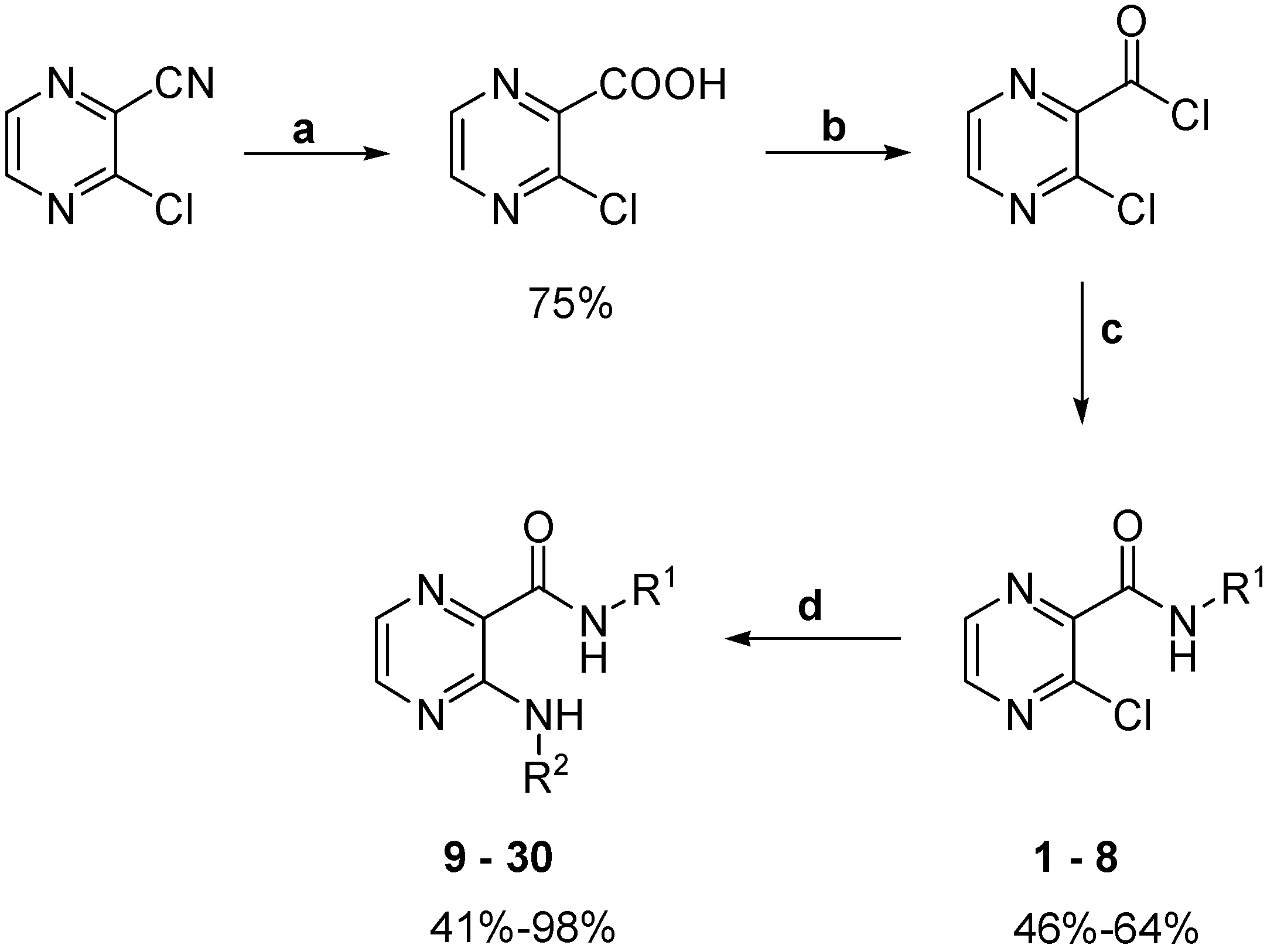
Synthesis and Biological Evaluation of N-Alkyl-3-(alkylamino)-pyrazine-2-carboxamides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
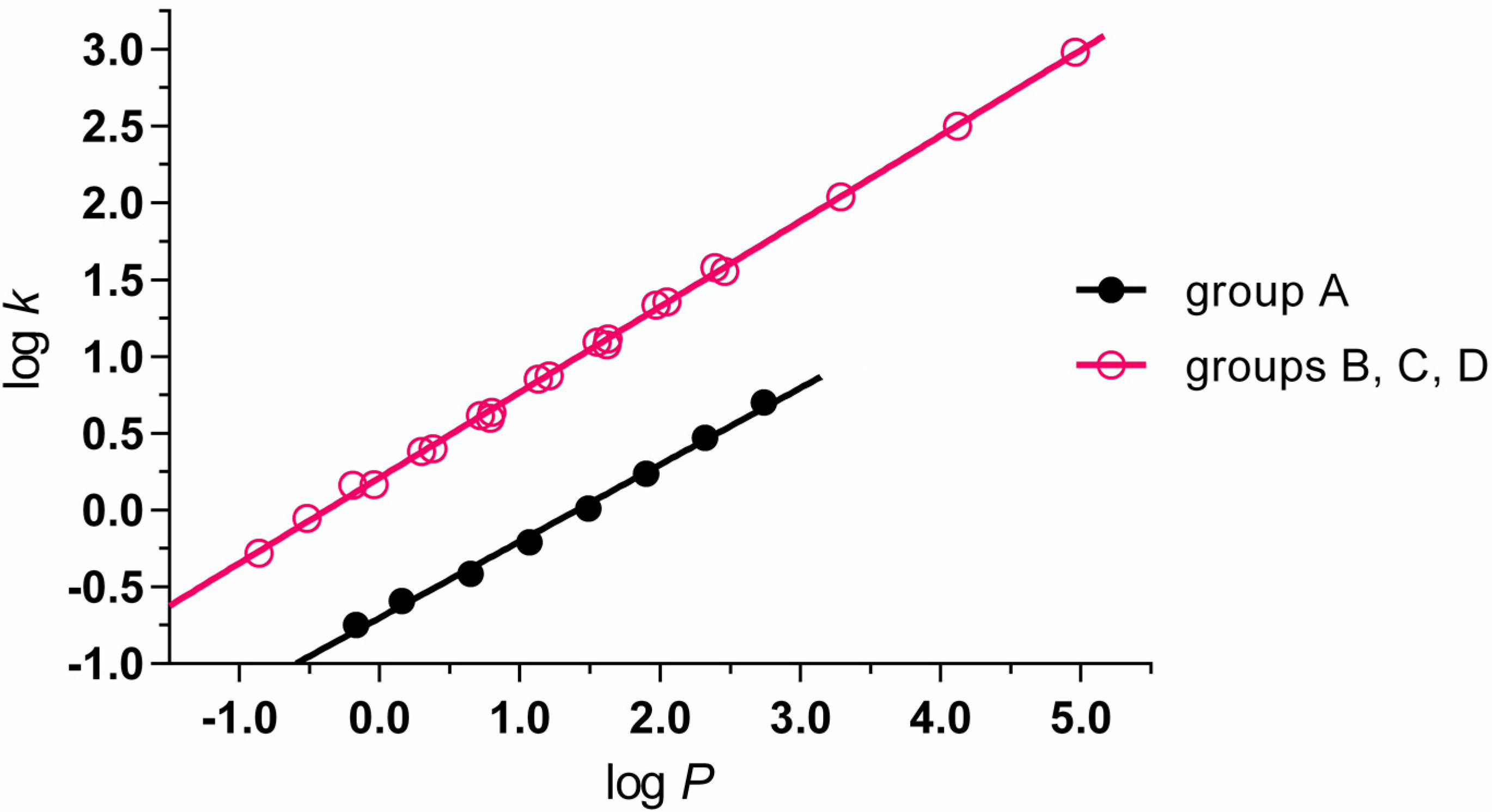
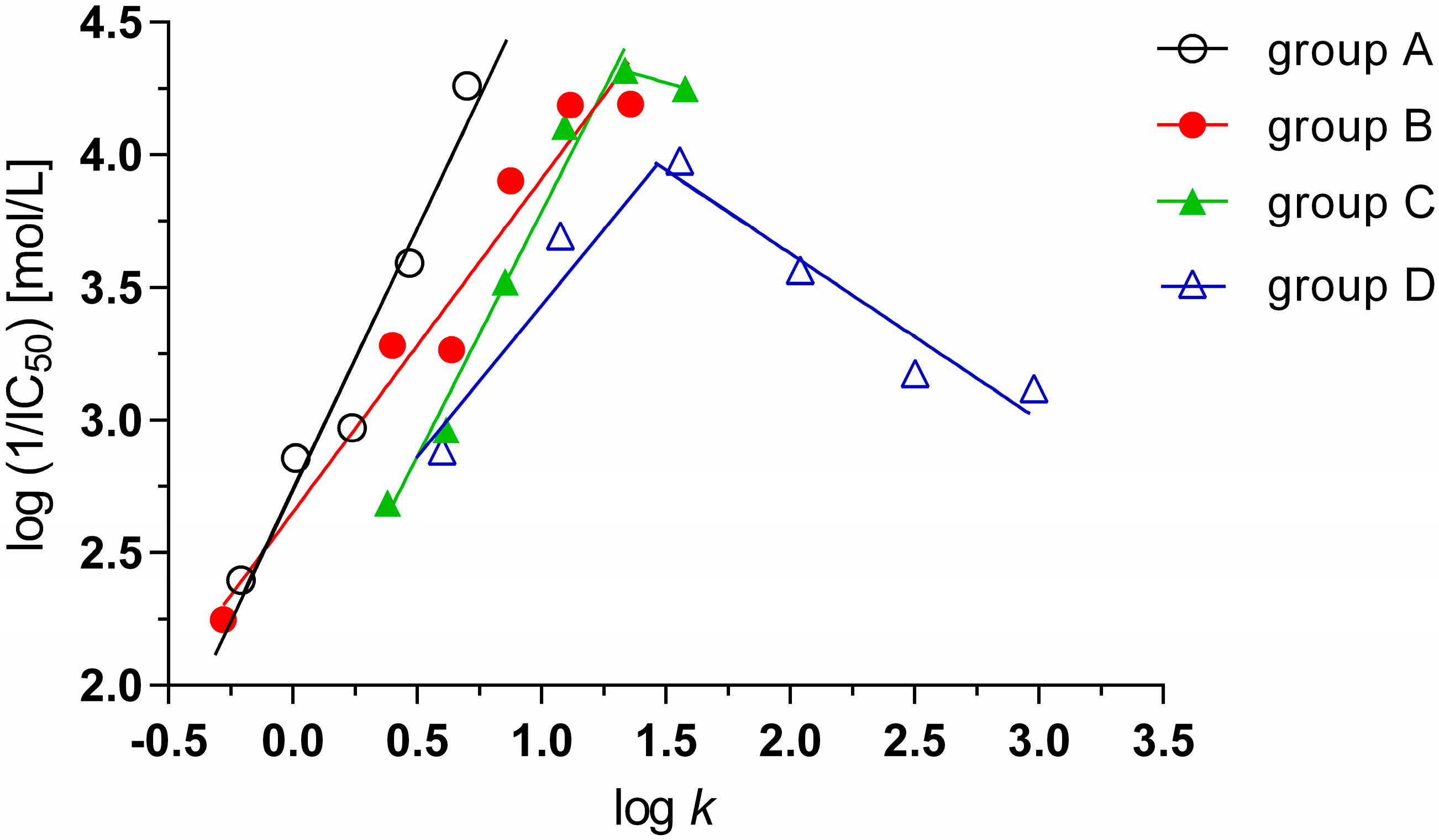
2.2. Lipophilicity
| No. | Structure | R1 | R2 | log P | log k | MIC M. tuberculosis [µg/mL] | IC50 [mmol/L] | |
|---|---|---|---|---|---|---|---|---|
| Group A | 1 | 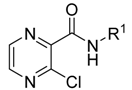 | CH3 | - | −0.17 | −0.747 | >100 | ND |
| 2 | C2H5 | - | 0.16 | −0.591 | >100 | ND | ||
| 3 | C3H7 | - | 0.65 | −0.414 | >100 | ND | ||
| 4 | C4H9 | - | 1.07 | −0.209 | >100 | 4.014 | ||
| 5 | C5H11 | - | 1.49 | 0.012 | >100 | 1.390 | ||
| 6 | C6H13 | - | 1.90 | 0.239 | 100 | 1.067 | ||
| 7 | C7H15 | - | 2.32 | 0.470 | 100 | 0.256 | ||
| 8 | C8H17 | - | 2.74 | 0.702 | 50 | 0.055 | ||
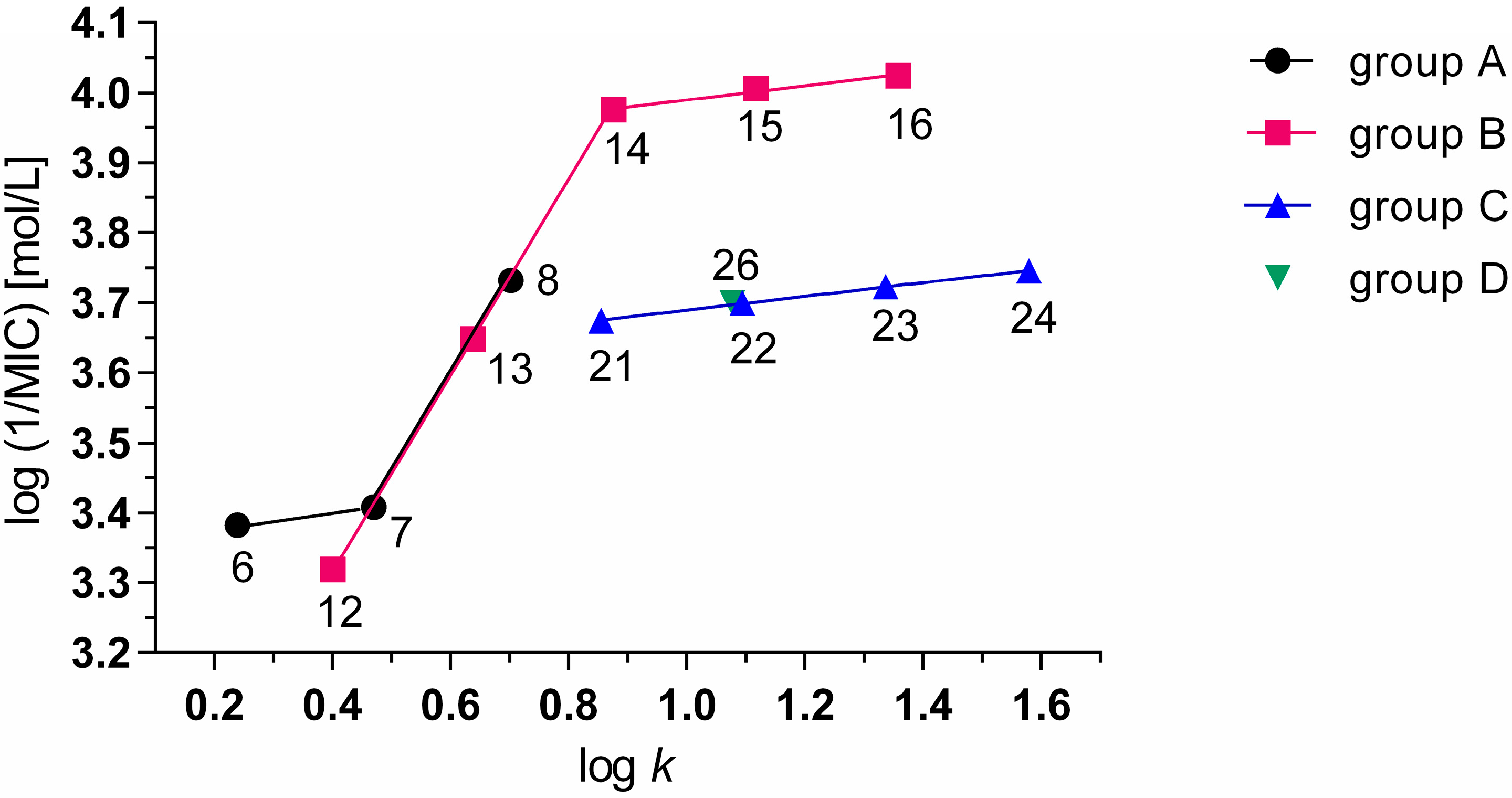
| Group B | 9 |  | CH3 | CH3 | −0.86 | −0.279 | >100 | 5.646 |
| 10 | C2H5 | −0.52 | −0.052 | >100 | ND | |||
| 11 | C3H7 | −0.04 | 0.166 | >100 | ND | |||
| 12 | C4H9 | 0.38 | 0.401 | 100 | 0.523 | |||
| 13 | C5H11 | 0.80 | 0.638 | 50 | 0.544 | |||
| 14 | C6H13 | 1.21 | 0.876 | 25 | 0.125 | |||
| 15 | C7H15 | 1.63 | 1.118 | 25 | 0.065 | |||
| 16 | C8H17 | 2.05 | 1.359 | 25 | 0.064 | |||
| Group C | 17 | C2H5 | CH3 | −0.52 | −0.063 | >100 | ND | |
| 18 | C2H5 | −0.19 | 0.163 | >100 | ND | |||
| 19 | C3H7 | 0.30 | 0.382 | >100 | 2.047 | |||
| 20 | C4H9 | 0.72 | 0.617 | >100 | 1.090 | |||
| 21 | C5H11 | 1.13 | 0.855 | 50 | 0.301 | |||
| 22 | C6H13 | 1.55 | 1.094 | 50 | 0.078 | |||
| 23 | C7H15 | 1.97 | 1.337 | 50 | 0.048 | |||
| 24 | C8H17 | 2.39 | 1.579 | 50 | 0.056 | |||
| Group D | 25 | C3H7 | C3H7 | 0.79 | 0.600 | >100 | 1.303 | |
| 26 | C4H9 | C4H9 | 1.62 | 1.077 | 50 | 0.202 | ||
| 27 | C5H11 | C5H11 | 2.46 | 1.557 | >100 | 0.105 | ||
| 28 | C6H13 | C6H13 | 3.29 | 2.041 | >100 | 0.272 | ||
| 29 | C7H15 | C7H15 | 4.12 | 2.503 | >100 | 1.123 | ||
| 30 | C8H17 | C8H17 | 4.96 | 2.981 | >100 | 0.752 | ||
| DCMU | 2.2 | 0.002 | ||||||
| INH | −0.64 | 0.1−0.39 | ||||||
2.3. Biological Activity
2.3.1. Antimycobacterial Activity
2.3.2. Antibacterial and Antifungal Activity
| Compound No. | MIC [µmol/L] |
|---|---|
| 6 | 250 |
| 7 | 125 |
| 8 | 62.5 |
| 13 | 250 |
| 14 | 250 |
| 15 | 125 |
| 21 | 250 |
| 22 | 125 |
| 23 | 125 |
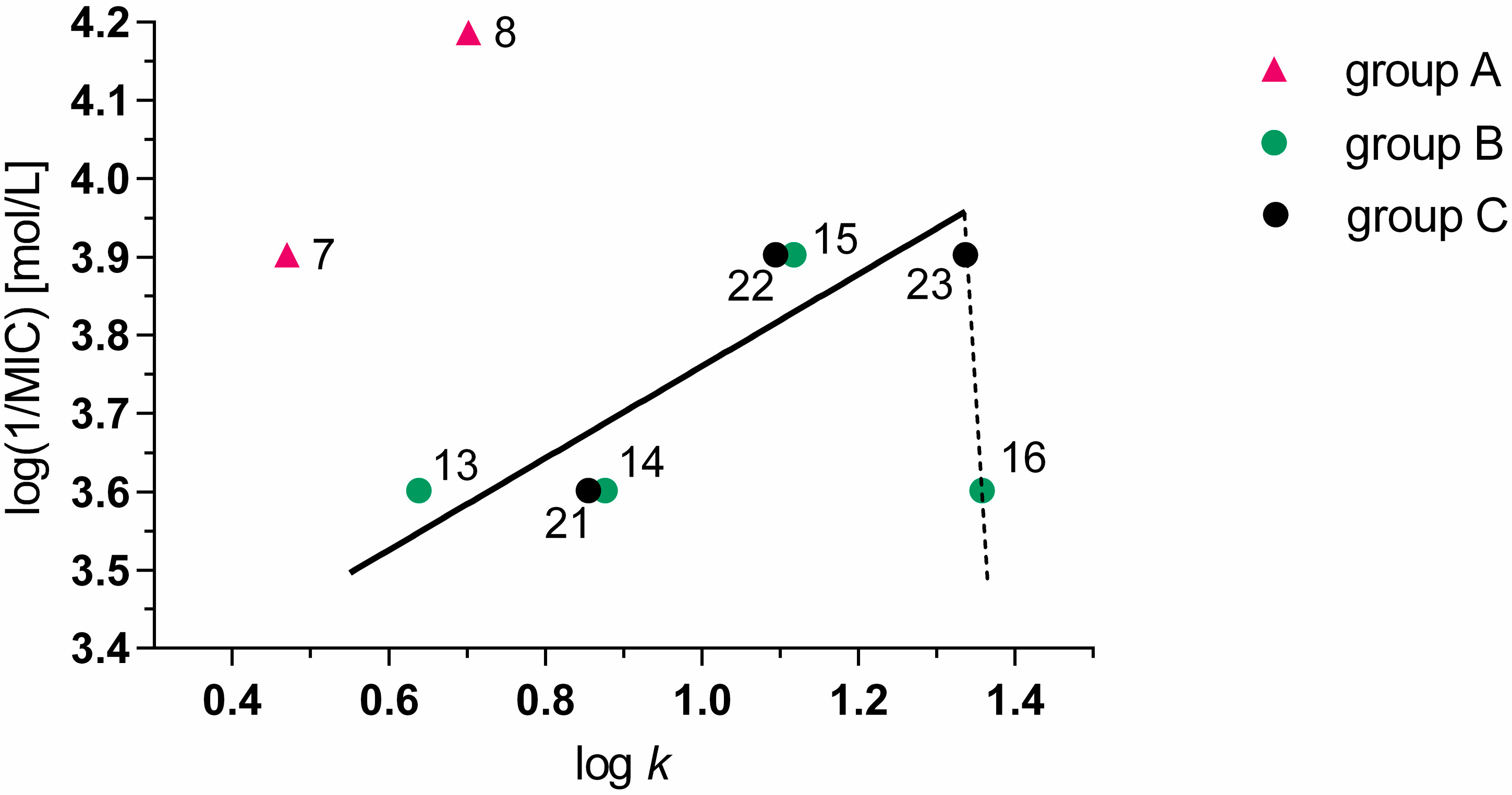
2.3.4. Cytotoxicity Assay
2.3.5. Evaluation of Photosynthetic Electron Transport (PET) Inhibiting Activity
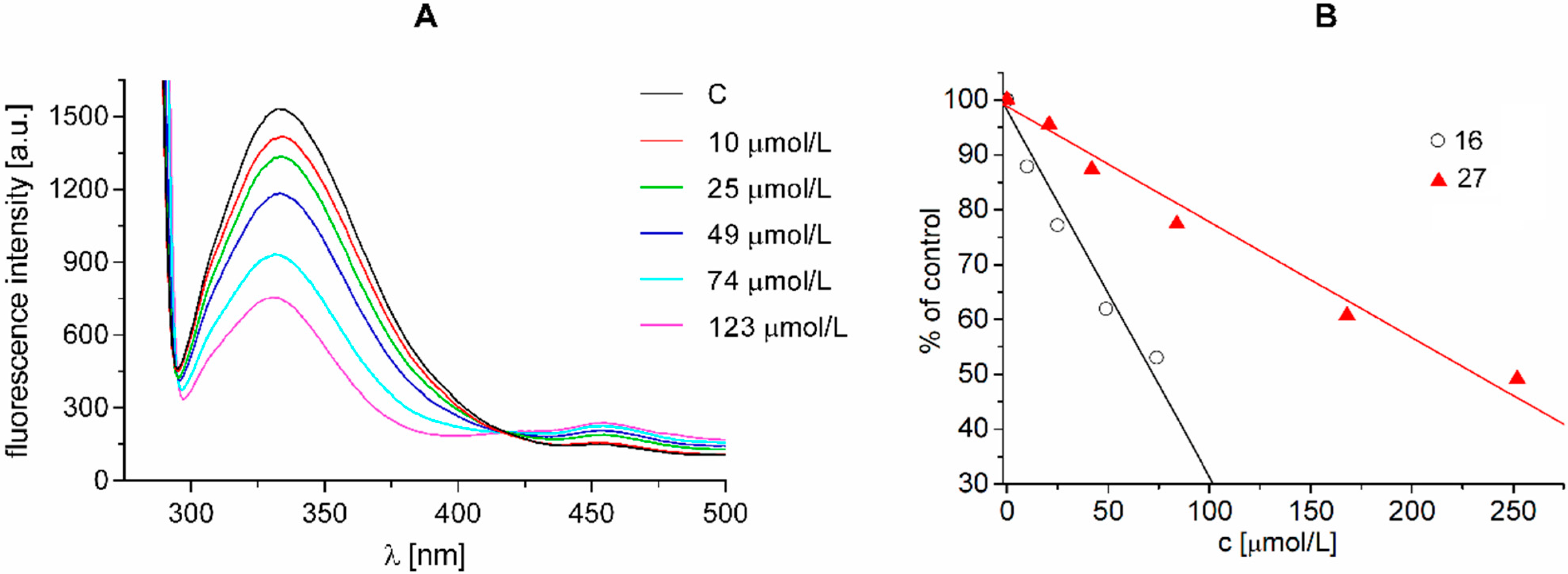
3. Experimental Section
3.1. General Information
3.2. Synthesis
3.3. Analytical Data
3.4. Determination of Lipophilicity by HPLC (Log k)
3.5. Biological Assays
3.5.1. Evaluation of in Vitro Antimycobacterial Activity
3.5.2. Evaluation of in Vitro Antibacterial Activity
3.5.3. Evaluation of in Vitro Antifungal Activity
3.5.5. Study of Inhibition of Photosynthetic Electron Transport (PET) in Spinach Chloroplasts
3.5.6. Study of Fluorescence of Aromatic Amino Acids in Spinach Chloroplasts
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ukrainets, I.V.; Bereznyakova, N.L. Heterocyclic diuretics. Chem. Heterocycl. Compd. 2012, 48, 155–165. [Google Scholar] [CrossRef]
- Miniyar, P.B.; Murumkar, P.R.; Patil, P.S.; Barmade, M.A.; Bothara, K.G. Unequivocal role of pyrazine ring in medicinally important compounds: A review. Mini Rev. Med. Chem. 2013, 13, 1607–1625. [Google Scholar] [PubMed]
- Mueller, R.; Rappert, S. Pyrazines: Occurrence, formation and biodegradation. Appl. Microbiol. Biot. 2010, 85, 1315–1320. [Google Scholar] [CrossRef]
- Dolezal, M.; Zitko, J. Pyrazine derivatives: A patent review (June 2012-present). Expert Opin. Ther. Pat. 2014, 25, 33–47. [Google Scholar]
- Kucerova-Chlupacova, M.; Kunes, J.; Buchta, V.; Vejsova, M.; Opletalova, V. Novel pyrazine analogs of chalcones: Synthesis and evaluation of their antifungal and antimycobacterial activity. Molecules 2015, 20, 1104–1117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.F.; Liu, Y.; Zhang, C.L.; Zhang, H.; Wang, B.; Xu, J.; Fu, L.; Yin, D.L.; Cooper, C.B.; Ma, Z.K.; et al. Synthesis and biological evaluation of novel 2-methoxypyridylamino-substituted riminophenazine derivatives as antituberculosis agents. Molecules 2014, 19, 4380–4394. [Google Scholar] [CrossRef] [PubMed]
- Rychtarcikova, Z.; Kratky, M.; Gazvoda, M.; Komloova, M.; Polanc, S.; Kocevar, M.; Stolarikova, J.; Vinsova, J. N-Substituted 2-isonicotinoylhydrazinecarboxamides—New antimycobacterial active molecules. Molecules 2014, 19, 3851–3868. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Mishra, A.K.; Malonia, S.K.; Chauhan, D.S.; Sharma, V.D.; Venkatesan, K.; Katoch, V.M. The paradox of pyrazinamide: An update on the molecular mechanisms of pyrazinamide resistance in Mycobacteria. J. Commun. Dis. 2006, 38, 288–298. [Google Scholar] [PubMed]
- Carel, C.; Nukdee, K.; Cantaloube, S.; Bonne, M.; Diagne, C.T.; Laval, F.; Daffe, M.; Zerbib, D. Mycobacterium tuberculosis proteins involved in mycolic acid synthesis and transport localize dynamically to the old growing pole and septum. PLoS ONE 2014, 9, e97148. [Google Scholar] [CrossRef] [PubMed]
- Sayahi, H.; Pugliese, K.M.; Zimhony, O.; Jacobs, W.R., Jr.; Shekhtman, A.; Welch, J.T. Analogs of the antituberculous agent pyrazinamide are competitive inhibitors of NADPH binding to M. tuberculosis fatty acid synthase I. Chem. Biodivers. 2012, 9, 2582–2596. [Google Scholar] [CrossRef] [PubMed]
- Servusova, B.; Paterova, P.; Mandikova, J.; Kubicek, V.; Kucera, R.; Kunes, J.; Dolezal, M.; Zitko, J. Alkylamino derivatives of pyrazinamide: Synthesis and antimycobacterial evaluation. Bioorg. Med. Chem. Lett. 2014, 24, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Good, N.E. Inhibitors of Hill reaction. Plant Physiol. 1961, 36, 788–803. [Google Scholar] [CrossRef] [PubMed]
- Kralova, K.; Sersen, F.; Cizmarik, J. Inhibitory effect of piperidinoethylesters of alkoxyphenylcarbamic acids on photosynthesis. Gen. Physiol. Biophys. 1992, 11, 261–267. [Google Scholar] [PubMed]
- Dolezal, M.; Miletin, M.; Kunes, J.; Kralova, K. Substituted amides of pyrazine-2-carboxylic acids: Synthesis and biological activity. Molecules 2002, 7, 363–373. [Google Scholar] [CrossRef]
- Draber, W.; Tietjen, K.; Kluth, J.F.; Trebst, A. Herbicides in photosynthesis research. Angew. Chem. Int. Ed. 1991, 30, 1621–1633. [Google Scholar] [CrossRef]
- Barber, J.; Marder, J.B. Photosynthesis and the application of molecular genetics. Biotechnol. Genet. Eng. Rev. 1986, 4, 355–404. [Google Scholar] [CrossRef]
- Shipman, L.L. Theoretical-study of the binding-site and mode of action for photosystem-ii herbicides. J. Theor. Biol. 1981, 90, 123–148. [Google Scholar] [CrossRef]
- Kral’ova, K.; Sersen, F.; Kubicova, L.; Waisser, K. Inhibition of photosynthetic electron transport in spinach chloroplasts by 3-and 4-halogeno substituted benzanilides and thiobenzanilides. J. Trace Microprobe Tech. 2000, 18, 251–256. [Google Scholar]
- Kral’ova, K.; Sersen, F.; Miletin, M.; Dolezal, M. Inhibition of photosynthetic electron transport in spinach chloroplasts by 2,6-disubstituted pyridine-4-thiocarboxamides. Chem. Pap. Chem. Zvesti 2002, 56, 214–217. [Google Scholar]
- Servusova, B.; Eibinova, D.; Dolezal, M.; Kubicek, V.; Paterova, P.; Pesko, M.; Kral’ova, K. Substituted N-benzylpyrazine-2-carboxamides: Synthesis and biological evaluation. Molecules 2012, 17, 13183–13198. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.R.; Andrews, K.L.; Frohn, M.J.; Harrington, P.E.; Pickrell, A.J.; Rzasa, R.M. Nitrogen-Heterocyclic Compounds as Phosphodiesterase 10 Inhibitors. U.S. Patent WO 2,011,143,129, 17 November 2011. [Google Scholar]
- Zhu, J.L.; Wong, H.; Zhang, Z.X.; Yin, Z.W.; Kadow, J.; Meanwell, N.A.; Wang, T. Malononitrile as a carbonyl synthon: A one-pot preparation of heteroaryl amide via a SNAr-oxidation-displacement strategy. Tetrahedron Lett. 2004, 45, 5909–5911. [Google Scholar] [CrossRef]
- Albert, A.; Brown, D.J.; Wood, H.C.S. Pteridine studies. Part VIII. The degradation of pteridine. Methylation of the hydroxypteridines and degradation of the products. J. Chem. Soc. 1956, 2066–2075. [Google Scholar] [CrossRef]
- Jandourek, O.; Dolezal, M.; Kunes, J.; Kubicek, V.; Paterova, P.; Pesko, M.; Buchta, V.; Kralova, K.; Zitko, J. New potentially active pyrazinamide derivatives synthesized under microwave conditions. Molecules 2014, 19, 9318–9338. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, F.; Kopecka-Leitmanova, A.; Sersen, F.; Balgavy, P. Interaction of surfactants with model and biological-membranes. 8. Amine oxides. 24. Cutoff effect in antimicrobial activity and in membrane perturbation efficiency of the homologous series of N,N-dimethylalkylamine oxides. J. Pharm. Pharmacol. 1990, 42, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Balgavy, P.; Devinsky, F. Cut-off effects in biological activities of surfactants. Adv. Colloid Interface 1996, 66, 23–63. [Google Scholar] [CrossRef]
- Przestalski, S.; Sarapuk, J.; Kleszczynska, H.; Gabrielska, J.; Hladyszowski, J.; Trela, Z.; Kuczera, J. Influence of amphiphilic compounds on membranes. Acta Biochim. Pol. 2000, 47, 627–638. [Google Scholar] [PubMed]
- Sarapuk, J.; Kubica, K. Cut-off phenomenon. Cell. Mol. Biol. Lett. 1998, 5, 261–269. [Google Scholar]
- Moreland, D.E. Research on biochemistry of herbicides—An historical overview. Z. Naturforsch. C 1993, 48, 121–131. [Google Scholar]
- Kral’ova, K.; Sersen, F.; Devinsky, F.; Lacko, I. Photosynthesis-inhibiting effects of cationic biodegradable gemini surfactants. Tenside Surf. Deterg. 2010, 47, 288–293. [Google Scholar] [CrossRef]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Govender, R.; Keltosova, S.; Chambel, B.; Pereira, D.; Kollar, P.; Imramovsky, A.; et al. Antibacterial and herbicidal activity of ring-substituted 2-hydroxynaphthalene-1-carboxanilides. Molecules 2013, 18, 9397–9419. [Google Scholar] [CrossRef] [PubMed]
- Kralova, K.; Sersen, F.; Cizmarik, J. Dimethylaminoethyl alkoxyphenylcarbamates as photosynthesis inhibitors. Chem. Pap. Chem. Zvesti 1992, 46, 266–268. [Google Scholar]
- Kralova, K.; Bujdakova, H.; Cizmarik, J. Antifungal and antialgal activity of piperidonopropyl esters of alkoxy-substituted phenylcarbamic acids. Pharmazie 1995, 50, 440–441. [Google Scholar] [PubMed]
- Sersen, F.; Kralova, K. Concentration-dependent inhibitory and stimulating effects of amphiphilic ammonium salts upon photosynthetic activity of spinach chloroplasts. Gen. Physiol. Biophys. 1996, 15, 27–36. [Google Scholar] [PubMed]
- Izawa, S. Acceptors and donors for chloroplast electron transport. In Methods in Enzymology; Colowick, P., Kaplan, N.O., Eds.; Academic Press: New York, NY, USA; London, UK, 1980; Volume 69, Part C; pp. 413–434. [Google Scholar]
- Purcell, M.; Leroux, G.; Carpentier, R. Interaction of the electron donor diphenylcarbazide with the herbicide-binding niche of photosystem II. Biochim. Biophys. Acta Int. J. Biochem. Biophys. 1991, 1058, 374–378. [Google Scholar] [CrossRef]
- Borse, T.H.; Maheshwari, V.L.; Baviskar, M.P. Effect of diphenyl carbazide on the metribuzin induced inhibition of photosystem-II photochemistry. J. Plant Biochem. Biotechnol. 2000, 9, 119–121. [Google Scholar] [CrossRef]
- Kamachi, H.; Tamura, N.; Inoue, H. Putative second binding site of DCMU on the oxidizing side of photosystem II in photosystem II membranes depleted of functional Mn. Plant Cell Physiol. 1992, 33, 437–443. [Google Scholar]
- Renger, G. The action of 3-(3,4-dichlorophenyl)-l,l-dimethylurea on the water-splitting enzyme system Y of photosynthesis. Biochim. Biophys. Acta 1973, 314, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Carpentier, R.; Fuerst, E.P.; Nakatani, H.Y.; Arntzen, C.J. A second site for herbicide action in photosystem II. Biochim. Biophys. Acta 1985, 808, 293–299. [Google Scholar] [CrossRef]
- Callis, P.R. Binding phenomena and fluorescence quenching. II: Photophysics of aromatic residues and dependence of fluorescence spectra on protein conformation. J. Mol. Struct. 2014, 1077, 22–29. [Google Scholar] [CrossRef]
- Kral’ova, K.; Sersen, F.; Pesko, M.; Waisser, K.; Kubicova, L. 5-Bromo- and 3,5-dibromo-2-hydroxy-N-phenylbenzamides—Inhibitors of photosynthesis. Chem. Pap. 2014, 68, 46–52. [Google Scholar]
- Zitko, J.; Servusova, B.; Paterova, P.; Mandikova, J.; Kubicek, V.; Kucera, R.; Hrabcova, V.; Kunes, J.; Soukup, O.; Dolezal, M. Synthesis, Antimycobacterial activity and in vitro cytotoxicity of 5-chloro-N-phenylpyrazine-2-carboxamides. Molecules 2013, 18, 14807–14825. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N.; Barry, A.L. Optimal dilution susceptibility testing conditions, recommendations for MIC interpretation, and quality-control guidelines for the ampicillin-sulbactam combination. J. Clin. Microbiol. 1987, 25, 1920–1925. [Google Scholar] [PubMed]
- National Committee for Clinical Laboratory Standards. Method for Antifungal Disc Diffusion Susceptibility Testing of Yeasts: Approved Guideline M44-A; NCCLS: Wayne, PA, USA, 2004. [Google Scholar]
- Naesens, L.; Stephens, C.E.; Andrei, G.; Loregian, A.; De Bolle, L.; Snoeck, R.; Sowell, J.W.; De Clercq, E. Antiviral properties of new arylsulfone derivatives with activity against human betaherpesviruses. Antivir. Res. 2006, 72, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Naesens, L.; Vanderlinden, E.; Roth, E.; Jeko, J.; Andrei, G.; Snoeck, R.; Pannecouque, C.; Illyes, E.; Batta, G.; Herczegh, P.; et al. Anti-influenza virus activity and structure-activity relationship of aglycoristocetin derivatives with cyclobutenedione carrying hydrophobic chains. Antivir. Res. 2009, 82, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Vanderlinden, E.; Goktas, F.; Cesur, Z.; Froeyen, M.; Reed, M.L.; Russell, C.J.; Cesur, N.; Naesens, L. Novel inhibitors of influenza virus fusion: Structure-activity relationship and interaction with the viral hemagglutinin. J. Virol. 2010, 84, 4277–4288. [Google Scholar] [CrossRef] [PubMed]
- Masarovicova, E.; Kralova, K. Approaches to measuring plant photosynthesis activity. In Handbook of Photosynthesis, 2nd ed.; Pessarakli, M., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 617–656. [Google Scholar]
- Kralova, K.; Sersen, F.; Sidoova, E. Photosynthesis inhibition produced by 2-alkylthio-6-R-benzothiazoles. Chem. Pap. 1992, 46, 348–350. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semelkova, L.; Konecna, K.; Paterova, P.; Kubicek, V.; Kunes, J.; Novakova, L.; Marek, J.; Naesens, L.; Pesko, M.; Kralova, K.; et al. Synthesis and Biological Evaluation of N-Alkyl-3-(alkylamino)-pyrazine-2-carboxamides. Molecules 2015, 20, 8687-8711. https://doi.org/10.3390/molecules20058687
Semelkova L, Konecna K, Paterova P, Kubicek V, Kunes J, Novakova L, Marek J, Naesens L, Pesko M, Kralova K, et al. Synthesis and Biological Evaluation of N-Alkyl-3-(alkylamino)-pyrazine-2-carboxamides. Molecules. 2015; 20(5):8687-8711. https://doi.org/10.3390/molecules20058687
Chicago/Turabian StyleSemelkova, Lucia, Klara Konecna, Pavla Paterova, Vladimir Kubicek, Jiri Kunes, Lucie Novakova, Jan Marek, Lieve Naesens, Matus Pesko, Katarina Kralova, and et al. 2015. "Synthesis and Biological Evaluation of N-Alkyl-3-(alkylamino)-pyrazine-2-carboxamides" Molecules 20, no. 5: 8687-8711. https://doi.org/10.3390/molecules20058687
APA StyleSemelkova, L., Konecna, K., Paterova, P., Kubicek, V., Kunes, J., Novakova, L., Marek, J., Naesens, L., Pesko, M., Kralova, K., Dolezal, M., & Zitko, J. (2015). Synthesis and Biological Evaluation of N-Alkyl-3-(alkylamino)-pyrazine-2-carboxamides. Molecules, 20(5), 8687-8711. https://doi.org/10.3390/molecules20058687









