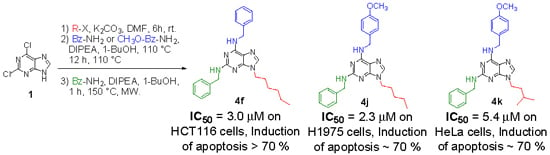
Synthesis and Pharmacophore Modelling of 2,6,9-Trisubstituted Purine Derivatives and Their Potential Role as Apoptosis-Inducing Agents in Cancer Cell Lines
Abstract
:1. Introduction
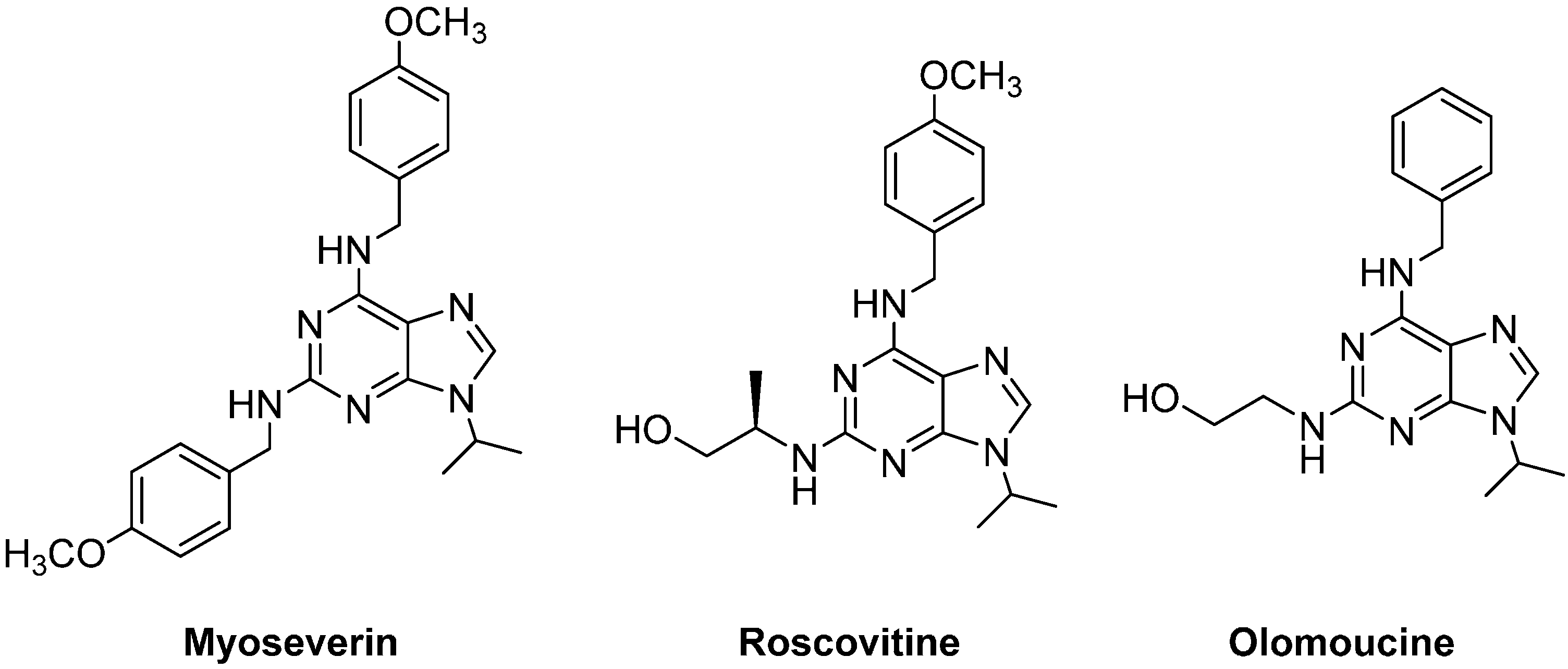
2. Results and Discussion
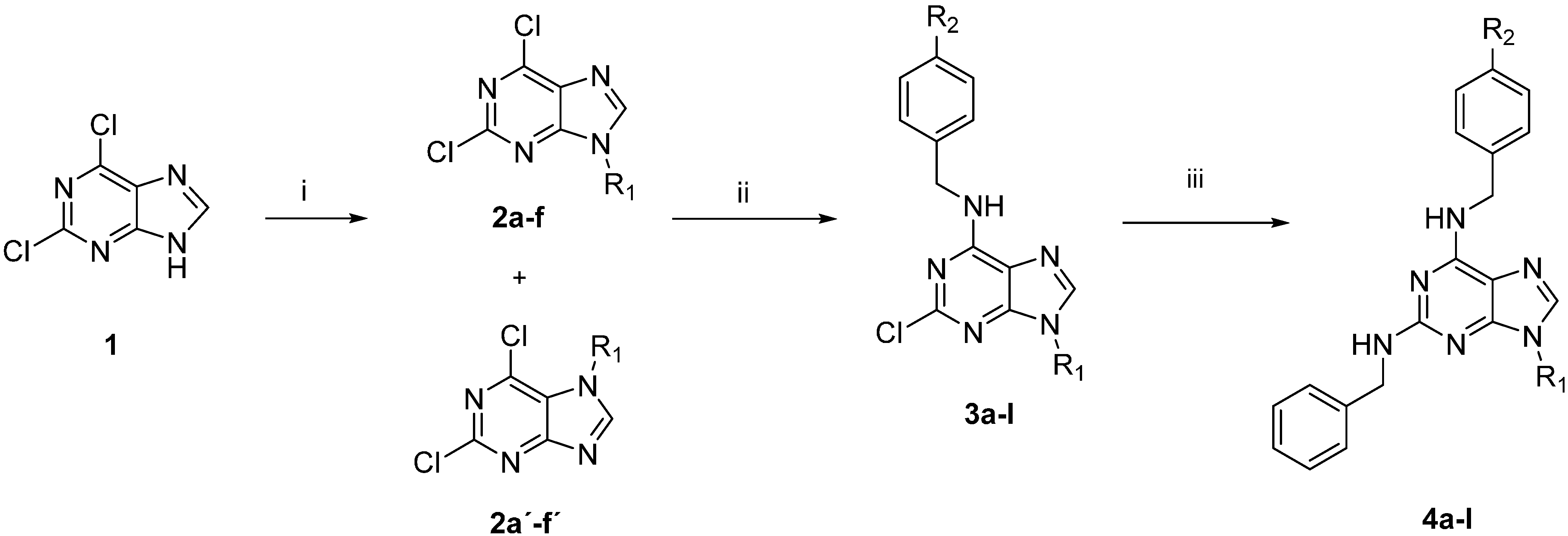
2.1. Synthesis
| Entry | R1 | R2 | IC50 (µM) a | ||||
|---|---|---|---|---|---|---|---|
| H1975 | HL-60 | HCT116 | HeLa | Vero | |||
| 4a | i-Propyl | H | 14.0 ± 0.26 | 26.0 ± 0.13 | 13.0 ± 0.12 | 32.0 ± 0.13 | 20.0 ± 0.11 |
| 4b | Butyl | H | 28.0 ± 0.08 | >50.0 | 7.2 ± 0.16 | 18.0 ± 0.14 | 5.6 ± 0.15 |
| 4c | i-Butyl | H | 36.0 ± 0.27 | >50.0 | >50.0 | >50.0 | 22.0 ± 0.23 |
| 4d | Pentyl | H | 12.0 ± 0.19 | >50.0 | 11.0 ± 0.11 | 10.1 ± 0.14 | 2.5 ± 0.20 |
| 4e | i-Pentyl | H | 8.6 ± 0.18 | >50.0 | 5.1 ± 0.14 | 2.7 ± 0.13 | 2.5 ± 0.14 |
| 4f | Hexyl | H | 9.9 ± 0.18 | >50.0 | 3.0 ± 0.12 | 5.3 ± 0.13 | 26.0 ± 0.12 |
| 4g | i-Propyl | OCH3 | >50.0 | 22.0 ± 0.13 | 7.1 ± 0.11 | 10.7 ± 0.18 | 15.0 ± 0.23 |
| 4h | Butyl | OCH3 | 11.0 ± 0.25 | >50.0 | 7.0 ± 0.16 | 6.3 ± 0.17 | 17.0 ± 0.20 |
| 4i | i-Butyl | OCH3 | 3.4 ± 0.28 | >50.0 | 1.6 ± 0.12 | 4.9 ± 0.16 | 1.9 ± 0.24 |
| 4j | Pentyl | OCH3 | 2.3 ± 0.13 | >50.0 | 8.0 ± 0.21 | 9.5 ± 0.14 | 26.0 ± 0.18 |
| 4k | i-Pentyl | OCH3 | 5.6 ± 0.23 | >50.0 | 11.0 ± 0.26 | 5.4 ± 0.32 | 34.0 ± 0.13 |
| 4l | Hexyl | OCH3 | >50.0 | >50.0 | 6.8 ± 0.14 | 9.0 ± 0.13 | 15.0 ± 0.06 |
| Etoposide | - | - | 8.0 ± 0.33 | 6.2 ± 0.18 | 2.8 ± 0.24 | 8.2 ± 0.33 | >25.0 |

2.2. Cytotoxic Studies
| Compound | Selectivity Index (SI) a | Log P b | ||
|---|---|---|---|---|
| (Vero/H1975) | (Vero/HCT116) | (Vero/HeLa) | ||
| 4a | 1.43 | 1.54 | 0.63 | 4.35 |
| 4b | 0.20 | 0.78 | 0.31 | 4.94 |
| 4c | 0.61 | 0.44 | 0.44 | 4.86 |
| 4d | 0.21 | 0.23 | 0.25 | 5.38 |
| 4e | 0.29 | 0.49 | 0.93 | 5.30 |
| 4f | 2.63 | 8.67 | 4.91 | 5.83 |
| 4g | 0.30 | 2.11 | 1.40 | 4.31 |
| 4h | 1.55 | 2.43 | 2.70 | 4.90 |
| 4i | 0.56 | 1.19 | 0.39 | 4.81 |
| 4j | 11.30 | 3.25 | 2.74 | 5.34 |
| 4k | 6.07 | 3.09 | 6.30 | 5.26 |
| 4l | 0.30 | 2.21 | 1.67 | 5.78 |
| Etoposide | 3.10 | 4.03 | 3.05 | - |
2.3. Pharmacophore Elucidation
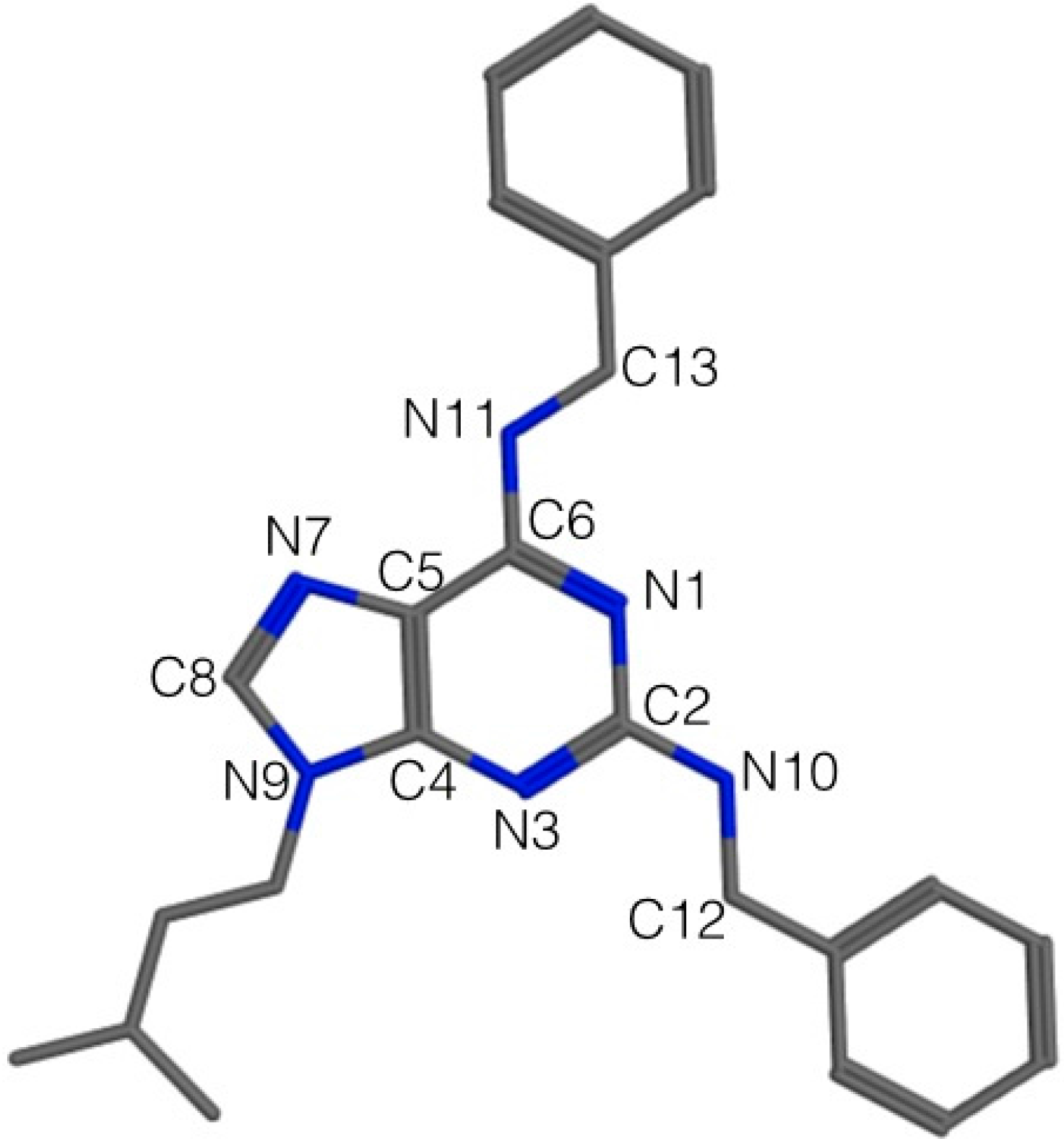
| Structure Feature | Pharmacophoric Features | ||
|---|---|---|---|
| Hela | H1975 | HTC116 | |
| Purine N1 | * Acc | * Acc | - |
| Purine N3 | * Acc | Acc | - |
| Purine N7 | Acc | - | Acc |
| Alkyl moiety on N9 | - | - | Aro/Hyd |
| N10 | - | * Don | - |
| N11 | - | - | Don |
| Benzyl moiety on C2 | Aro/Hyd | Aro/Hyd | Aro/Hyd |
| Benzyl moiety on C2 | Aro/Hyd | Aro/Hyd | Aro/Hyd |
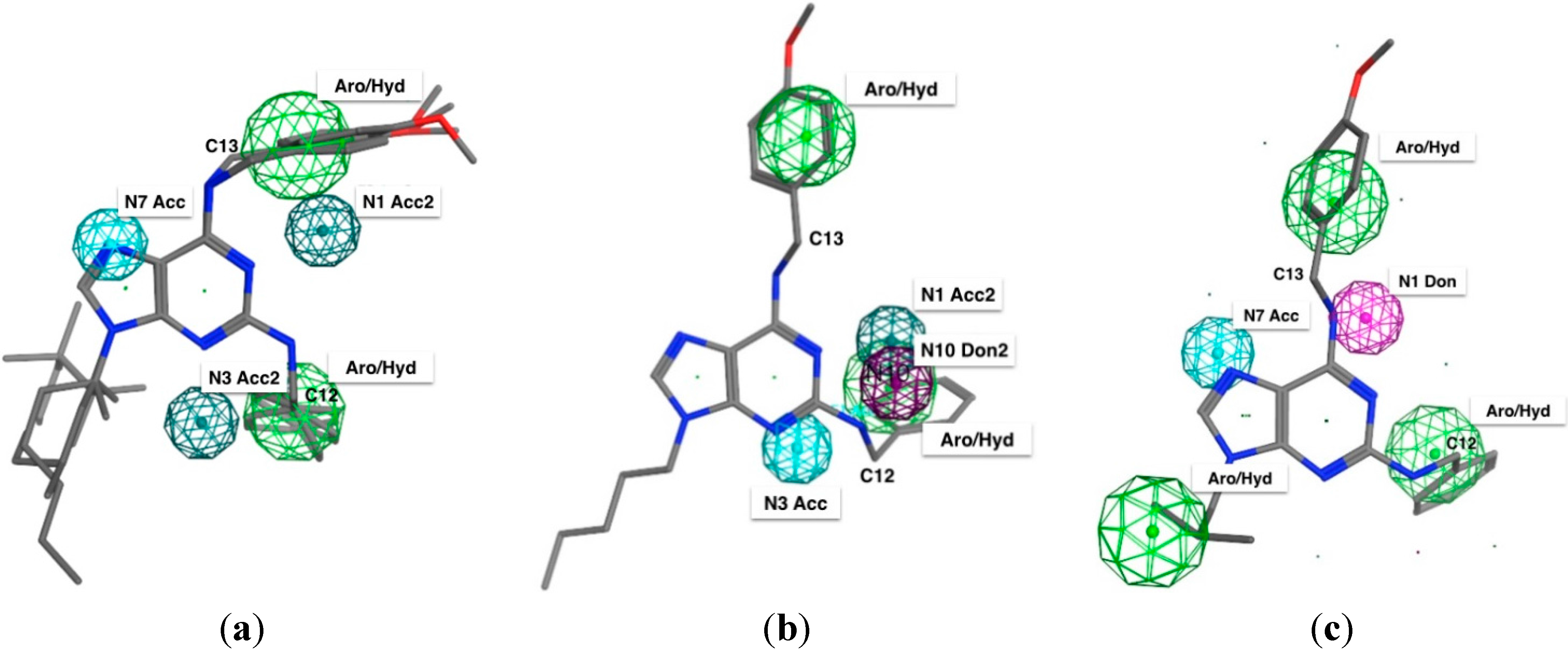
2.4. Apoptosis Assay
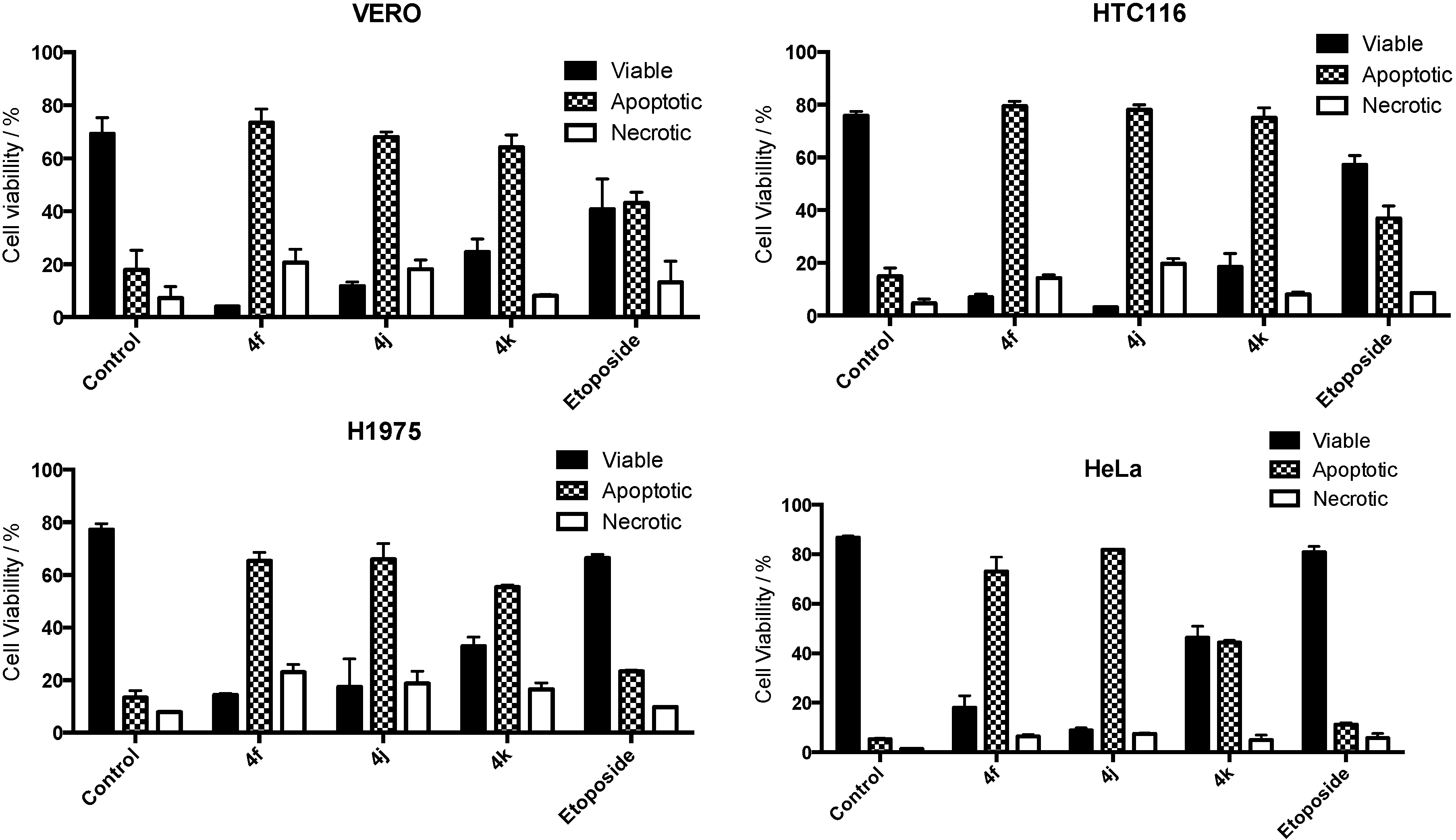
3. Experimental Section
3.1. General Information
3.2. General Synthetic Procedure to Obtain N-Alkyl Purines 2a–f and 2a'–f'
3.3. General Synthetic Procedure to Obtain N-Benzyl-9-Alkyl-2-Chloro-9H-Purin-6-Amines 3a–l
3.4. General Synthetic Procedure to Obtain N2,N6-Dibenzyl-9-Alkyl-9H-Purine-2,6-Diamine Derivatives 4a–l
3.5. Anticancer Activity
3.5.1. Cell Lines Culture
3.5.2. Cytotoxicity Study
3.5.3. Viability Assays with Propidium Iodide
3.5.4. Statical Analysis
3.6. Pharmacophore Elucidation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Welsch, M.E.; Snyder, S.A.; Stockwell, B.R. Privileged scaffolds for library design and drug discovery. Curr. Opin. Chem. Biol. 2010, 14, 347–361. [Google Scholar]
- Rosemeyer, H. The Chemodiversity of Purine as a Constituent of Natural Products. Chem. Biodivers. 2004, 1, 361–401. [Google Scholar]
- Legraverend, M.; Grierson, D.S. The purines: Potent and versatile small molecule inhibitors and modulators of key biological targets. Bioorg. Med. Chem. 2006, 14, 3987–4006. [Google Scholar]
- Hansen, S.W.; Skovsgaard, T.; Sorensen, J.B. Treatment of small cell lung cancer with 6-mercaptopurine: A phase II study. Cancer Treat. Rep. 1985, 69, 555. [Google Scholar]
- Munshi, P.N.; Lubin, M.; Bertino, J.R. 6-Thioguanine: A drug with unrealized potential for cancer therapy. Oncologist 2014, 19, 760–765. [Google Scholar]
- Masson, C. Treatment of herpes with acyclovir. Presse Med. 1983, 12, 1399–1400. [Google Scholar]
- Wang, X.; Wang, L.; Wu, N.; Ma, X.; Xu, J. Clinical efficacy of oral ganciclovir for prophylaxis and treatment of recurrent herpes simplex keratitis. Chin. Med. J. 2015, 128, 46–50. [Google Scholar]
- Vince, R. Synthesis and anti-HIV activity of carbovir and related carbocyclic nucleosides. Nucleic Acids Symp. Ser. 1991, 25, 193–194. [Google Scholar]
- De Clercq, E. In search of a selective antiviral chemotherapy. Clin. Microbiol. Rev. 1997, 10, 674–693. [Google Scholar]
- Flechner, S.M.; Zhou, L.; Derweesh, I.; Mastroianni, B.; Savas, K.; Goldfarb, D.; Modlin, C.S.; Krishnamurthi, V.; Novick, A. The impact of sirolimus, mycophenolate mofetil, cyclosporine, azathioprine, and steroids on wound healing in 513 kidney-transplant recipients. Transplantation 2003, 76, 1729–1734. [Google Scholar]
- Chang, Y.T.; Gray, N.S.; Rosania, G.R.; Sutherlin, D.P.; Kwon, S.; Norman, T.C.; Sarohia, R.; Leost, M.; Meijer, L.; Schultz, P.G. Synthesis and application of functionally diverse 2,6,9-trisubstituted purine libraries as CDK inhibitors. Chem. Biol. 1999, 6, 361–375. [Google Scholar]
- Chang, Y.T.; Wignall, S.M.; Rosania, G.R.; Gray, N.S.; Hanson, S.R.; Su, A.I.; Merlie, J.; Moon, H.S.; Sangankar, S.B.; Perez, O.; et al. Synthesis and biological evaluation of myoseverin derivatives: Microtubule assembly inhibitors. J. Med. Chem. 2001, 44, 4497–4500. [Google Scholar]
- Popowycz, F.; Schneider, C.; Debonis, S.; Skoufias, D.A.; Kozielski, F.; Galmarini, C.M.; Joseph, B. Synthesis and antiproliferative evaluation of pyrazolo[1,5-a]-1,3,5-triazine myoseverin derivatives. Bioorg. Med. Chem 2009, 17, 3471–3478. [Google Scholar]
- Gucky, T.; Jorda, R.; Zatloukal, M.; Bazgier, V.; Berka, K.; Reznickova, E.; Beres, T.; Strnad, M.; Krystof, V. A novel series of highly potent 2,6,9-trisubstituted purine cyclin-dependent kinase inhibitors. J. Med. Chem. 2013, 56, 6234–6247. [Google Scholar]
- Sroka, I.M.; Heiss, E.H.; Havlicek, L.; Totzke, F.; Aristei, Y.; Pechan, P.; Kubbutat, M.H.; Strnad, M.; Dirsch, V.M. A novel roscovitine derivative potently induces G1-phase arrest in platelet-derived growth factor-BB-activated vascular smooth muscle cells. Mol. Pharmacol. 2010, 77, 255–261. [Google Scholar]
- Yenugonda, V.M.; Deb, T.B.; Grindrod, S.C.; Dakshanamurthy, S.; Yang, Y.; Paige, M.; Brown, M.L. Fluorescent cyclin-dependent kinase inhibitors block the proliferation of human breast cancer cells. Bioorg. Med. Chem. 2011, 19, 2714–2725. [Google Scholar]
- Benson, C.; White, J.; de Bono, J.; O’>Donnell, A.; Raynaud, F.; Cruickshank, C.; McGrath, H.; Walton, M.; Workman, P.; Kaye, S.; et al. A phase I trial of the selective oral cyclin-dependent kinase inhibitor seliciclib (CYC202; R-Roscovitine), administered twice daily for 7 days every 21 days. Br. J. Cancer. 2007, 96, 29–37. [Google Scholar]
- Le Tourneau, C.; Faivre, S.; Laurence, V.; Delbaldo, C.; Vera, K.; Girre, V.; Chiao, J.; Armour, S.; Frame, S.; Green, S.R.; et al. Phase I evaluation of seliciclib (R-roscovitine), a novel oral cyclin-dependent kinase inhibitor, in patients with advanced malignancies. Eur. J. Cancer 2010, 46, 3243–3250. [Google Scholar]
- Krystof, V.; McNae, I.W.; Walkinshaw, M.D.; Fischer, P.M.; Muller, P.; Vojtesek, B.; Orsag, M.; Havlicek, L.; Strnad, M. Antiproliferative activity of olomoucine II, a novel 2,6,9-trisubstituted purine cyclin-dependent kinase inhibitor. Cell. Mol. Life Sci. 2005, 62, 1763–1771. [Google Scholar]
- Raynaud, F.I.; Whittaker, S.R.; Fischer, P.M.; McClue, S.; Walton, M.I.; Barrie, S.E.; Garrett, M.D.; Rogers, P.; Clarke, S.J.; Kelland, L.R.; et al. In vitro and in vivo pharmacokinetic-pharmacodynamic relationships for the trisubstituted aminopurine cyclin-dependent kinase inhibitors olomoucine, bohemine and CYC202. Clin. Cancer Res. 2005, 11, 4875–4887. [Google Scholar]
- Massard, C.; Soria, J.C.; Anthoney, D.A.; Proctor, A.; Scaburri, A.; Pacciarini, M.A.; Laffranchi, B.; Pellizzoni, C.; Kroemer, G.; Armand, J.P.; et al. A first in man, phase I dose-escalation study of PHA-793887, an inhibitor of multiple cyclin-dependent kinases (CDK2, 1 and 4) reveals unexpected hepatotoxicity in patients with solid tumors. Cell Cycle 2011, 10, 963–970. [Google Scholar]
- Ederhy, S.; Izzedine, H.; Massard, C.; Dufaitre, G.; Spano, J.P.; Milano, G.; Meuleman, C.; Besse, B.; Boccara, F.; Kahyat, D.; et al. Cardiac side effects of molecular targeted therapies: Towards a better dialogue between oncologists and cardiologists. Crit. Rev. Oncol. Hematol. 2011, 80, 369–379. [Google Scholar]
- Gray, N.S.; Wodicka, L.; Thunnissen, A.M.; Norman, T.C.; Kwon, S.; Espinoza, F.H.; Morgan, D.O.; Barnes, G.; le Clerc, S.; Meijer, L.; et al. Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors. Science 1998, 281, 533–538. [Google Scholar]
- Vandromme, L.; Legraverend, M.; Kreimerman, S.; Lozach, O.; Meijer, L.; Grierson, D.S. A Pd(0) based cross-coupling approach to the synthesis of 2-amidopurines and their evaluation as CDK inhibitors. Bioorg. Med. Chem. 2007, 15, 130–141. [Google Scholar]
- Bork, J.T.; Lee, J.W.; Chang, Y.T. The combinatorial synthesis of purine, pyrimidine and triazinebased libraries. QSAR Comb. Sci. 2004, 23, 245–260. [Google Scholar]
- Morales, F.; Ramirez, A.; Conejo-Garcia, A.; Morata, C.; Marchal, J.A.; Campos, J.M. Anti-proliferative activity of 2,6-dichloro-9- or 7-(ethoxycarbonylmethyl)-9H- or 7H-purines against several human solid tumour cell lines. Eur. J. Med. Chem. 2014, 76, 118–124. [Google Scholar]
- Ciszewski, L.; Waykole, L.; Prashad, M.; Repic, O. A practical synthesis of 2-arylamino-6-alkylaminopurines from 2,6-dichloropurine. Org. Process Res. Dev. 2006, 10, 799–802. [Google Scholar]
- Takvorian, A.G.; Combs, A.P. Microwave-assisted organic synthesis using minivials to optimize and expedite the synthesis of diverse purine libraries. J. Comb. Chem. 2004, 6, 171–174. [Google Scholar]
- Huang, H.; Liu, H.; Chen, K.; Jiang, H. Microwave-Assisted Rapid Synthesis of 2,6,9-Substituted Purines. J. Comb. Chem. 2007, 9, 197–199. [Google Scholar]
- NCI/NIH Developmental Therapeutcs Program. Available online: http://dtp.nci.nih.gov/branches/btb/handlingprep.html (accessed on 15 January 2015).
- Aboul-Fadl, T.; Bin-Jubair, F.A.; Aboul-Wafa, O. Schiff bases of indoline-2,3-dione (isatin) derivatives and nalidixic acid carbohydrazide, synthesis, antitubercular activity and pharmacophoric model building. Eur. J. Med. Chem. 2010, 45, 4578–4586. [Google Scholar]
- Saxena, S.; Chaudhaery, S.S.; Varshney, K.; Saxena, A.K. Pharmacophore-based virtual screening and docking studies on Hsp90 inhibitors. SAR QSAR Environ. Res. 2010, 21, 445–462. [Google Scholar]
- Sample Availability: Samples of all compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón-Arancibia, J.; Espinosa-Bustos, C.; Cañete-Molina, Á.; Tapia, R.A.; Faúndez, M.; Torres, M.J.; Aguirre, A.; Paulino, M.; Salas, C.O. Synthesis and Pharmacophore Modelling of 2,6,9-Trisubstituted Purine Derivatives and Their Potential Role as Apoptosis-Inducing Agents in Cancer Cell Lines. Molecules 2015, 20, 6808-6826. https://doi.org/10.3390/molecules20046808
Calderón-Arancibia J, Espinosa-Bustos C, Cañete-Molina Á, Tapia RA, Faúndez M, Torres MJ, Aguirre A, Paulino M, Salas CO. Synthesis and Pharmacophore Modelling of 2,6,9-Trisubstituted Purine Derivatives and Their Potential Role as Apoptosis-Inducing Agents in Cancer Cell Lines. Molecules. 2015; 20(4):6808-6826. https://doi.org/10.3390/molecules20046808
Chicago/Turabian StyleCalderón-Arancibia, Jeannette, Christian Espinosa-Bustos, Álvaro Cañete-Molina, Ricardo A. Tapia, Mario Faúndez, Maria Jose Torres, Adam Aguirre, Margot Paulino, and Cristian O. Salas. 2015. "Synthesis and Pharmacophore Modelling of 2,6,9-Trisubstituted Purine Derivatives and Their Potential Role as Apoptosis-Inducing Agents in Cancer Cell Lines" Molecules 20, no. 4: 6808-6826. https://doi.org/10.3390/molecules20046808
APA StyleCalderón-Arancibia, J., Espinosa-Bustos, C., Cañete-Molina, Á., Tapia, R. A., Faúndez, M., Torres, M. J., Aguirre, A., Paulino, M., & Salas, C. O. (2015). Synthesis and Pharmacophore Modelling of 2,6,9-Trisubstituted Purine Derivatives and Their Potential Role as Apoptosis-Inducing Agents in Cancer Cell Lines. Molecules, 20(4), 6808-6826. https://doi.org/10.3390/molecules20046808