Synthesis of 5-Alkoxythieno[2,3-e][1,2,4]triazolo[4,3-c]pyrimidine Derivatives and Evaluation of Their Anticonvulsant Activities
Abstract
:1. Introduction
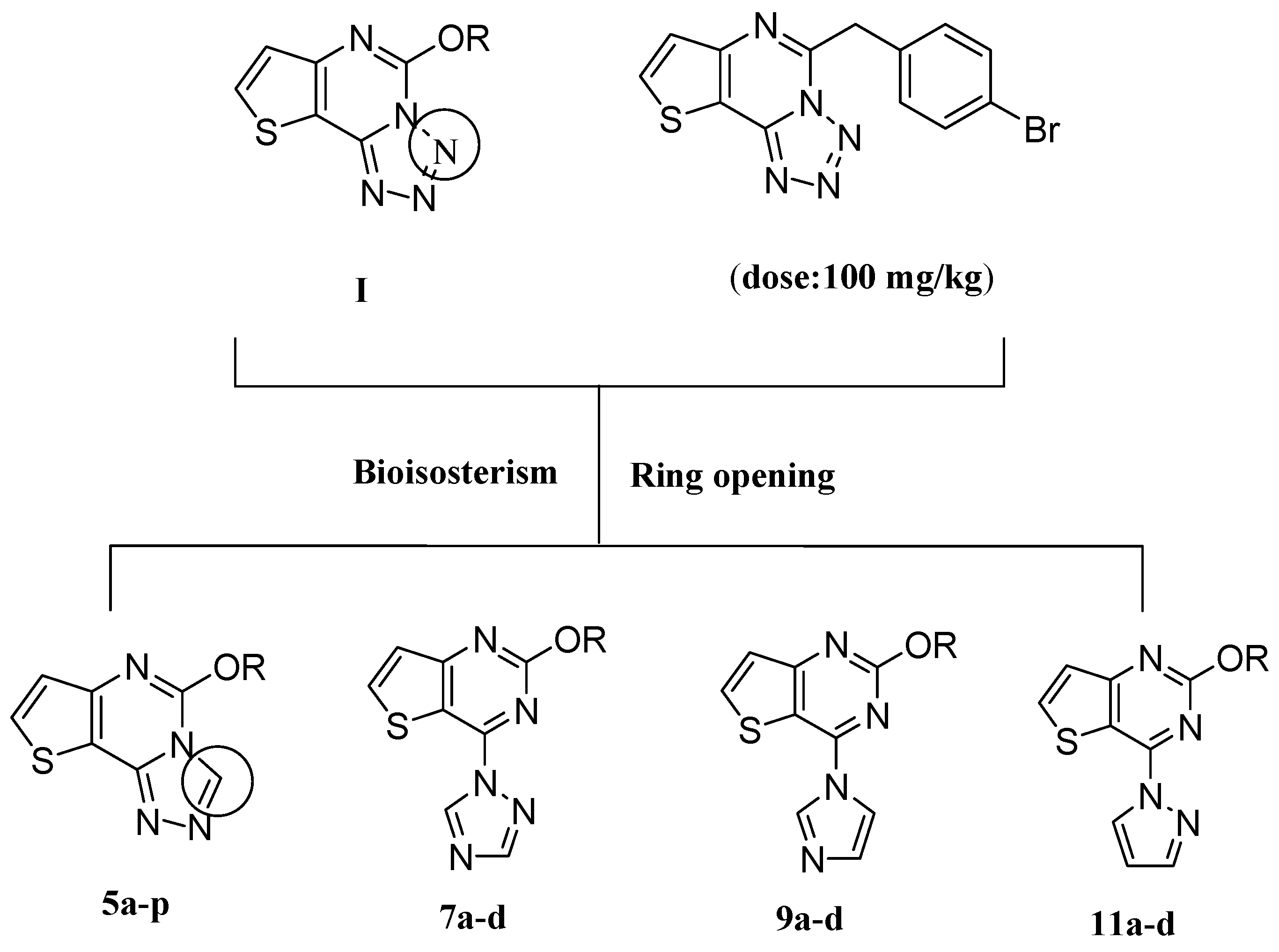
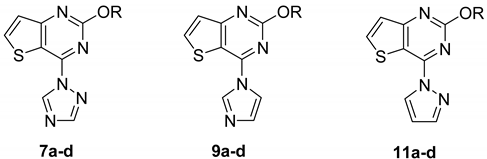
2. Results and Discussion
2.1. Chemistry
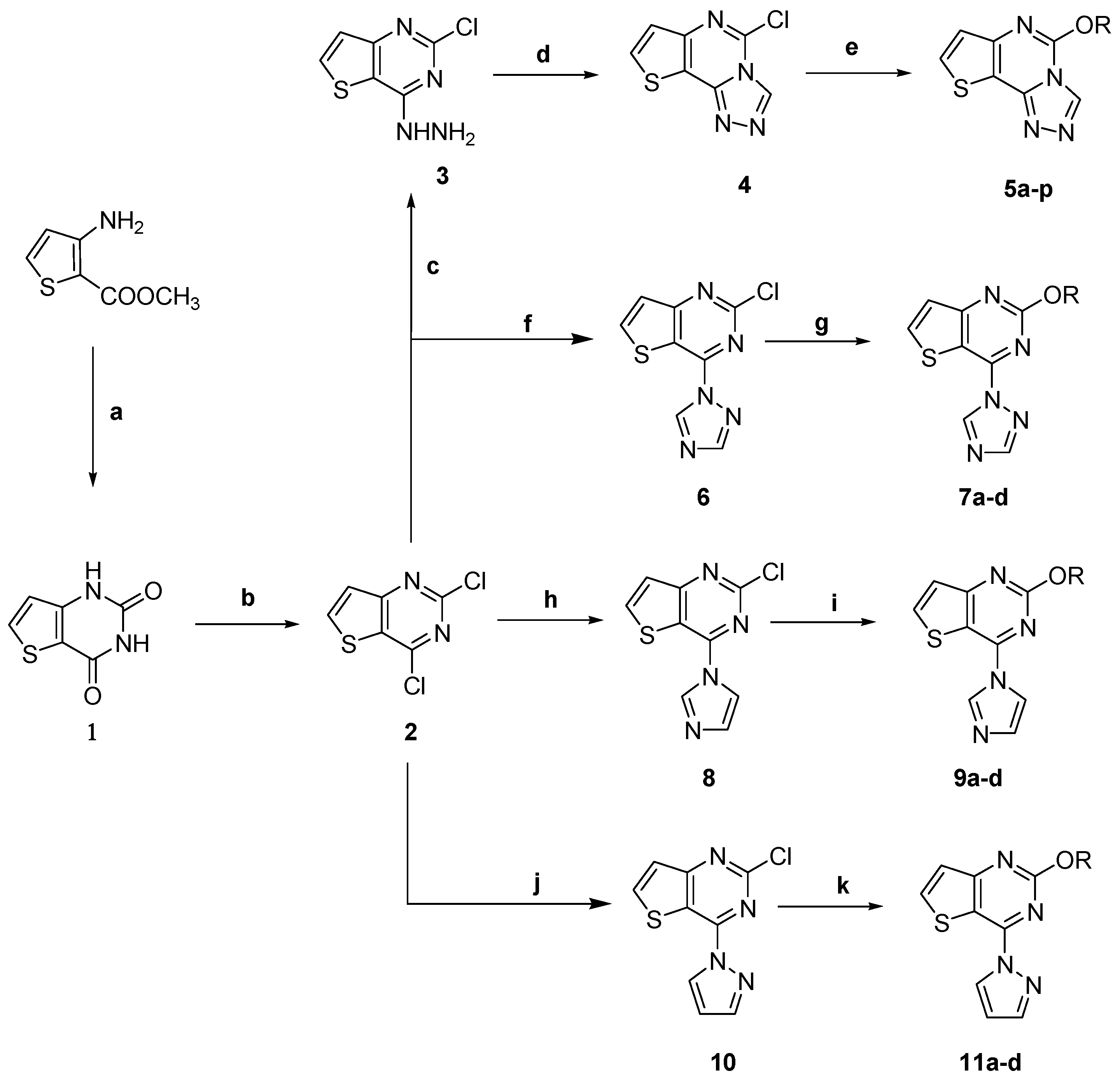
2.2. Pharmacology
2.2.1. Anticonvulsant Activity
| Compound | R | MES a | scPTZ b | TOX c | |||
|---|---|---|---|---|---|---|---|
| 0.5 h | 4 h | 0.5 h | 4 h | 0.5 h | 4 h | ||
| 5a | -C6H5 | 300 | 300 | - | - | 300 | - |
| 5b | -C6H4(o-Cl) | 100 | 100 | 300 | - | - | - |
| 5c | -C6H4(p-Cl) | 30 | 100 | 100 | 300 | 300 | - |
| 5d | -C6H4(m-Cl) | 30 | 100 | 100 | - | 100 | 300 |
| 5e | -C6H4(o-F) | 100 | 300 | - | - | - | - |
| 5f | -C6H4(m-F) | 30 | 100 | 100 | 300 | 300 | 300 |
| 5g | -C6H4(p-F) | 100 | 100 | 100 | 300 | - | - |
| 5h | -C6H4(o-CH3) | 100 | 100 | - | - | 300 | 300 |
| 5i | -C6H4(m-CH3) | 100 | - | - | - | - | - |
| 5j | -C6H4(p-CH3) | 300 | - | - | - | 300 | - |
| 5k | -C6H4(o-OCH3) | 100 | 100 | 300 | - | - | - |
| 5l | -C6H4(m-OCH3) | 100 | - | - | - | 300 | - |
| 5m | -C6H4(p-OCH3) | 300 | 300 | - | - | - | - |
| 5n | -C6H4(p-Br) | 300 | - | - | - | 300 | - |
| 5o | -C6H4(m-CF3) | 30 | 100 | 100 | 300 | 300 | 300 |
| 5p | -C6H3(2,4-Cl) | 30 | 100 | 100 | - | 100 | 300 |
| I | - | 100 | 300 | - | - | - | - |
| Compound | R | MES | scPTZ | TOX | |||
|---|---|---|---|---|---|---|---|
| 0.5 h | 4 h | 0.5 h | 4 h | 0.5 h | 4 h | ||
| 7a | -C6H4(m-Cl) | 300 | - | - | - | 300 | 300 |
| 7b | -C6H4(m-F) | 100 | 300 | 300 | - | 300 | - |
| 7c | -C6H4(m-CF3) | 30 | 100 | 100 | 300 | 300 | 300 |
| 7d | -C6H3(2,4-Cl ) | - | - | - | 300 | - | - |
| 9a | -C6H4(m-Cl) | - | - | 300 | - | 300 | - |
| 9b | -C6H4(m-F) | 300 | - | - | - | 300 | - |
| 9c | -C6H4(m-CF3) | 100 | 300 | 300 | - | 300 | 300 |
| 9d | -C6H3(2,4-Cl ) | - | - | 300 | - | - | - |
| 11a | -C6H4(m-Cl) | 300 | - | - | - | - | - |
| 11b | -C6H4(m-F) | - | - | - | 300 | - | - |
| 11c | -C6H4(m-CF3) | 100 | 300 | 300 | - | 300 | - |
| 11d | -C6H3(2,4-Cl ) | - | 300 | - | - | 300 | - |
| Compound | ED50 (MES) a | ED50 (scPTZ) | TD50 (NT) b | PI c | |
|---|---|---|---|---|---|
| MES scPTZ | |||||
| 5c | 18.5 (16.0–21.5) | 85.2 (78.1–92.9) | 294.6 (257.7–336.7) | 15.8 | 3.4 |
| 5f | 31.7 (23.6–42.5) | >100 | 328.6 (280.1–385.5) | 10.3 | <3.2 |
| 5o | 11.5 (8.6–15.4) | 58.9 (49.1–70.7) | 204.6 (179.0–233.8) | 17.7 | 3.4 |
| 5p | 12.9 (12.9–23.2) | 65.5 (52.5–72.9) | 112.3 (89.8–140.4) | 8.7 | 1.7 |
| 7c | 30.4 (27.2–34.1) | 71.6 (45.9–135) | 237.1 (200.1–280.3) | 7.8 | 3.3 |
| Carbamazepine | 11.8 (8.5–16.4) | – | 76.1 (55.8–103.7) | 6.4 | - |
| Ethosuximide | – | 106 (93–121) | 343 (311–378) | 3.2 | |
| Compound | R | Time (h) | ED50 (MES) | TD50 (NT) | PI |
|---|---|---|---|---|---|
| 5c | -C6H4(p-Cl) | 1.5 | 39.4 (34.0–45.6) | 642.2 (609.7–689.6) | 16.2 |
| 5o | -C6H4(m-CF3) | 1.5 | 28.4 (23.1–32.6) | 510.5 (484.4–648.5) | 17.9 |
| CBZ | - | 1.5 | 27.3(19.7–37.9) | 328.6 (229.9–469.7) | 12.0 |
2.2.2. Structure-Activity Relationship
3. Experimental Section
3.1. General Information
3.2. Chemistry
3.2.1. Synthesis of Thieno[3,2-d]pyrimidine-2,4(1H,3H)-dione (1)
3.2.2. Synthesis of 2,4-Dichlorothieno[3,2-d]pyrimidine (2)
3.2.3. Synthesis of 1-(2-Chlorothieno[3,2-d]pyrimidin-4-yl)hydrazine (3)
3.2.4. Synthesis of 5-Chlorothieno[2,3-e][1,2,4]triazolo[4,3-c]pyrimidine (4)
3.2.5. General Procedure for the Synthesis of 5-Alkoxythieno[2,3-e][1,2,4]triazolo[4,3-c]pyrimidine Derivatives 5a–p
3.2.6. General Procedure for the Synthesis of 2-Alkoxy-4-(1H-1,2,4-triazol-1-yl)thieno[3,2-d]pyrimidine 7a–d, 2-Alkoxy-4-(1H-imidazol-1-yl)thieno[3,2-d]pyrimidine 9a–d and 2-Alkoxy-4-(1H-pyrazol-1-yl)thieno[3,2-d]pyrimidine Derivatives 11a–d
3.3. Pharmacology
3.3.1. Anticonvulsant Effects in the MES Test
3.3.2. Neurotoxicity (NT) Screening
3.3.3. The Subcutaneous Metrazol Seizure Test (scMET)
3.3.4. Pharmacological Evaluation of Compounds 5c and 5o Administered Orally to Mice
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Scheurer, M.L.; Pedley, T.A. The evaluation and treatment of seizures. N. Engl. J. Med. 1990, 323, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Klitgaard, H.; Twyman, R.E.; Schmidt, D. New avenues for anti-epileptic drug discovery and development. Nat. Rev. Drug Discov. 2013, 12, 757–776. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Bahare, R.S.; Khan, S.A. Synthesis and anticonvulsant evaluation of N-(benzo[d]thiazol 2-ylcarbamoyl)-2-methyl-4-oxoquinazoline-3(4H)-carbothioamide derivatives: A hybrid pharmacophore approach. Eur. J. Med. Chem. 2013, 67, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mattson, R.H. Drug treatment of partial epilepsy. Adv. Neurol. 1992, 57, 643–650. [Google Scholar] [PubMed]
- Wesnes, K.A.; Edgar, C.; Dean, A.D.; Wroe, S.J. The cognitive and psychomotor effects of remacemide and carbamazepine in newly diagnosed epilepsy. Epilepsy Behav. 2009, 14, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Meador, K.J. Newer anticonvulsants: Dosing strategies and cognition in treating patients with mood disorders and epilepsy. Clin. Psychiatry 2003, 64, 30–34. [Google Scholar] [CrossRef]
- Stefan, H.; Feuerstein, T. Novel anticonvulsant drugs. Pharmacol. Ther. 2007, 113, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Bilotta, F.; Gelb, A.W.; Stazi, E.; Titi, L.; Paoloni, F.P.; Rosa, G. Pharmacological perioperative brain neuroprotection: A qualitative review of randomized clinical trials. Br. J. Anaesth. 2013, 110 (Suppl. 1), i113–i120. [Google Scholar] [CrossRef]
- Chazot, P.L. Safinamide for the treatment of Parkinson’s disease, epilepsy and restless legs syndrome. Curr. Opin. Investig. Drugs 2013, 8, 570–579. [Google Scholar]
- Shu, B.; Zheng, Y.; Wang, S.B.; Deng, X.Q.; Quan, Z.S. Design, synthesis, and anticonvulsant activity evaluation of 4-(3-alkoxy-phenyl)-2,4-dihydro-[1,2,4]triazol-3-ones. Arch. Pharm. 2013, 346, 127–133. [Google Scholar] [CrossRef]
- Zhang, C.B.; Yang, C.W.; Deng, X.Q.; Quan, Z.S. Design and synthesis of 6-alkyoxyl[1,2,4] triazolo[1,5-a]quinazoline derivatives with anticonvulsant activity. Med. Chem. Res. 2012, 21, 3294–3300. [Google Scholar] [CrossRef]
- Deng, X.Q.; Dong, Z.Q.; Song, M.X.; Shu, B.; Wang, S.B.; Quan, Z.S. Synthesis and anticonvulsant activities of some triazolothiadiazole derivatives. Arch. Pharm. 2012, 345, 565–573. [Google Scholar] [CrossRef]
- Wei, C.X.; Deng, X.Q.; Chai, K.Y.; Sun, Z.G.; Quan, Z.S. Synthesis and anticonvulsant activity of 1-formamide-triazolo[4,3-a]quinoline derivatives. Arch. Pharm. Res. 2010, 33, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.; Adhikari, A.V.; Shetty, N.S. Synthesis and antimicrobial activities of novel quinoline derivatives carrying 1,2,4-triazole moiety. Eur. J. Med. Chem. 2009, 44, 4637–4647. [Google Scholar] [CrossRef] [PubMed]
- Bayrak, H.; Demirbas, A.; Demirbas, N.; Karaoglu, S.A. Synthesis of some new 1,2,4-triazoles starting from isonicotinic acid hydrazide and evaluation of their antimicrobial activities. Eur. J. Med. Chem. 2009, 44, 4362–4366. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.P.; Zhang, H.B.; Qian, H.; Lin, L.; Huang, W.L.; Ni, S.J. Synthesis and Biological Evaluation of Aromatase Inhibitors. Lett. Drug Des. Discov. 2009, 6, 181–185. [Google Scholar] [CrossRef]
- Owen, C.P.; Dhanani, S.; Patel, C.H.; Ahmed, S. Synthesis, Biochemical Evaluation and Rationalisation of the Inhibitory Activity of a Series of 4-Substituted Phenyl Alkyl Triazole-Based Compounds as Potential Inhibitors of 17a-Hydroxylase/17,20-Lyase (P45017a). Lett. Drug Des. Discov. 2007, 4, 479–483. [Google Scholar] [CrossRef]
- Wang, S.B.; Deng, X.Q.; Zheng, Y.; Yuan, Y.P.; Quan, Z.S.; Guan, L.P. Synthesis and evaluation of anticonvulsant and antidepressant activities of 5-alkoxytetrazolo[1,5-c]thieno[2,3-e]pyrimidine derivatives. Eur. J. Med. Chem. 2012, 56, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Bian, M.; Deng, X.Q.; Wang, S.B.; Quan, Z.S. Synthesis and Anticonvulsant Activity Evaluation of 5-Phenyl-[1,2,4]triazolo[4,3-c]quinazolin-3-amines. Arch. Pharm. 2013, 346, 119–126. [Google Scholar] [CrossRef]
- Kelley, J.L.; Krochmal, M.P.; Linn, J.A.; McLean, E.W.; Soroko, F.E. 6-(Alkylamino)-9-benzyl-9H-purines A new class of anticonvulsant agents. J. Med. Chem. 1988, 31, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Qu, G.R.; Zhang, H.L.; Niu, H.Y.; Xue, Z.K.; Lv, X.X.; Guo, H.M. Synthesis of C6-azolyl purine nucleosides via C–N coupling reaction of unprotected 6-chloropurine nucleosides and N-heterocycles under catalyst- and solvent-free conditions. Green Chem. 2012, 14, 1877–1879. [Google Scholar] [CrossRef]
- Krall, R.J.; Penry, J.K.; White, B.G.; Kupferberg, H.J. Antiepileptic drug development: II. Anticonvulsant drug screening. Epilepsia 1978, 19, 409–428. [Google Scholar] [CrossRef]
- Poter, R.J.; Cereghino, J.J.; Gladding, G.D.; Hessie, B.J.; Kupferberg, H.J.; Scoville, B. Antiepileptic Drug Development Program. Clevel. Clin. Q. 1984, 51, 293–305. [Google Scholar] [CrossRef]
- White, H.S.; Woodhead, J.H.; Wilcox, K.S.; Stables, J.P.; Kupferberg, H.J.; Wolf, H.H. Antiepileptic Drugs, 5th ed.; Levy, R.H., Mattson, B.S., Meldrum, E., Perucca, E., Eds.; Lippincott Williams & Wilkins Publishers: New York, NY, USA, 2002; pp. 36–48. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.-B.; Piao, G.-C.; Zhang, H.-J.; Quan, Z.-S. Synthesis of 5-Alkoxythieno[2,3-e][1,2,4]triazolo[4,3-c]pyrimidine Derivatives and Evaluation of Their Anticonvulsant Activities. Molecules 2015, 20, 6827-6843. https://doi.org/10.3390/molecules20046827
Wang S-B, Piao G-C, Zhang H-J, Quan Z-S. Synthesis of 5-Alkoxythieno[2,3-e][1,2,4]triazolo[4,3-c]pyrimidine Derivatives and Evaluation of Their Anticonvulsant Activities. Molecules. 2015; 20(4):6827-6843. https://doi.org/10.3390/molecules20046827
Chicago/Turabian StyleWang, Shi-Ben, Guang-Chun Piao, Hong-Jian Zhang, and Zhe-Shan Quan. 2015. "Synthesis of 5-Alkoxythieno[2,3-e][1,2,4]triazolo[4,3-c]pyrimidine Derivatives and Evaluation of Their Anticonvulsant Activities" Molecules 20, no. 4: 6827-6843. https://doi.org/10.3390/molecules20046827
APA StyleWang, S.-B., Piao, G.-C., Zhang, H.-J., & Quan, Z.-S. (2015). Synthesis of 5-Alkoxythieno[2,3-e][1,2,4]triazolo[4,3-c]pyrimidine Derivatives and Evaluation of Their Anticonvulsant Activities. Molecules, 20(4), 6827-6843. https://doi.org/10.3390/molecules20046827