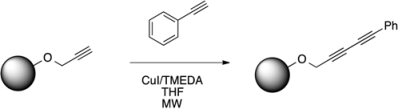
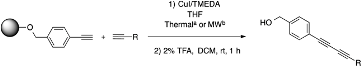
Optimization of Solid-Supported Glaser-Hay Reactions in the Microwave
Abstract
:1. Introduction
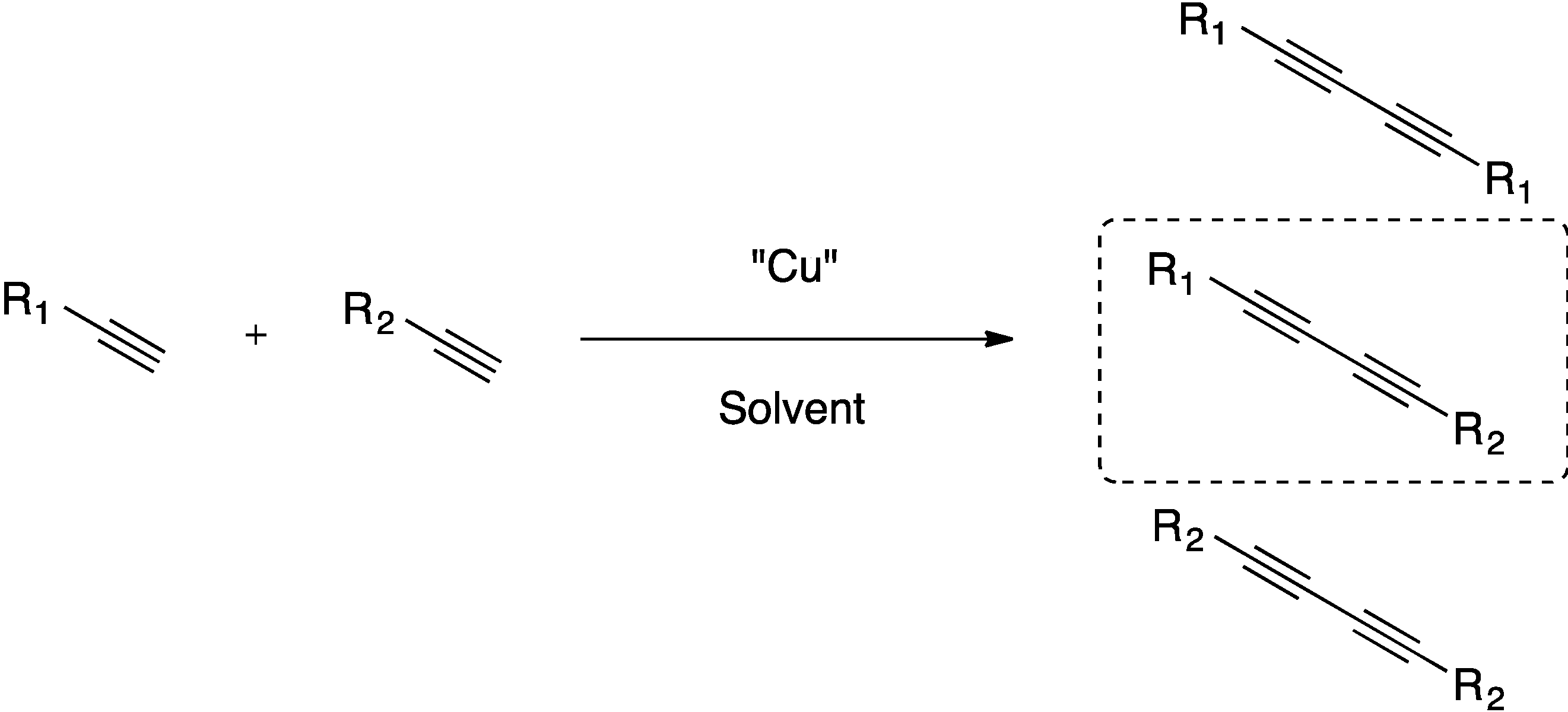
2. Results and Discussion
2.1. Optimization of Microwave Conditions
| Temperature (°C) | Time (min) | Yield (%) |
|---|---|---|
| 40 | 20 | 0 |
| 60 | 5 | 26 |
| 60 | 10 | 74 |
| 80 | 5 | 12 |
| 80 | 10 | 77 |
| 80 | 20 | 77 |
| 100 | 5 | 0 |
| 100 | 10 | 63 |
2.2. Comparison to Thermal Conditions
| Alkyne | Product | % Yield Thermal a | %Yield Microwave b |
|---|---|---|---|
 | 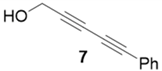 | 95% | 77% |
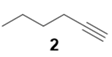 | 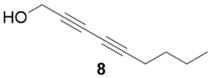 | 84% | 67% |
 | 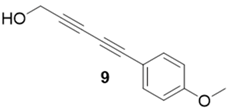 | 99% | 75% |
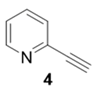 |  | 55% | 90% |
 |  | 40% | 92% |
 |  | 99% | 90% |
| Alkyne | Product | % Yield Thermal a | %Yield Microwave b |
|---|---|---|---|
 |  | 96% | 78% |
 |  | 98% | 76% |
 | 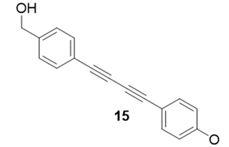 | 73% | 76% |
 |  | 53% | 86% |
 | 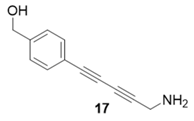 | 96% | 86% |
 | 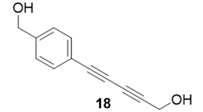 | 40% | 86% |
3. Experimental Section
3.1. General
3.2. Alkyne Immobilization Protocol
3.3. General Glaser-Hay Coupling Protocol
3.4. Analytical Data
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vilhelmsen, M.; Jensen, J.; Tortzen, C.; Nielsen, M. The Glaser-Hay Reaction: Optimization and Scope Based on C-13 NMR Kinetics Experiments. Eur. J. Org. Chem. 2013, 701–711. [Google Scholar] [CrossRef]
- Glaser, C. Beitrage zur Kenntiniss des Acetyenylbenzols. Ber. Dtsch. Chem. Ges. 1896, 2, 422–424. [Google Scholar] [CrossRef]
- Hay, A.S. Polymerization by oxidative coupling.II. Oxidation of 2, 6-disubstituted phenols. J. Poly. Sci. 1962, 58, 581. [Google Scholar]
- Hay, A.S. Oxidative Coupling of Acetylenes. J. Org. Chem. 1962, 27, 3320. [Google Scholar] [CrossRef]
- Wong, W. Recent advances in luminescent transition metal polyyne polymers. J. Inorg. Organomet. Polym. Mater. 2005, 15, 197–219. [Google Scholar] [CrossRef]
- Shi Shun, A.L.K.; Tykwinski, R.R. Synthesis of naturally occurring polyynes. Angew. Chem. Int. Ed. 2006, 45, 1034–1057. [Google Scholar] [CrossRef]
- Slepkov, A.D.; Hegmann, F.A.; Eisler, S.; Elliott, E.; Tykwinski, R.R. The surprising nonlinear optical properties of conjugated polyyne oligomers. J. Chem. Phys. 2004, 120, 6807–6810. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Lowary, T.; Tykwinski, R.R. Naturally occurring and synthetic polyyne glycosides. Can. J. Chem. Rev. 2009, 87, 1565–1582. [Google Scholar] [CrossRef]
- Klebansky, A.; Dolgopolsky, I.; Dobler, Z. The Role of Complex Compounds and Cations of Complex-Forming Components in the Polymerization of Acetylene. Dokl. Akad. Nauk SSSR 1957, 114, 323–326. [Google Scholar]
- Yin, W.; He, C.; Chen, M.; Zhang, H.; Lei, A. Nickel-Catalyzed Oxidative Coupling Reactions of Two Different Terminal Alkynes Using O-2 as the Oxidant at Room Temperature: Facile Syntheses of Unsymmetric 1,3-Diynes. Org. Lett. 2009, 11, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Montierth, J.; DeMario, D.; Kurth, M.; Schore, N. The polymer-supported Cadiot-Chodkiewicz coupling of acetylenes to produce unsymmetrical diynes. Tetrahedron 1998, 54, 11741–11748. [Google Scholar] [CrossRef]
- Chodkiewicz, W.; Cadiot, P.; Willemart, A. Diethynyl-Arenes. Comptes Rendus Hebd. Seances Acad. Sci. 1957, 245, 2061–2062. [Google Scholar]
- Zheng, Q.; Hua, R.; Wan, Y. An Alternative CuCl—Piperidine-Catalyzed Oxidative Homocoupling of Terminal Alkynes Affording 1,3-Diynes in Air. Appl. Organomet. Chem. 2010, 24, 314–316. [Google Scholar]
- Wang, L.; Yan, J.; Li, P.; Wang, M.; Su, C. The Effect of Amines on Oxidative Homo-Coupling of Terminal Alkynes Promoted by Copper Salts. J. Chem. Res. 2005, 2, 112–115. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Zhang, G.; Liu, Q. A Mild Copper-Mediated Glaser-Type Coupling Reaction Under the Novel CuI/NBS/DIPEA Promoting System. Tetrahedron Lett. 2009, 50, 4033–4036. [Google Scholar] [CrossRef]
- Lampkowski, J.S.; Durham, C.E.; Padilla, M.S.; Young, D.D. Preparation of Asymmetrical Polyynes by a Solid-Supported Glaser-Hay Reaction. Org. Biomol. Chem. 2014, 13, 424–427. [Google Scholar] [CrossRef]
- Tripp, V.T.; Lampkowski, J.S.; Tyler, R.; Young, D.D. Development of Solid-Supported Glaser-Hay Couplings. ACS Comb. Sci. 2014, 16, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.A.; Kremsner, J.M.; Kappe, C.O. Nonthermal Microwave Effects Revisited: On the Importance of Internal Temperature Monitoring and Agitation in Microwave Chemistry. J. Org. Chem. 2008, 73, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Hoz, A; Loupy, A (Eds.) Microwaves in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2012.
- Senaiar, R.; Young, D.D.; Deiters, A. Pyridines via Solid-Supported [2 + 2 + 2] Cyclotrimerization. Chem. Commun. 2006, 1313–1315. [Google Scholar] [CrossRef]
- Young, D.D.; Deiters, A. A General Approach to Chemo- and Regioselective Cyclotrimerization Reactions. Angew. Chem. Int. Ed. 2007, 46, 5187–5190. [Google Scholar] [CrossRef]
- Bédard, A.C.; Collins, S.K. Microwave Accelerated Glaser-Hay Macrocyclizations at High Concentrations. Chem. Commun. 2012, 48, 6420–6422. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 7–18 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lampkowski, J.S.; Maza, J.C.; Verma, S.; Young, D.D. Optimization of Solid-Supported Glaser-Hay Reactions in the Microwave. Molecules 2015, 20, 5276-5285. https://doi.org/10.3390/molecules20045276
Lampkowski JS, Maza JC, Verma S, Young DD. Optimization of Solid-Supported Glaser-Hay Reactions in the Microwave. Molecules. 2015; 20(4):5276-5285. https://doi.org/10.3390/molecules20045276
Chicago/Turabian StyleLampkowski, Jessica S., Johnathan C. Maza, Sanjana Verma, and Douglas D. Young. 2015. "Optimization of Solid-Supported Glaser-Hay Reactions in the Microwave" Molecules 20, no. 4: 5276-5285. https://doi.org/10.3390/molecules20045276
APA StyleLampkowski, J. S., Maza, J. C., Verma, S., & Young, D. D. (2015). Optimization of Solid-Supported Glaser-Hay Reactions in the Microwave. Molecules, 20(4), 5276-5285. https://doi.org/10.3390/molecules20045276