Synthesis, Antimicrobial and Hypoglycemic Activities of Novel N-(1-Adamantyl)carbothioamide Derivatives
Abstract
:1. Introduction
2. Results and Discussion
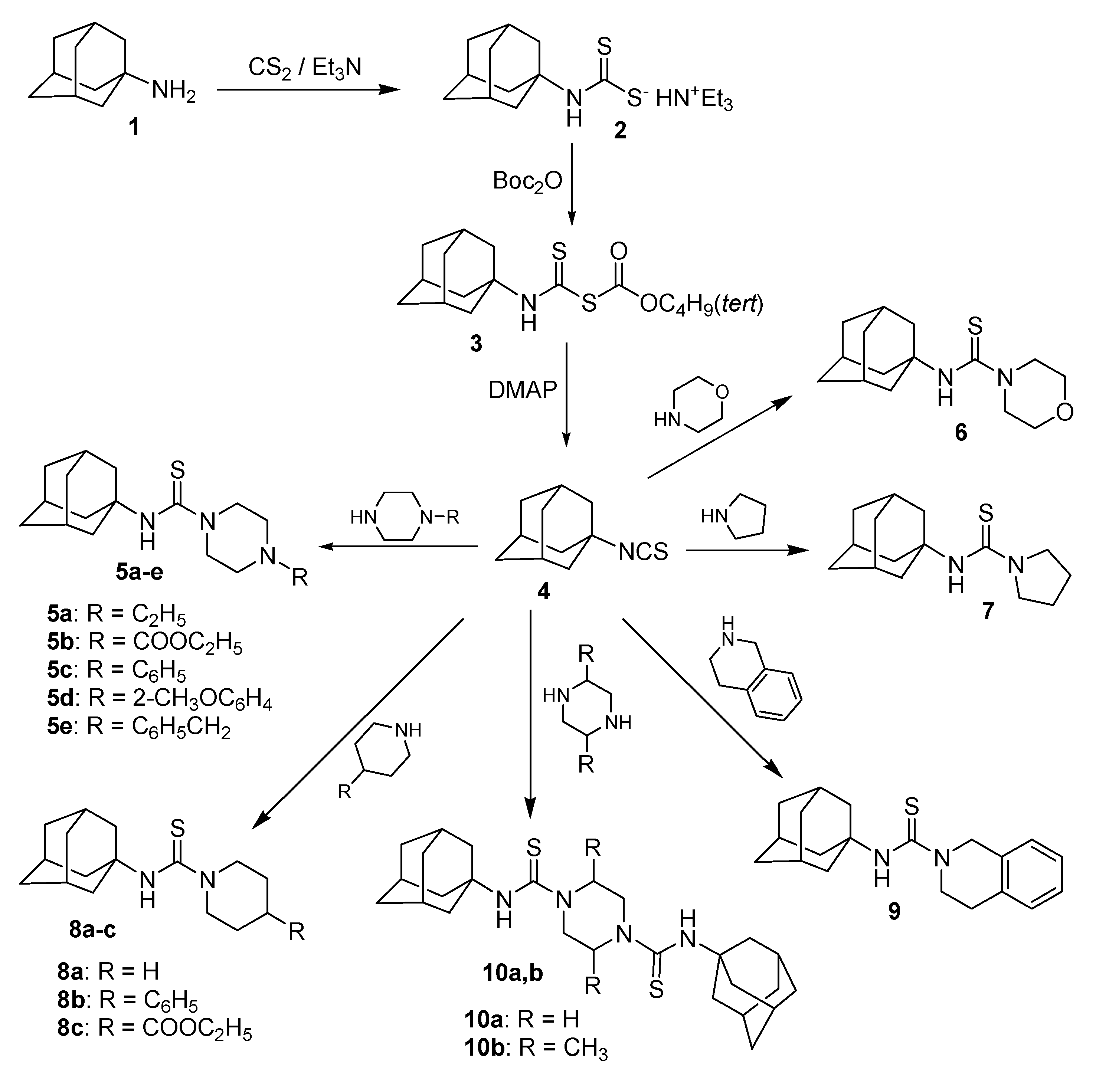
2.1. Chemical Synthesis
| Comp. No. | R | Cryst. Solv. | Mp (°C) | Yield (%) | Molecular Formula (Mol. Wt.) |
|---|---|---|---|---|---|
| 5a | C2H5 | EtOH | 150–152 | 77 | C17H29N3S (307.50) |
| 5b | COOC2H5 | EtOH | 121–123 | 68 | C18H29N3O2S (351.51) |
| 5c | C6H5 | EtOH | 174–176 | 91 | C21H29N3S (355.54) |
| 5d | 2-CH3OC6H4 | EtOH | 137–139 | 95 | C22H31N3OS (385.57) |
| 5e | C6H5CH2 | EtOH | 146–148 | 95 | C22H31N3S (369.57) |
| 6 | - | EtOH | 139–141 | 88 | C15H24N2OS (280.43) |
| 7 | - | EtOH | 175–177 | 74 | C15H24N2S (264.43) |
| 8a | H | EtOH | 145–147 | 78 | C16H26N2S (278.46) |
| 8b | C6H5 | EtOH | 137–139 | 90 | C22H30N2S (354.55) |
| 8c | COOC2H5 | EtOH | 166–168 | 78 | C19H30N2O2S (350.52) |
| 9 | - | EtOH | 147–149 | 88 | C20H26N2S (326.5) |
| 10a | H | DMF | 227–229 | 89 | C26H40N4S2 (472.75) |
| 10b | CH3 | DMF | 232–234 | 92 | C28H44N4S2 (500.81) |
| 14a | CH3 | EtOH | 200–202 | 38 | C19H29N5S2 (391.6) |
| 14b | C6H5 | DMF | >300 | 44 | C24H31N5S2 (453.67) |
| 15a | 2-OH | EtOH | 196–198 | 65 | C24H32N4O2S (440.6) |
| 15b | 3,4-Cl2 | EtOH/CHCl3 | 237–237 | 61 | C24H30Cl2N4OS (493.49) |
| 15c | 2,6-Cl2 | EtOH | 199–201 | 54 | C24H30Cl2N4OS (493.49) |
| 15d | 3,4-(OCH3)2 | EtOH | 154–156 | 48 | C26H36N4O3S (484.65) |
| 15e | 3,4,5-(OCH3)3 | EtOH | 128–130 | 43 | C27H38N4O4S (514.68) |
| 15f | 2-OH-5-OCH3 | EtOH/CHCl3 | 170–172 | 44 | C25H34N4O3S (470.63) |
| 15g | 3-OC2H5-4-OH | EtOH/CHCl3 | 194–196 | 58 | C26H36N4O3S (484.65) |
2.2. In Vitro Antimicrobial Activity
| Comp. No. | Clog P | Diameter of Growth Inhibition Zone (mm) a | |||||
|---|---|---|---|---|---|---|---|
| SA | BS | ML | EC | PA | CA | ||
| 5a | 4.32 | - | - | - | - | - | - |
| 5b | 4.32 | - | - | - | - | - | - |
| 5c | 4.77 | 20 (8) b | 24 (2) b | 16 | 15 | 14 | - |
| 5d | 4.79 | 19 (4) b | 22 (4) b | 17 | 12 | - | - |
| 5e | 5.52 | 15 | 19 (8) b | 12 | 15 | 12 | - |
| 6 | 3.23 | 22 (2) b | 20 (1) b | 19 (4) b | 15 | 12 | - |
| 7 | 4.06 | 18 (8) b | 20 (2) b | 18 (4) b | 15 | 13 | - |
| 8a | 4.62 | 12 | 14 | 12 | - | - | - |
| 8b | 6.03 | 16 | 16 | 14 | - | - | - |
| 8c | 4.01 | 12 | 13 | - | - | - | - |
| 9 | 5.48 | - | - | - | - | - | - |
| 10a | 6.86 | 25 (32) b | 28 (16) b | 20 (32) b | 18 | 16 | - |
| 10b | 7.90 | 26 (32) b | 24 (8) b | 23 (32) b | 19 (64) b | 12 | - |
| 14a | 3.40 | - | - | - | - | - | - |
| 14b | 5.29 | - | - | - | - | - | - |
| 15a | 5.07 | 18 (16) b | 22 (2) b | 15 | 12 | - | - |
| 15b | 5.77 | 12 | 12 | - | - | - | - |
| 15c | 4.70 | 14 | 17 | 12 | - | - | - |
| 15d | 4.41 | - | - | - | - | - | - |
| 15e | 4.03 | - | - | - | - | - | - |
| 15f | 5.14 | 22 (8) b | 27 (1) b | 19 (8) b | 13 | 11 | 11 |
| 15g | 4.79 | 19 (8) b | 25 (2) b | 18 (8) b | 12 | 12 | 13 |
| Gentamicin | 26 (2) b | 25 (2) b | 18 (2) b | 20 (0.5) b | 19 (1) b | NT | |
| Ampicillin | 23 (2) b | 21 (0.5) b | 19 (2) b | 17 (2) b | 16 (2) b | NT | |
| Clotrimazole | NT | NT | NT | NT | NT | 21 (2) b | |
2.3. In Vivo Hypoglycemic Activity
| Treatment | Results | ||
|---|---|---|---|
| C0 (mg/dL) a | C24 (mg/dL) a | % Glucose Reduction b | |
| Group 1 c | 302.6 ± 11.64 | 287.2 ± 16.85 | 5.09% |
| Group 2 d | 295.4 ± 17.52 | 183.0 ± 13.38 * | 38.05% |
| 5c (10 mg/kg) | 291.6 ± 15.23 | 189.0 ± 22.16 * | 35.19% (92.48) |
| 5c (20 mg/kg) | 319.6 ± 7.85 | 207.0 ± 13. 84 * | 35.23% (46.30) |
| 6 (10 mg/kg) | 283.8 ± 11.16 | 263.8 ± 16.66 | 7.05% (18.52) |
| 6 (20 mg/kg) | 296.6 ± 12.92 | 196.0 ± 9.67 * | 33.92% (44.58) |
| 8b (10 mg/kg) | 264.2 ± 5.49 | 257.4 ± 9.45 | 2.57% (6.75) |
| 8b (20 mg/kg) | 303.3 ± 16.35 | 291.2 ± 8.18 | 4.05% (5.32) |
| 9 (10 mg/kg) | 282.8 ± 13.90 | 275.8 ± 15.52 | 2.48% (6.52) |
| 9 (20 mg/kg) | 276.2 ± 14.17 | 264.6 ± 7.35 | 4.20% (5.52) |
| 14a (10 mg/kg) | 274.8 ± 14.59 | 277.4 ± 7.19 | 0.95% (2.50) |
| 14a (20 mg/kg) | 286.4 ± 24.56 | 271.4 ± 21.85 | 5.24% (6.89) |
| 15b (10 mg/kg) | 282.0 ± 14.21 | 287.8 ± 13.37 | −2.06% |
| 15b (20 mg/kg) | 293.2 ± 15.66 | 286.8 ± 17.16 | 2.18% (2.87) |
2.4. Oral Acute Toxicity Testing of Compound 5c
3. Experimental Section
3.1. General
3.2. Synthesis of N-(1-Adamantyl)-4-substituted piperazine-1-carbothioamides 5a–e, N-(1-Adamantyl)morpholine-4-carbothioamide 6, N-(1-Adamantyl)pyrrolidine-1-carbothioamide 7, N-(1-Adamantyl)-4-substituted piperidine-1-carbothioamides 8a–c and N-(1-Adamantyl)-1,2,3,4-tetrahydroisoquinoline-2-carbothioamide 9
3.3. Synthesis of N,N'-Bis(1-adamantyl)piperazine-1,4-dicarbothioamide 10a and trans-N,N'-Bis(1-adamantyl)-2,5-dimethylpiperazine-1,4-dicarbothioamide 10b
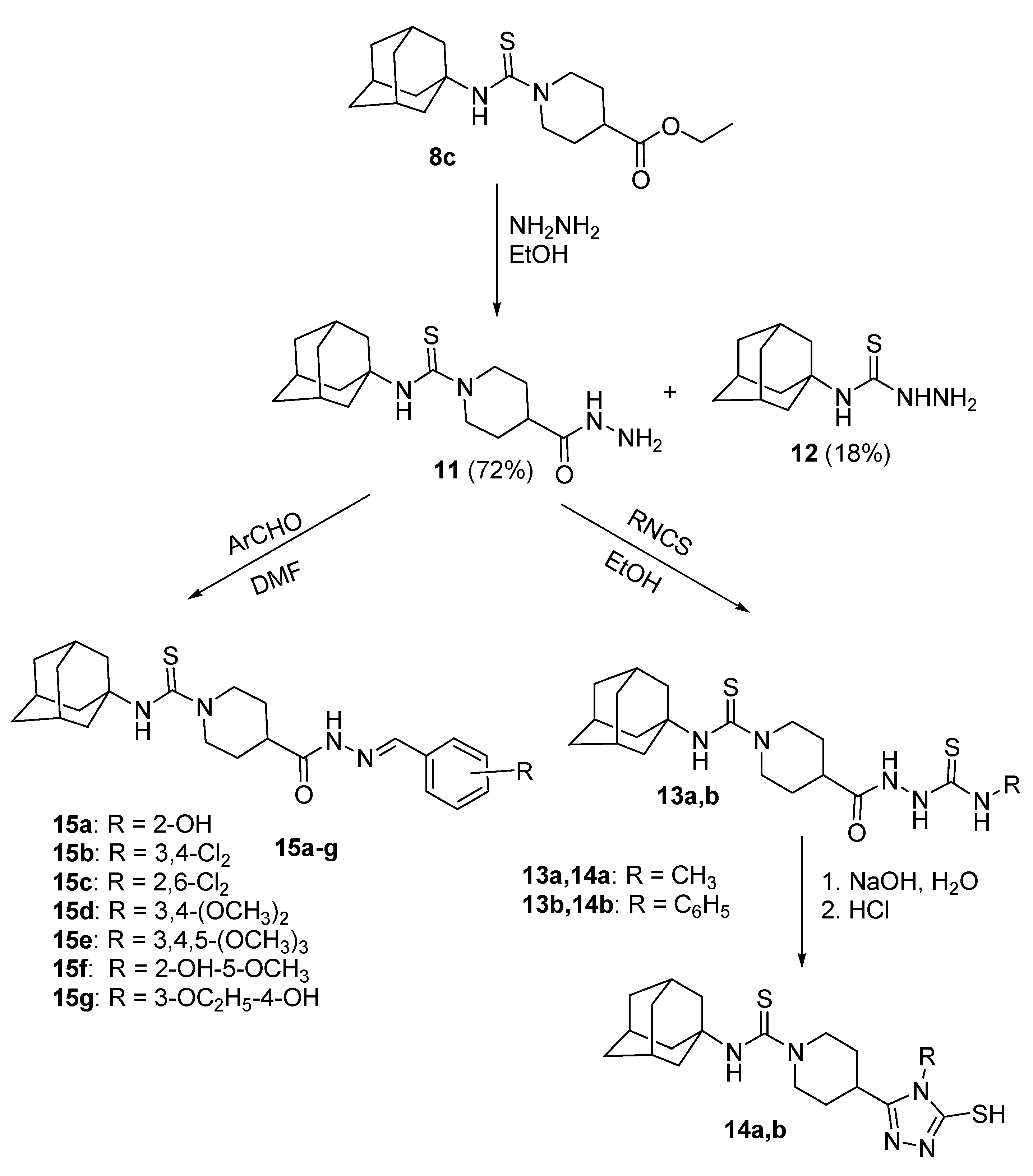
3.4. Synthesis of 1-(1-Adamantylthiocarbamoyl)piperidine-4-carbohydrazide 11 and 4-(1-Adamanty)-3-thiosemicarbazide 12
3.5. Synthesis of N-(1-Adamantyl)piperidine-4-(5-mercapto-4-phenyl-1,2,4-triazol-3-yl)-1-carbothioamide 14a,b
3.6. Synthesis of 1-[(1-Adamantyl)thiocarbamoyl)]-N'-(arylidene)piperidine-4-carbohydrazides 15a–g
3.7. Determination of the in Vitro Antimicrobial Activity (Agar Disc-Diffusion Method)
3.8. Determination of the Minimal Inhibitory Concentration (MIC)
3.9. Determination of the in Vivo Hypoglycemic Activity
3.10. Determination of the Oral Acute Toxicity of Compound 5c
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, J.; Obando, D.; Liao, V.; Lifa, T.; Codd, R. The many faces of the adamantyl group in drug design. Eur. J. Med. Chem. 2011, 46, 1949–1963. [Google Scholar] [PubMed]
- Lamoureux, G.; Artavia, G. Use of the adamantane structure in medicinal chemistry. Curr. Med. Chem. 2010, 17, 2967–2978. [Google Scholar] [CrossRef] [PubMed]
- Davies, W.L.; Grunnert, R.R.; Haff, R.F.; McGahen, J.W.; Neumeyer, E.M.; Paulshock, M.; Watts, J.C.; Wood, T.R.; Hermann, E.C.; Hoffmann, C.E. Antiviral activity of 1-adamantamine (amantadine). Science 1964, 144, 862–863. [Google Scholar] [CrossRef] [PubMed]
- Togo, Y.; Hornick, R.B.; Dawkins, A.T. Studies on induced influenza in man. I. Double blind studies designed to assess prophylactic efficacy of amantadine hydrochloride against A2/Rockville/1/65 strain. J. Am. Med. Assoc. 1968, 203, 1089–1094. [Google Scholar] [CrossRef]
- Wendel, H.A.; Snyder, M.T.; Pell, S. Trial of amantadine in epidemic influenza. Clin. Pharmacol. Ther. 1966, 7, 38–43. [Google Scholar] [PubMed]
- Schwab, R.S.; England, A.C., Jr.; Poskanzer, D.C.; Young, R.R. Amantadine in the treatment of Parkinson’s disease. J. Am. Med. Assoc. 1969, 208, 1168–1170. [Google Scholar] [CrossRef]
- Owen, J.C.E.; Whitton, P.S. Effect of amantadine and budipune on antidepressant drug-evoked changes in extracellular dopamine in the frontal cortex of freely moving rats. Brain Res. 2006, 1117, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, S.; Baldini, J.T.; Bannister, R. Treatment of influenza. The therapeutic efficacy of rimantadine HCl in a naturally occurring influenza A2 outbreak. Am. J. Med. Sci. 1969, 257, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, K.S.; Sokol, M.S.; Ingram, R.L.; Subramanian, R.; Fort, R.C. Tromantadine: Inhibitor of Early and Late Events in Herpes Simplex Virus Replication. Antimicrob. Agents Chemother. 1982, 22, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Burstein, M.E.; Serbin, A.V.; Khakhulina, T.V.; Alymova, I.V.; Stotskaya, L.L.; Bogdan, O.P.; Manukchina, E.E.; Jdanov, V.V.; Sharova, N.K. Inhibition of HIV-1 replication by newly developed adamantane-containing polyanionic agents. Antivir. Res. 1999, 41, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Van Derpoorten, K.; Balzarini, J.; de Clercq, E.; Poupaert, J.H. Anti-HIV activity of N-1-adamantyl-4-aminophthalimide. Biomed. Pharmacother. 1997, 51, 464–468. [Google Scholar] [CrossRef] [PubMed]
- El-Emam, A.A.; Al-Deeb, O.A.; Al-Omar, M.A.; Lehmann, J. Synthesis, antimicrobial, and anti-HIV-1 activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazoline-2-thiones. Bioorg. Med. Chem. 2004, 12, 5107–5113. [Google Scholar] [CrossRef] [PubMed]
- Balzarini, J.; Orzeszko, B.; Mauri, J.K.; Orzeszko, A. Synthesis and anti-HIV studies of 2-adamantyl-substituted thiazolidin-4-ones. Eur. J. Med. Chem. 2007, 42, 993–1003. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.Y.; Yue, P.; Chen, X.; Hong, W.K.; Lotan, R. The synthetic retinoid CD437 selectively induces apoptosis in human lung cancer cells while sparing normal human lung epithelial cells. Cancer Res. 2002, 62, 2430–2436. [Google Scholar] [PubMed]
- Antoniadou-Vyza, E.; Tsitsa, P.; Hytiroglou, E.; Tsantili-Kakoulidou, A. New adamantan-2-ol and adamantan-1-methanol derivatives as potent antibacterials, synthesis, antibacterial activity and lipophilicity studies. Eur. J. Med. Chem. 1996, 31, 105–110. [Google Scholar] [CrossRef]
- Omar, K.; Geronikaki, A.; Zoumpoulakis, P.; Camoutsis, C.; Soković, M.; Ćirić, A.; Glamoćlija, J. Novel 4-thiazolidinone derivatives as potential antifungal and antibacterial drugs. Bioorg. Med. Chem. 2010, 18, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Scherman, M.S.; North, E.J.; Jones, V.; Hess, T.N.; Grzegorzewicz, A.E.; Kasagami, T.; Kim, I.H.; Merzlikin, O.; Lenaerts, A.J.; Lee, R.E.; et al. Screening a library of 1600 adamantyl ureas for anti-Mycobacterium tuberculosis activity in vitro and for better physical chemical properties for bioavailability. Bioorg. Med. Chem. 2012, 20, 3255–3262. [Google Scholar] [CrossRef] [PubMed]
- El-Emam, A.A.; Al-Tamimi, A.-M.S.; Al-Omar, M.A.; Al-Rashood, K.A.; Habib, E.E. Synthesis and antimicrobial activity of novel 5-(1-adamantyl)-2-aminomethyl-4-substituted-1,2,4-triazoline-3-thiones. Eur. J. Med. Chem. 2013, 68, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Al-Deeb, O.A.; Al-Omar, M.A.; El-Brollosy, N.R.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial, and anti-inflammatory activities of novel 2-[3-(1-adamantyl)-4-substituted-5-thioxo-1,2,4-triazolin-1-yl]acetic acids, 2-[3-(1-adamantyl)-4-substituted-5-thioxo-1,2,4-triazolin-1-yl]propionic acids and related derivatives. Arzneim.-Forsch./Drug Res. 2006, 56, 40–47. [Google Scholar]
- Kadi, A.A.; El-Brollosy, N.R.; Al-Deeb, O.A.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial, and anti-inflammatory activities of novel 2-(1-adamantyl)-5-substituted-1,3,4-oxadiazoles and 2-(1-adamantylamino)-5-substituted-1,3,4-thiadiazoles. Eur. J. Med. Chem. 2007, 42, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Kadi, A.A.; Al-Abdullah, E.S.; Shehata, I.A.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial and anti-inflammatory activities of novel 5-(1-adamantyl)-1,3,4-thiadiazole derivatives. Eur. J. Med. Chem. 2010, 45, 5006–5011. [Google Scholar] [CrossRef] [PubMed]
- Al-Abdullah, E.S.; Asiri, H.H.; Lahsasni, S.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial, and anti-inflammatory activity, of novel S-substituted and N-substituted 5-(1-adamantyl)-1,2,4-triazole-3-thiols. Drug Des. Dev. Ther. 2014, 8, 505–518. [Google Scholar]
- Al-Abdullah, E.S.; Al-Tuwaijri, H.M.; Hassan, H.M.; Haiba, M.E.; Habib, E.E.; El-Emam, A.A. Antimicrobial and hypoglycemic activities of novel N-Mannich bases derived from 5-(1-adamantyl)-4-substituted-1,2,4-triazoline-3-thiones. Int. J. Mol. Sci. 2015, 15, 22995–23010. [Google Scholar] [CrossRef]
- Jia, L.; Tomaszewski, J.E.; Hanrahan, C.; Coward, L.; Noker, P.; Gorman, G.; Nikonenko, B.; Protopopova, M. Pharmacodynamics and pharmacokinetics of SQ109, a new diamine-based antitubercular drug. Br. J. Pharmacol. 2005, 144, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Protopopova, M.; Hanrahan, C.; Nikonenko, B.; Samala, R.; Chen, P.; Gearhart, J.; Einck, L.; Nacy, C.A. Identification of a new antitubercular drug candidate, SQ109, from a combinatorial library of 1,2-ethylenediamines. J. Antimicrob. Chemother. 2005, 56, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Villhauer, E.B.; Brinkman, J.A.; Naderi, G.B.; Burkey, B.F.; Dunning, B.E.; Prasad, K.; Mangold, B.L.; Russell, M.E.; Hughes, T.E. 1-(3-Hydroxy-1-adamantyl)aminoacetyl-2-cyano-(S)-pyrrolidine: A potent, selective, and orally bioavailable dipeptidyl peptidase IV inhibitor with antihyperglycemic properties. J. Med. Chem. 2003, 46, 2774–2789. [Google Scholar] [CrossRef] [PubMed]
- Augeri, D.J.; Robl, J.A.; Betebenner, D.A.; Magnin, D.R.; Khanna, A.; Robertson, J.G.; Wang, A.; Simpkins, L.M.; Taunk, P.; Huang, Q.; et al. Discovery and preclinical profile of saxagliptin (BMS-477118): A highly potent, long-acting, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J. Med. Chem. 2005, 48, 5025–5037. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.; Aster, S.D.; Brown, K.; Carbin, L.; Graham, D.W.; Hermanowski-Vosatka, A.; LeGrand, C.B.; Mundt, S.S.; Robbins, M.A.; Schaeffer, J.M.; et al. Adamantyl triazoles as selective inhibitors of 11β-hydroxysteroid dehydrogenase type 1. Bioorg. Med. Chem. Lett. 2005, 15, 4359–4362. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Hoffman, J.; Le, P.; Nair, S.K.; Cripps, S.; Matthews, J.; Smith, C.; Yang, M.; Kupchinsky, S.; Dress, K.; et al. The development and SAR of pyrrolidine carboxamide 11β-HSD1 inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 2897–2902. [Google Scholar] [CrossRef] [PubMed]
- Cott, J.S.; Choormun, J. 11β-Hydroxysteroid dehydrogenase Type 1 (11β-HSD-1) inhibitors in developmen. In New Therapeutic Strategies for Type-2 Diabetes, Small Molecule Approaches; Jones, R.N., Ed.; RSC: Cambridge, UK, 2012; Chapter 5; pp. 109–133. [Google Scholar]
- El-Emam, A.A.; Ibrahim, T.M. Synthesis, anti-inflammatory and analgesic activity of certain 3-(1-adamantyl)-4-substituted-5-mercapto-1,2,4-triazole derivatives. Arzneim.-Forsch./Drug Res. 1991, 41, 1260–1264. [Google Scholar]
- Kouatly, O.; Geronikaki, A.; Kamoutsis, C.; Hadjipavlou-Litina, D.; Eleftheriou, P. Adamantane derivatives of thiazolyl-N-substituted amide, as possible non-steroidal anti-inflammatory agents. Eur. J. Med. Chem. 2009, 44, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Baxter, A.; Bent, J.; Bowers, K.; Braddock, M.; Brough, S.; Fagura, M.; Lawson, M.; McInally, T.; Mortimore, M.; Robertson, M.; et al. Hit-to-lead studies: The discovery of potent adamantane amide P2X7 receptor antagonists. Bioorg. Med. Chem. Lett. 2003, 13, 4047–4050. [Google Scholar] [CrossRef] [PubMed]
- Joubert, J.; van Dyk, S.; Green, I.R.; Malan, S.F. Synthesis and evaluation of fluorescent heterocyclic aminoadamantanes as multifunctional neuroprotective agents. Bioorg. Med. Chem. 2011, 19, 3935–3944. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, B.; Winn, M.; Rohde, J.; Shuai, Q.; Wang, J.; Fung, S.; Monzon, K.; Chiou, W.; Stolarik, D.; Imade, H.; et al. Adamantane sulfone and sulfonamide 11-β-HSD1 inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Rohde, J.J.; Pliushchev, M.A.; Sorensen, B.K.; Wodka, D.; Shuai, Q.; Wang, J.; Wang, S.; Monzon, K.; Patel, J.R.; Shuai, Q.; et al. Discovery and metabolic stabilization of potent and selective 2-amino-N-(adamant-2-yl)acetamide 11β-hydroxysteroid dehydrogenase type 1 inhibitors. J. Med. Chem. 2007, 50, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Bahare, R.S.; Khan, S.A. Design, synthesis and anticonvulsant evaluation of N-(benzo[b]thiazol-2-ylcarbamoyl)-2-methyl-4-oxoquinazoline-3(4H)-carbothioamide derivatives: A hybrid pharmacophore approach. Eur. J. Med. Chem. 2013, 67, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.-L.; Wu, Y.; Liu, C.-J.; Wei, C.-Y.; Tao, J.-C.; Liu, H.-M. Synthesis and in vitro cytotoxic activity evaluation of novel heterocycle bridged carbothioamide type derivatives as antitumor agents. Bioorg. Med. Chem. Lett. 2013, 23, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Munch, H.; Hansen, J.S.; Pittelkow, M.; Christensen, J.B.; Boas, U.A. A new efficient synthesis of isothiocyanates from amines using di-tert-butyl dicarbonate. Tetrahedron Lett. 2008, 49, 3117–3119. [Google Scholar] [CrossRef]
- Spilovska, K.; Korabecny, J.; Kral, J.; Horova, A.; Musilek, K.; Soukup, O.; Drtinova, L.; Gazova, Z.; Siposova, K.; Kuca, K. 7-Methoxytacrine-adamantylamine heterodimers as cholinesterase inhibitors in Alzheimer’s disease treatment-synthesis, biological evaluation and molecular modeling studies. Molecules 2013, 18, 2397–2418. [Google Scholar] [CrossRef] [PubMed]
- El-Emam, A.A.; Al-Abdullah, E.S.; Al-Tuwaijri, H.M.; Chidan Kumar, C.S.; Fun, H.-K. N-(Adamantan-1-yl)-1,2,3,4-tetrahydroisoquinoline-2-carbothioamide. Acta Crystallogr. 2013, E69, o1815. [Google Scholar]
- Woods, G.L.; Washington, J.A. Antibacterial susceptibility tests: Dilution and disk diffusion methods. In Manual of Clinical Microbiology; Murray, P.R., Baron, E.J., Pfaller, M.A., Tenover, F.C., Yolken, R.H., Eds.; American Society of Microbiology: Washington, DC, USA, 1995. [Google Scholar]
- Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard Document M2-A3; National Committee for Clinical Laboratory Standards (NCCLS): Villanova, PA, USA, 1985.
- Rossini, A.A.; Like, A.A.; Chick, W.L.; Appel, M.C.; Cahill, G.F., Jr. Studies of streptozotocin-induced insulitis and diabetes. Proc. Natl. Acad. Sci. USA 1977, 74, 2485–2489. [Google Scholar] [CrossRef] [PubMed]
- Olajide, O.A.; Awe, S.O.; Makinde, J.M.; Morebise, O. Evaluation of the antidiabetic property of Morinda lucida leaves in streptozotocin diabetes rats. J. Pharm. Pharmacol. 1999, 51, 1321–1324. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, J.T.; Wilcoxon, T. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–104. [Google Scholar] [PubMed]
- Serradas, P.; Bailbe, D.; Portha, B. Long-term gIiclazide treatment improves the in vitro glucose-induced insulin release in rats with type 2 (non-insulin-dependent) diabetes induced by neonatal streptozotocin. Diabetologia 1989, 32, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the all compounds are available from the correspondent author.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Abdullah, E.S.; Al-Tuwaijri, H.M.; Hassan, H.M.; Al-Alshaikh, M.A.; Habib, E.E.; El-Emam, A.A. Synthesis, Antimicrobial and Hypoglycemic Activities of Novel N-(1-Adamantyl)carbothioamide Derivatives. Molecules 2015, 20, 8125-8143. https://doi.org/10.3390/molecules20058125
Al-Abdullah ES, Al-Tuwaijri HM, Hassan HM, Al-Alshaikh MA, Habib EE, El-Emam AA. Synthesis, Antimicrobial and Hypoglycemic Activities of Novel N-(1-Adamantyl)carbothioamide Derivatives. Molecules. 2015; 20(5):8125-8143. https://doi.org/10.3390/molecules20058125
Chicago/Turabian StyleAl-Abdullah, Ebtehal S., Hanaa M. Al-Tuwaijri, Hanan M. Hassan, Monirah A. Al-Alshaikh, Elsayed E. Habib, and Ali A. El-Emam. 2015. "Synthesis, Antimicrobial and Hypoglycemic Activities of Novel N-(1-Adamantyl)carbothioamide Derivatives" Molecules 20, no. 5: 8125-8143. https://doi.org/10.3390/molecules20058125
APA StyleAl-Abdullah, E. S., Al-Tuwaijri, H. M., Hassan, H. M., Al-Alshaikh, M. A., Habib, E. E., & El-Emam, A. A. (2015). Synthesis, Antimicrobial and Hypoglycemic Activities of Novel N-(1-Adamantyl)carbothioamide Derivatives. Molecules, 20(5), 8125-8143. https://doi.org/10.3390/molecules20058125





