Targeting the Cryptococcus neoformans var. grubii Cell Wall Using Lectins: Study of the Carbohydrate-Binding Domain
Abstract
:1. Introduction
2. Results and Discussion
| Cryptococcus neoformans var. Grubii *URM | WGA lectin | Con A lectin | PNA lectin | UEA-I lectin |
|---|---|---|---|---|
| 5809 | +++ | ++ | + | + |
| 5810 | +++ | ++ | + | + |
| 5811 | +++ | ++ | + | + |
| 5813 | +++ | ++ | + | + |
| 5814 | +++ | ++ | + | + |
| 5815 | +++ | ++ | + | + |
| 5816 | +++ | ++ | + | + |
| 5818 | +++ | ++ | + | + |
| 5819 | +++ | ++ | + | + |
| 5820 | +++ | ++ | + | + |
| 5821 | +++ | ++ | + | + |
| 5822 | +++ | ++ | + | + |
| 5823 | +++ | ++ | + | + |
| 5824 | +++ | ++ | + | + |
| 5825 | +++ | ++ | + | + |
| 6895 | +++ | ++ | + | + |
| 6896 | +++ | ++ | + | + |
| 6897 | +++ | ++ | + | + |
| 6898 | +++ | ++ | + | + |
| 6899 | +++ | ++ | + | + |
| 6900 | +++ | ++ | + | + |
| 6901 | +++ | ++ | + | + |
| 6902 | +++ | ++ | + | + |
| 6903 | +++ | ++ | + | + |
| 6904 | +++ | ++ | + | + |
| 6905 | +++ | ++ | + | + |
| 6906 | +++ | ++ | + | + |
| 6907 | +++ | ++ | + | + |
| 6908 | +++ | ++ | + | + |
| 6909 | +++ | ++ | + | + |
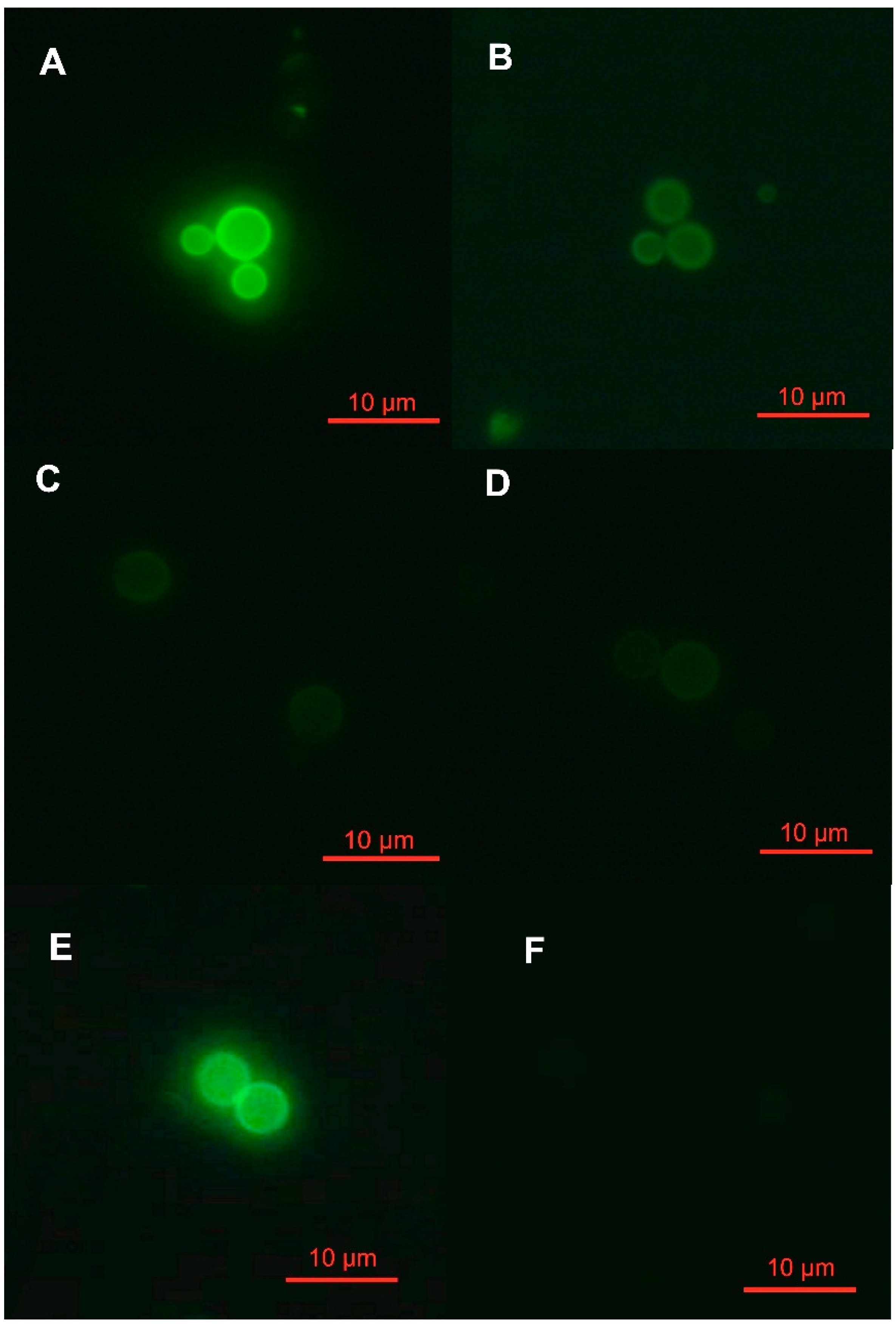
3. Experimental Section
3.1. Cryptococcus Strains and Growth Conditions
3.2. Cryptococcal Cell Wall Lectin Binding
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, T.B.; Perlin, D.; Xue, C. Molecular mechanisms of cryptococcal meningitis. Virulence 2012, 3, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Vu, K.; Eigenheer, R.A.; Phinney, B.S.; Gelli, A. Cryptococcus neoformans promotes its transmigration into the central nervous system by inducing molecular and cellular changes in brain endothelial cells. Infect. Immun. 2013, 81, 3139–3147. [Google Scholar] [CrossRef] [PubMed]
- Santi, L.; Beys-da-Silva, W.O.; Berger, M.; Calzolari, D.; Guimarães, J.A.; Moresco, J.J.; Yates, J.R. Proteomic profile of Cryptococcus neoformans biofilm reveals changes in metabolic processes. J. Proteome Res. 2014, 13, 1545–1559. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.F.G.; Lima-Neto, R.G.; Macêdo, D.P.C.; Beltrão, E.I.C.; Neves, R.P. Carbohydrate profile of fungal cell wall surfasse glycoconjugates of Trichophyton tonsurans and other keratinophilic filamentous fungi using lectins. Mycoses 2011, 54, 789–794. [Google Scholar] [CrossRef]
- Patil, R.T.; Sangwan, J.; Juya, D.; Lathwa, S. Meningitis due to Cryptococcus gattii in an immunocompetent patient. J. Clin. Diagn. Res. 2013, 7, 2274–2275. [Google Scholar] [PubMed]
- Seco-Rovira, V.; Beltrán-Frutos, E.; Ferrer, C.; Sánchez-Huertas, M.M.; Madrid, J.F.; Saez, F.J.; Pastor, L.M. Lectin histochemistry as a tool to identify apoptotic cells in the seminiferous epithelium of Syrian hamster (Mesocricetus auratus) subjected to short photoperiod. Reprod. Domest. Anim. 2013, 48, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cerdeira, C.; Arenas, R.; Moreno-Coutiño, G.; Vásquez, E.; Fernández, R.; Chang, P. Micosis sistémicas en pacientes con virus de la inmunodeficiencia humana/SIDA. Actas Dermosifiliogr. 2014, 105, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Lima-Neto, R.G.; Beltrão, E.I.; Oliveira, P.C.; Neves, R.P. Adherence of Candida albicans and Candida parapsilosis to epithelial cells correlates with fungal cell surface carbohydrates. Mycoses 2011, 54, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.L.; Alvarez, M.; Fonseca, F.L.; Casadevall, A. Binding of the wheat germ lectin to Cryptococcus neoformans suggests an association of chitinlike structures with yeast budding and capsular glucuronoxylomannan. Eukaryot. Cell 2008, 7, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, F.L.; Guimarães, A.J.; Kmetzsch, L.; Dutra, F.F.; Silva, F.D.; Taborda, C.P.; Araujo, G.S.; Frases, S.; Staats, C.C.; Bozza, M.T.; et al. Binding of the wheat germ lectin to Cryptococcus neoformans chitooligomers affects multiple mechanisms required for fungal pathogenesis. Fungal Genet. Biol. 2013, 60, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Gooday, G.W. Cell walls. In The Growing Fungus; Gow, N.A.R., Gadd, G.M., Eds.; Chapman Hall: London, UK, 1995; pp. 43–62. [Google Scholar]
- Pietrella, D.; Cherniak, D.; Strappani, C.; Perito, S.; Mosci, P.; Bistoni, F.; Vecchiarelli, A. Role of mannoprotein in induction and regulation of immunity to Cryptococcus neoformans. Infect. Immun. 2001, 69, 2808–2814. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.J.; Bird, R.A.; Kelly, S.L.; Nishimura, K.; Poyner, D.; Taylor, S.; Smith, S.N. FITC-lectin avidity of Cryptococcus neoformans cell wall and capsular components. Mycologia 2004, 96, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Brito Ximenes, P.; Beltrão, E.I.C.; Macêdo, D.P.C.; Buonafina, M.D.S.; De Lima-Neto, R.G.; Neves, R.P. Targeting the Cryptococcus neoformans var. grubii Cell Wall Using Lectins: Study of the Carbohydrate-Binding Domain. Molecules 2015, 20, 3776-3782. https://doi.org/10.3390/molecules20033776
De Brito Ximenes P, Beltrão EIC, Macêdo DPC, Buonafina MDS, De Lima-Neto RG, Neves RP. Targeting the Cryptococcus neoformans var. grubii Cell Wall Using Lectins: Study of the Carbohydrate-Binding Domain. Molecules. 2015; 20(3):3776-3782. https://doi.org/10.3390/molecules20033776
Chicago/Turabian StyleDe Brito Ximenes, Pamella, Eduardo Isidoro Carneiro Beltrão, Danielle Patrícia Cerqueira Macêdo, Maria Daniela Silva Buonafina, Reginaldo Gonçalves De Lima-Neto, and Rejane Pereira Neves. 2015. "Targeting the Cryptococcus neoformans var. grubii Cell Wall Using Lectins: Study of the Carbohydrate-Binding Domain" Molecules 20, no. 3: 3776-3782. https://doi.org/10.3390/molecules20033776
APA StyleDe Brito Ximenes, P., Beltrão, E. I. C., Macêdo, D. P. C., Buonafina, M. D. S., De Lima-Neto, R. G., & Neves, R. P. (2015). Targeting the Cryptococcus neoformans var. grubii Cell Wall Using Lectins: Study of the Carbohydrate-Binding Domain. Molecules, 20(3), 3776-3782. https://doi.org/10.3390/molecules20033776





