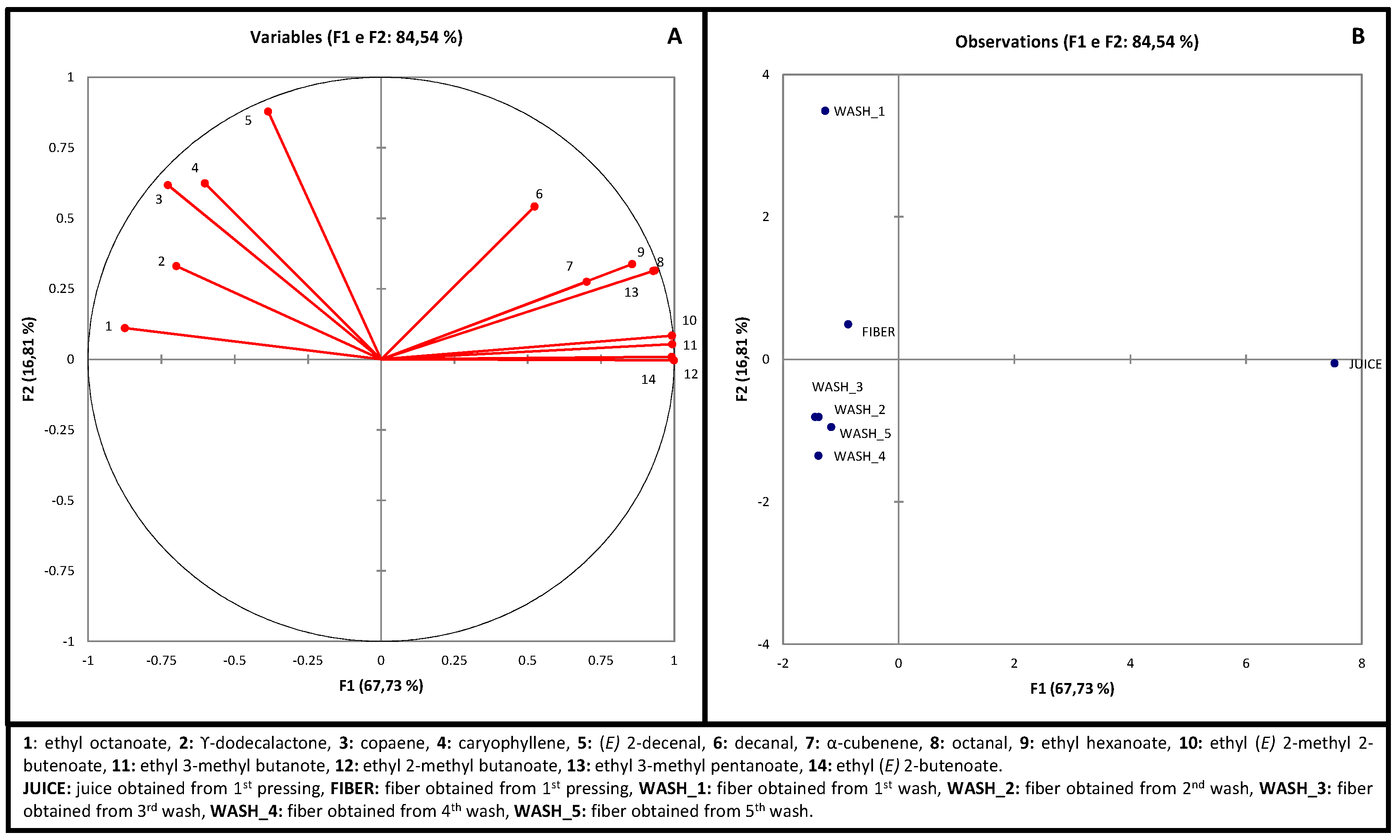
| Fiber | Wash_1 | Wash_2 | Wash_3 | Wash_4 | Wash_5 | |
|---|---|---|---|---|---|---|
| Panel Mean | 4.68 a | 4.22 a | 2.96 b | 2.54 b | 2.18 b | 2.46 b |
3. Experimental Section
3.1. Raw Material
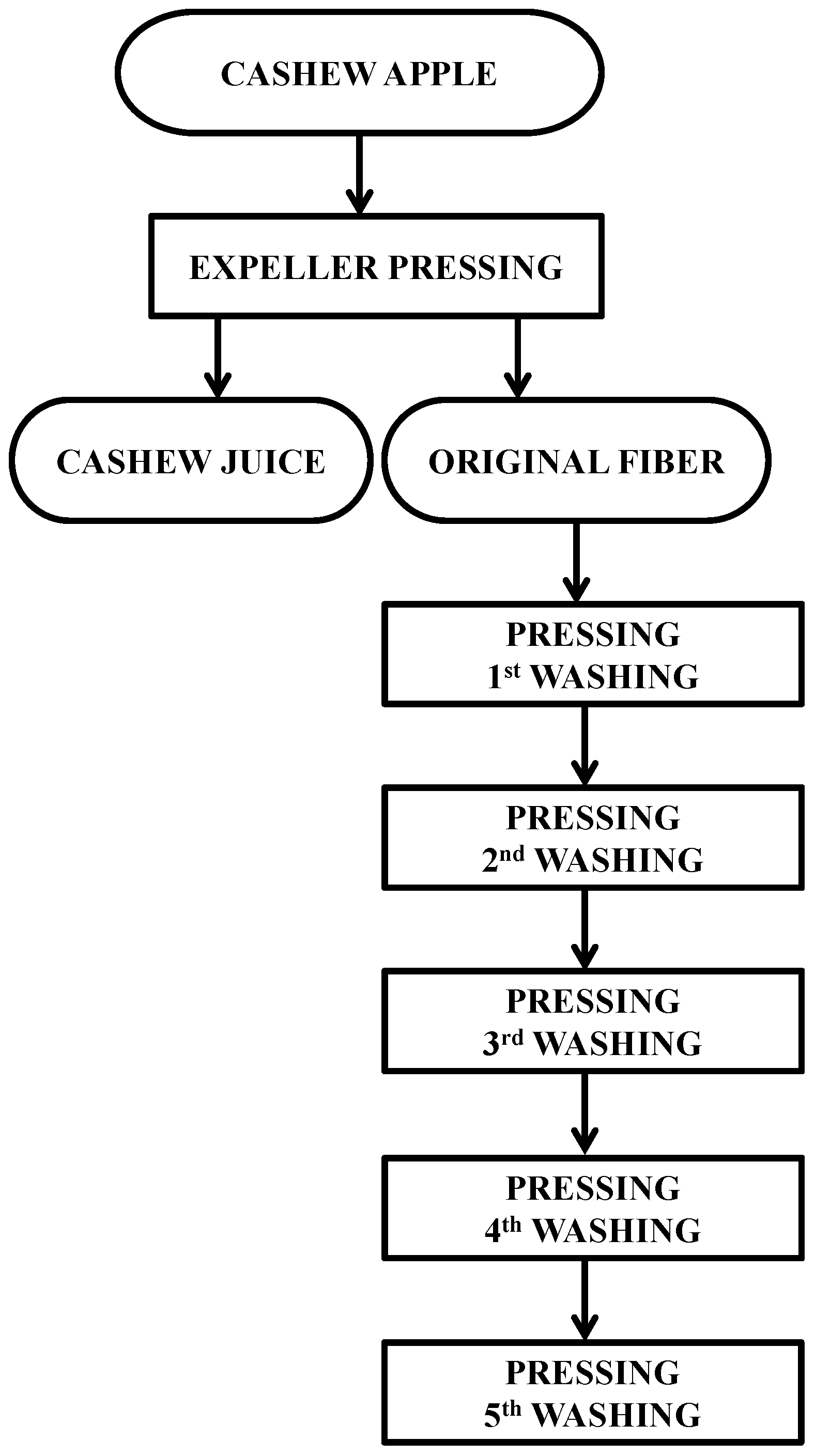
3.2. Cashew Apple Processing
3.3. Isolation of Volatile Compounds
3.4. Analysis of Volatiles by Gas Chromatography-Mass Spectrometry (GC-MS)
3.5. Identification of Volatiles
3.6. Sensory Analysis
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pinho, L.X.; Afonso, M.R.A.; Carioca, J.O.B.; Costa, J.M.C.; Rybka, A.C.P. Desidratação e aproveitamento de resíduo de pedúnculo de caju como adição de fibra na elaboração de hambúrguer. Aliment. Nutr. 2011, 22, 571–576. [Google Scholar]
- Lima, J.R. Physical chemical and sensory characterization of vegetal hamburger elaborated from cashew apple. Ciênc. Agrotecnol. 2008, 32, 191–195. [Google Scholar] [CrossRef]
- Barros, N.V.A.; Costa, N.Q.; Porto, R.G.C.L.; Morgano, M.A.; Araújo, M.A.M.; Araújo, R.S.R.M. Elaboração de hambúrguer enriquecido com fibras de caju. Bol. CEPPA 2012, 30, 315–325. [Google Scholar]
- Lima, J.R. Hambúrguer de caju: Elaboração e Características. In Comunicado Técnico 131; Embrapa: Fortaleza, CE, Brazil, 2007. [Google Scholar]
- Oliveira, C.F.P.; Malta, H.L.; Jesus, M.A.C.L.; Cruz, R.S.; Cardoso, F.S.N. Desenvolvimento, avaliação sensorial e físico-química de barra de cereal de caju. Rev. Brasil. Tecnol. Agroind. 2013, 7, 934–942. [Google Scholar] [CrossRef]
- Pinheiro, H.D.; Soares, L.S. Processo inovador no marketing dos derivados do caju no mercado varejista de Teresina. Inf. Cient. FAPEPI 2007, 13, 3–4. [Google Scholar]
- Lima, J.R.; Modesto, A.L.G.; Garruti, D.S.; Firmino, D.S.; Araújo, I.M.S.; Moraes, I.V.M. Elaboração de hambúrguer vegetal de fibra de caju e feijão-caupi. In Comunicado Técnico 203; Embrapa: Fortaleza, CE, Brazil, 2013. [Google Scholar]
- Franco, M.R.B.; Janzantti, N.S. Aroma de Frutas Tropicais: Avanços na metodologia instrumental da pesquisa do sabor. In Aroma e sabor de alimentos: Temas atuais; Franco, M.R.B., Ed.; Editora Varela: São Paulo, Brazil, 2003; pp. 26–34. [Google Scholar]
- Garruti, D.S.; Franco, M.R.B.; Da Silva, M.A.A.P.; Janzantti, N.S.; Alves, G.L. Evaluation of volatile flavor compounds from cashew apple (Anacardium occidentale L.) juice by the OSME gas chromatography; olfactometry technique. J. Sci. Food Agric. 2003, 83, 1455–1462. [Google Scholar] [CrossRef]
- Sampaio, K.L.; Garruti, D.S.; Franco, M.R.B.; Janzantti, N.S.; Da Silva, M.A.A.P. Aroma volatiles recovered in the water phase of cashew apple (Anacardium occidentale L.) juice during concentration. J. Sci. Food Agric. 2011, 91, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, K.L.; Biasoto, A.C.T.; Marques, E.J.N.; Da Silva, M.A.A.P. Perfil de compostos voláteis em polpa pasteurizada de caju. In Proceeding of Anais... XXIII Congresso Brasileiro de Ciência e Tecnologia De Alimentos, Centro de Convenções da Unicamp, Campinas-SP, Brazil, 01–04 May 2012; 2012. [Google Scholar]
- Macleod, A.J.; Troconis, N.G. Volatile flavour components of cashew “apple” (Anacardium occidentale). Pthytochemistry 1982, 21, 2527–2530. [Google Scholar] [CrossRef]
- Biasoto, A.C.T. Dinâmica da perda e formação de compostos voláteis durante a concentração de suco de caju (Anacardium occidentale L.) e impacto sobre o perfil sensorial da bebida. Ph.D. Thesis, Universidade Estadual de Campinas, Campinas-SP, Brazil, 2013. [Google Scholar]
- Garruti, D.S.; Facundo, H.V.V.; Souza Neto, M.A.; Wagner, R. Changes in the key odour-active compounds and sensory profile of cashew apple juice during processing. In Expression of Multidisciplinary Flavour Science; Blank, I., Wüst, M., Yeretzian, C., Eds.; ZHAW: Wädenswil, Switzerland, 2010; pp. 215–218. [Google Scholar]
- Valim, M.F.; Rouseff, L.R.; Lin, J. Gas cromatographic-olfatometric characterization of aroma compounds in two types of cashew apple nectar. J. Agric. Food Chem. 2003, 51, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- NIST, National Institute of Standards and Technology. Database Standard Reference Number 69. Available online: http://webbook.nist.gov/chemistry/ (accessed on 12 August 2014).
- Macfie, H.J.; Bratchell, N.; Greenhoff, K.; Vallis, L. Designs to balance the effect of order of presentation and first-order carry-over effects in hall tests. J. Sens. Stud. 1989, 4, 129–148. [Google Scholar] [CrossRef]
- SAS (Statistical Analysis System) for Windows; Version 9.2 [CD-ROM]; SAS Institute Inc.: Cary, NC, USA, 2008.
- Sample Availability: Samples of cashew juice and cashew fibers are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nobre, A.C.d.O.; De Almeida, Á.S.S.d.S.; Lemos, A.P.D.; Magalhães, H.C.R.; Garruti, D.D.S. Volatile Profile of Cashew Apple Juice Fibers from Different Production Steps. Molecules 2015, 20, 9803-9815. https://doi.org/10.3390/molecules20069803
Nobre ACdO, De Almeida ÁSSdS, Lemos APD, Magalhães HCR, Garruti DDS. Volatile Profile of Cashew Apple Juice Fibers from Different Production Steps. Molecules. 2015; 20(6):9803-9815. https://doi.org/10.3390/molecules20069803
Chicago/Turabian StyleNobre, Ana Carolina de Oliveira, Áfia Suely Santos da Silva De Almeida, Ana Paula Dajtenko Lemos, Hilton César Rodrigues Magalhães, and Deborah Dos Santos Garruti. 2015. "Volatile Profile of Cashew Apple Juice Fibers from Different Production Steps" Molecules 20, no. 6: 9803-9815. https://doi.org/10.3390/molecules20069803
APA StyleNobre, A. C. d. O., De Almeida, Á. S. S. d. S., Lemos, A. P. D., Magalhães, H. C. R., & Garruti, D. D. S. (2015). Volatile Profile of Cashew Apple Juice Fibers from Different Production Steps. Molecules, 20(6), 9803-9815. https://doi.org/10.3390/molecules20069803





