Abstract
The synthesis, tautomerism and antibacterial activity of novel barbiturates is reported. In particular, 3-acyl and 3-carboxamidobarbiturates exhibited antibacterial activity, against susceptible and some resistant Gram-positive strains of particular interest is that these systems possess amenable molecular weight, rotatable bonds and number of proton-donors/acceptors for drug design as well as less lipophilic character, with physicochemical properties and ionic states that are similar to current antibiotic agents for oral and injectable use. Unfortunately, the reduction of plasma protein affinity by the barbituric core is not sufficient to achieve activity in vivo. Further optimization to reduce plasma protein affinity and/or elevate antibiotic potency is therefore required, but we believe that these systems offer unusual opportunities for antibiotic drug discovery.
1. Introduction
The use of natural products as leads for antibacterial drug discovery is enjoying a resurgence of interest, forced by the failure of existing drug discovery strategies, the particular requirements of antibacterial therapies, the emergence of virulent bacterial strains and the paucity of new development candidates working their way through the drug development pipeline [1,2]. In this regard, the tetramic acid scaffold (especially with a 3-acyl side chain moiety) [3,4,5,6,7,8] is of particular interest, since naturally occurring 3-acyltetramic acids such as reutericyclin (bacterial membrane disruption) [5], streptolydigin (bacterial RNA polymerase (RNAP) inhibitor) [6], kibdelomycin (bacterial type II topoisomerase inhibitor) [7] and signermycin B (the dimerization domain of histidine kinase WalK inhibitor) [8] all exhibit antibacterial activity with novel modes of action. Aiming to develop both the biological activity and bioavailability of these systems, methodology for the modification of ring substituents in natural 3-acyltetramic acids (R1-R4 in 1a) [9,10,11,12,13,14] as well as the replacement of the 3-acyl side chain group by 3-carboxamide (1b) [13,15,16,17,18] and 3-enamine functionalities (1c, Figure 1) [13,19] has been reported. These investigations revealed that 3-acyl 1a and 3-carboxamide 1b substitutions can impart good antibacterial activity, resulting from novel modes of action including bacterial membrane disruption, inhibition of bacterial RNAP or undecaprenyl pyrophosphate synthase (UPPS), while 3-enamine 1c exhibits much weaker antibacterial activity and without a clear mode of action. Furthermore, it has been found that the 5-membered tetramic acid core scaffold may also be replaced by the 6-membered piperidine-2,4-dione unit with 3-acyl (1d) [14] and 3-carboxamide (1e) [16,18] pendant functionality, and that these systems show similar antibacterial activity and mode of action compared to tetramates 1a,b.
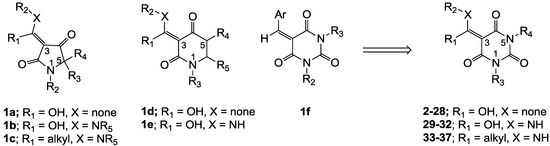
Figure 1.
Optimization of core scaffold.
Although 3-acyl and 3-carboxamide tetramate analogues reported in our previous papers [13,14,15] showed good antibacterial activity, novel modes of action and acceptable toxicity, their high plasma protein binding (PPB) affinity interfered with further biological study in vivo for antibiotic drug discovery. In order to overcome this PPB affinity, we decided to conduct further structural optimization of the core scaffold, by moving from the tetramic acid to the 6-membered barbituric acid system. This scaffold possesses similar chemical structure compared to tetramic acids and piperidine-2,4-diones (especially around the C(3) position), but importantly has additional polar functional groups at the 5- and 6-positions, and we expected that these groups might help to reduce PPB affinity. Further validation of this proposal comes from the fact that arylidene barbituric acids 1f are reported to have mild antibacterial activity [20,21,22,23]. In this paper, with the inspiration from related analogues 1a–f, novel barbituric acids 2–28 (with 3-acyl), 29–32 (with 3-carboxamide) and 33–37 (with 3-enamine) have been prepared and their tautomeric behavior, antibacterial activity and structure-activity relationships (SARs) have been studied. Furthermore, in order to understand trends of biological activity for further drug optimization, their physicochemical property-activity relationships have been investigated and compared with the tetramic acids reported in our previous papers [13,14,15,19] as well as clinical antibiotics. To the best of our knowledge, the antibacterial activity of 3-acylbarbituric acids with only limited functionality has been reported in the literature [20,21], and the 3-carboxamide and the 3-enamine analogues are as yet completely unreported.
2. Result and Discussion
2.1. Synthesis
The starting barbituric templates 40a–c were prepared by known methods, while templates 40d,e were commercially available (Scheme 1). Thus, urea 39a–c was condensed with malonic acid in the presence of acetic acid and acetic anhydride to provide barbituric acids 40a–c, respectively [24]. N-Disubstituted 40b,c, along with 3-acetyl 2b,c [20,25] as minor products, respectively, could be purified by flash column chromatography, while N-monosubstituted 40a was best obtained by precipitation in ethyl acetate solution. For this reaction, urea 39c was efficiently obtained from amine 38 and ethyl isocyanate [20,26], while ureas 39a,b were commercially available.
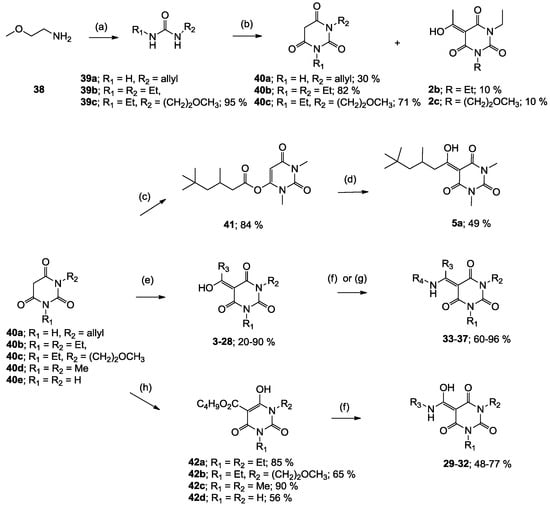
Scheme 1.
Synthesis of barbituric acid analogues. Reaction conditions; (a) ethyl isocyanate (1.0 eq), CH2Cl2, 0 °C; (b) malonic acid (1.0 eq), acetic acid, acetic anhydride, 60–90 °C; (c) 3,5,5-trimethylhexanoyl chloride (1.1 eq), triethylamine (1.2 eq), CH2Cl2, r.t.; (d) DMAP (1.2 eq), CH2Cl2, r.t.; (e) R3CO2H (1.1 eq), DCC (1.1 eq), DMAP (1.2 eq), CH2Cl2, r.t.; (f) RNH2 (1.0 eq), toluene, reflux; (g) RNH2 (1.1 eq), CH3OH, reflux; (h) butyl chloroformate (1.2 eq), DMAP (2.2 eq), CH2Cl2, r.t.; Abbreviation; DCC; N,N′-dicyclohexylcarbodiimide, DMAP; 4-(dimethylamino)pyridine.
With templates 40a–e in hand, the synthesis of 3-acyl, 3-carboxamide and 3-enamine tetramic acids using recently reported approaches were successfully applied to the synthesis of the corresponding barbituric acid analogues [13,14,15,19,27]. 3-Acyl analogues 3–28 (with the exception of 5a) were prepared via direct 3-acylation of templates 40a–e with the required carboxylic acid promoted by 1.1 equivalent of DCC and 1.2 equivalent of DMAP, while stepwise 3-acylation via O-acylation using the acid chloride in the presence of triethylamine followed by acyl migration promoted by DMAP (1.2 equivalent) gave analogue 5a. Although other synthetic methods for 3-acylbarbituric acids have been reported [20,21,28,29,30], this direct acylation approach provides efficient access to systems with a wide variety of substituents at the acyl group. Furthermore, it is also applicable to N-unsubstituted, -mono and di-substituted barbituric acids. 3-Enamines 33–37 were prepared by reaction of the corresponding 3-acyl analogue with an amine in refluxing toluene [25,31,32,33]. In the case of compound 36, methanol instead of toluene was required as solvent. Alternatively, 3-alkoxycarbonyl barbiturates 42a–d needed as starting materials for 3-carboxamides 29–32 were conveniently prepared from the corresponding barbituric acids by treatment with butyl chloroformate in the presence of 1.2 equivalents of DMAP. Conventional direct amine exchange of the 3-alkoxycarbonlys in toluene allowed preparation of 3-carboxamides 29–32. To the best of our knowledge, this is the first example of the preparation of 3-carboxamidobarbituric acids.
2.2. Tautomerism
Similar to tetramic acid derivatives [14,15,19,27], 3-acyl, 3-carboxamide, 3-alkoxycarbonyl and 3-enamine barbituric acids can exist as endo- and exo-enol and keto tautomers in solution (Figure 2). In the case of barbiturates derived from symmetrical barbituric acids 40b,d,e, one set of peaks in their NMR spectra was observed, while in the case of asymmetric barbituric acids 40a,c, split signals (rather than two sets from two tautomeric isomers) were observed. The enol tautomer was assigned from the chemical shift of the C(3)-carbon (80–90 ppm for sp2 carbon) as well as the absence of the H(3)-proton signal. The observation of one set of signals for endo- and exo-enol tautomers supports the fact that equilibration between endo- and exo-enol tautomers is fast on the NMR time scale, resulting in coalescence of the peaks of the two enol tautomers.
In order to identify the favored enol-form, the ground state energies of simplified analogues, 3-acyl 2a, 3-carboxamide 43a, 3-alkoxycarbonyl 43b and 3-enamine 43c in endo- and exo-enol tautomers were calculated (Figure 2). The exo-enol form of 3-acyl 2a and endo-enol form of 3-alkoxycarbonyl 43b were found to be more stable than the alternative enol form, and these results are similar to the favoured tautomer of tetramic acids [15,27]. In contrast, 3-carboxamidobarbiturate 43a favours the exo-enol tautomer, while the corresponding 3-carboxamidotetramate favours the endo-enol tautomer [15]. In the case of 3-enamine 43c, the ground state energy of the endo-enol tautomer is much less stable than the exo-enol tautomer (compare the geometry of 3-enamine 43c between endo- and exo-enol tautomers in Supplementary Figure 1g,h in the Supporting Information). From this computational result, 3-acyl, 3-carboxamide and 3-enamine barbituric acids all preferentially exist as exo-enol tautomers, and 3-alkoxycarbonyl as endo-enol tautomers. In addition, HMBC NMR spectra of representative analogues were acquired and the correlations for the main ring were established (Supplementary Figure 2 in Supporting Information). In this assignment, the free carbonyl (around 160 ppm) and hydrogen-bonded carbonyl (165–170 ppm) on C(2) and (C4) were readily identified.
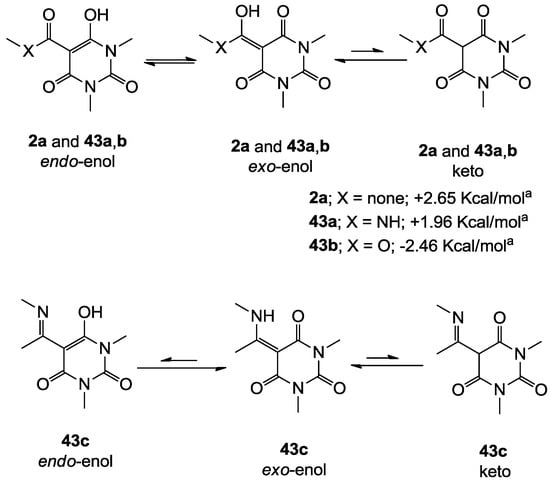
Figure 2.
Tautomeric behavior of barbituric acid analogues; the energy difference between endo- and exo-enol tautomers (∆E = Eendo − Eexo) was calculated by using DFT B3LYP (6-31G*) in Spartan 02.
2.3. Antibacterial Activity
Minimum inhibition concentration (MIC) values for the in vitro in vitro antibacterial activity of 73 barbiturates was determined (shown in Table 1) against Gram-positive bacteria such as Staphylococcus aureus (methicillin sensitive S1, vancomycin susceptible S26, non-resistant S4 and methicillin-resistant in vivo, MRSA, S2), Enterococcus faecalis (vancomycin susceptible, VSE, E1), E. faecium (vancomycin resistant, VRE, E2) and S. pneumonia (erythromycin susceptible P1 and multi drug resistant, MDRSP, P9) as well as Gram-negative bacteria such as Pseudomonas aeruginosa, Escherichia coli (efflux-positive Ec50 and -negative Ec49) and Haemophilus influenzae (efflux-positive H3 and -negative H4). In general, the activity trend for barbiturates is similar to that for tetramates [13,14,15,19,34]; firstly, none of the analogues was active against both P. aeruginosa and efflux-positive and -negative E. coli, (MIC ≥ 32 µg/mL) while the activity against the other strains depended on their ring substituents. Secondly, templates 40a–e, 3-alkoxycarbonyls 42a–d, and O-acyl derivative 41 did not exhibit antibacterial activity against any strains, while the activity of 3-acyls 2–28 (Figure 3), 3-carboxamides 29–32 and 3-enamines 33–37 (Figure 4) depended both upon the identity of the bacterial strains as well as their chemical substituents, with 3-acyls and 3-carboxamides tending to be more effective than 3-enamines. Third, N-disubstituted barbituric acids (especially 3-acyls) exhibited excellent activity whereas N-monosubstituted and N-unsubstituted analogues were inactive (see below in detail). These two results reveal that the functional group located on the C(3) position, as seen for tetramates (e.g., 3-acyl and 3-carboxamide), as well as the N-substitution in the barbituric acid templates, are critical factors for the observation of antibacterial activity. Lastly, of particular importance is that, depending on the substituents, the analogues exhibited excellent antibacterial selectivity against resistant and susceptible strains (S1, S26, S2, E1, E2, P1 and P9). By comparison, the activity of ciprofloxacin against MRSA S2 and VRE E2 and amoxicillin against MDRSP P9 dropped more than 50-fold compared to that of the non-resistant strain [15]. In conclusion, 3-acyls 7a,b and 3-carboxamides 32 possessing adamantyl groups exhibited excellent antibacterial activity against Gram-positive strains and Gram-negative H. influenzae (MIC; up to 0.25 µg/mL).
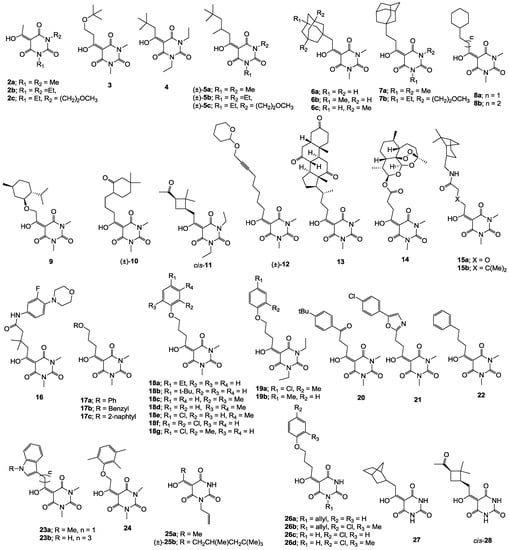
Figure 3.
3-Acylbarbituric acids (50 analogues).
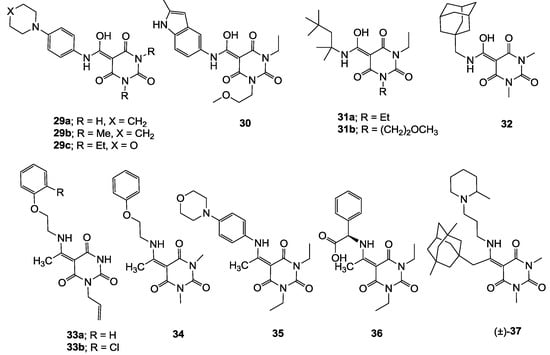
Figure 4.
3-Carboxamides (7 analogues) and 3-enamine (6 analogues) barbituric acids.
Despite replacement of the tetramic acid with the more hydrophilic barbituric acid core, PPB affinity of barbiturates was only slightly reduced when compared with that of tetramates [13,14,15,19]; overall, MICs of barbiturates against S. pneumonia P9 in the presence of 2.5% horse blood were only slightly shifted from the values without blood (Table 1). For example, (±)-5a,b, 6a,b, 7a,b, 8a,b and 18b,g against S. aureus S26 in the presence of 10% human serum exhibited weak activity (MIC = 64 µg/mL) which were approximately 4-fold worse compared to those without serum. Moreover, it appears that the 3-acyl group might be better than the 3-carboxamide group for PPB binding in this family ((±)-5b; 8 to 64 µg/mL versus 31a; 8 to >64 µg/mL, and 6a; 16 to 64 µg/mL and 7a; 1 to 64 µg/mL versus 32; 1 to >64 µg/mL by 10% human serum).

Table 1.
In vitro antibacterial activity (MIC, µg/mL) of barbituric acid analogues a−f.
| S1 | S26 | S26S | S4 | S2 | E1 | E2 | P1 | P9 | P9B | H3 | H4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (±)-5a | 16 | 16 | 64 | 16 | 16 | 16 | 16 | 8 | 8 | 8 | 8 | 4 |
| (±)-5b | 4 | 8 | 64 | 4 | 8 | 4 | 8 | 4 | 4 | 4 | 32 | 8 |
| (±)-5c | 8 | 32 | >64 | 32 | 16 | 16 | 16 | 16 | 16 | 16 | 64 | 32 |
| 6a | 8 | 16 | 64 | 8 | 16 | 8 | 8 | 8 | 8 | 8 | 8 | 2 |
| 6b | 4 | 8 | 64 | 4 | 8 | 4 | 8 | 4 | 4 | 4 | 16 | 4 |
| 6c | 8 | 8 | >64 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 64 | 16 |
| 7a | - c | 1 | 64 | 2 | 2 | 1 | 2 | 0.5 | 0.5 | 2 | 8 | 1 |
| 7b | 0.5 | 2 | 64 | 2 | 1 | 0.5 | 1 | 2 | 2 | 4 | 16 | 8 |
| 8a | 16 | 16 | 64 | 16 | 16 | 8 | 16 | 8 | 8 | 8 | 8 | 2 |
| 8b | 4 | 4 | 64 | 2 | 4 | 0.5 | 2 | 1 | 1 | 1 | 8 | 2 |
| 9 | 32 | 32 | >64 | 16 | 32 | 32 | 16 | 16 | 16 | 16 | >64 | 16 |
| 14 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | 64 | 64 | 8 | >64 | 64 |
| 15a | >64 | - c | - c | >64 | >64 | >64 | >64 | 32 | 32 | >64 | >64 | 64 |
| 15b | 32 | 64 | >64 | 32 | 32 | 32 | 32 | 32 | 16 | 16 | >64 | 8 |
| 16 | >64 | >64 | >64 | >64 | >64 | 64 | 64 | 32 | 32 | 32 | >64 | 8 |
| 17a | 64 | >64 | >64 | 64 | 64 | 64 | 64 | 32 | 32 | 32 | 16 | 8 |
| 17c | 8 | 8 | >64 | 8 | 8 | 8 | 4 | 4 | 4 | 4 | 16 | 2 |
| 18a | 32 | 64 | >64 | 64 | 64 | 32 | 32 | 32 | 32 | 32 | 32 | 4 |
| 18b | 4 | 4 | 64 | 4 | 4 | 2 | 4 | 2 | 2 | 4 | 16 | 2 |
| 18c | 64 | 64 | >64 | 64 | 64 | 64 | 16 | 32 | 32 | 64 | >64 | 32 |
| 18e | 8 | 8 | >64 | 8 | 8 | 4 | 4 | 4 | 4 | 8 | 16 | 2 |
| 18f | 4 | 4 | >64 | 4 | 4 | 4 | 4 | 2 | 2 | 2 | 16 | 2 |
| 18g | 8 | 8 | 64 | 4 | 4 | 4 | 4 | 2 | 2 | 4 | 8 | 2 |
| 19a | 1 | 2 | >64 | 2 | 2 | 1 | 2 | 0.5 | 0.5 | 4 | 16 | 8 |
| 19b | 16 | 16 | >64 | 16 | 16 | 8 | 16 | 8 | 8 | 8 | 32 | 8 |
| 20 | 4 | 8 | >64 | 8 | 8 | 4 | 2 | 2 | 2 | 4 | 16 | 2 |
| 22 | 64 | 64 | >64 | 32 | 64 | 32 | 32 | 32 | 32 | 32 | 8 | 4 |
| 23a | 64 | 64 | >64 | 64 | 32 | 32 | 64 | 64 | 64 | >64 | 32 | 16 |
| 26c | >64 | >64 | >64 | >64 | >64 | >64 | 64 | >64 | >64 | >64 | 32 | 64 |
| 26d | 64 | 64 | >64 | 64 | 32 | 64 | 64 | 64 | >64 | >64 | 32 | 16 |
| 29a | 32 | >64 | >64 | 64 | 32 | >64 | 64 | 64 | 64 | 64 | 32 | 16 |
| 30 | 32 | 64 | >64 | 16 | 32 | 64 | 64 | 64 | >64 | >64 | 32 | 16 |
| 31a | 8 | 8 | >64 | 8 | 4 | 8 | 8 | 8 | 8 | 8 | 64 | 8 |
| 31b | 2 | 4 | >64 | 4 | 4 | 4 | 8 | 4 | 4 | 4 | 64 | 4 |
| 32 | 1 | 1 | >64 | 0.5 | 1 | 0.25 | 0.5 | 0.25 | 0.25 | 0.5 | 2 | 0.25 |
| Lined | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 0.5 | 0.5 | 16 | 4 |
| Cipd | 0.12 | 0.5 | 0.5 | 0.12 | 16 | 1 | 32 | 1 | 1 | 1 | 0.5 | ≤0.06 |
| Amod | - c | - c | - c | - c | - c | - c | - c | >0.03 | 8 | - c | - c | - c |
Notes: a; Abbreviation; S1; S. aureus 1, ATCC13709 in vivo (methicillin sensitive), S26; S. aureus 26, ATCC25923 (vancomycin susceptible), S26S; S. aureus 26 in presence of 10% human serum, S4; S. aureus 4, Oxford, S2; S. aureus 2, MRSA in vivo (methicillin resistant), E1; E. faecalis 1, ATCC29212 VanS (vancomycin susceptible), E2; E. faecium 1, VanA (vancomycin resistant), P1; S. pneumonia 1, ATCC49619 (erythromycin susceptible), P9; S. pneumonia 9, PenR (penicillin and erythromycin resistant), P9B; S. pneumonia 9 in presence of 2.5% horse blood, H3; H. influenzae 3, ATCC31517 MMSA, H4; H. influenzae 4, LS2 Efflux knock-out, Line; linezolid, Cip; ciprofloxacin, Amo; amoxicillin, b; All analogues are inactive against E. coli 1, ATCC25922 (non Pathogenic strain), E. coli 50, Ec49 No Efflux and P. aeruginosa 1, ATCC27853 (MIC > 32 μg/mL), c; Not determined, d; reported in our previous papers [13,14,15,19], e; 3-Acyls 2a–c, 3, cis-11–13, 17b, 25a, (±)-25b and 26a,b, 3-carboxamides 29b,c, 3-enamines 33a,b, 35 and 36, 3-alkoxycarbonyls 42a-d, O-acyl 41 and barbituric acid templates 40a-e were inactive against all strains (MIC > 32 μg/mL), f; 3-Acyls 4, (±)-10, 18d, 21, 23b and 24 and 3-enamines 34 and (±)-37 were only mild active against H4 (8 ≤ MIC ≤ 32 μg/mL) while they were inactive against the other strains (MIC > 32 μg/mL).
2.4. Structure-Activity Relationships
Among the 3-acylbarbiturates, 3-acetyl derivatives 2a–c did not exhibit antibacterial activity, whereas branched alkyls (±)-5a–c possessing a bulky lipophilic group exhibited good antibacterial activity. In addition, the adamantylacetyl substituent, possessing a similar steric effect to the branched alkyl group, retained activity ((±)-5a versus 6a) while methyl substitutions at R1 and R2 in the adamantyl group did not affect the activity (6a–c). In contrast, replacements of the branched alkyl to smaller tert-butyl ((±)-5b versus 4) but also of adamantyl to the smaller cyclohexyl (7a versus 8a) decreased activity. Although the steric effect of 3, 9, (±)-10 and cis-11 is similar to active compounds (±)-5a, 7a, 8b and 6c, respectively, their activities were abolished, probably by the polar heteroatom in the 3-acyl group. However, the longer bridge between the 3-acyl and cyclic functionality provided better activity (6a versus 7a; 8a versus 8b). In addition, longer and polar N-substituents on the barbituric core gave similar activity (7a versus 7b; (±)-5a,b versus (±)-5c) while the longer and lipophilic N-diethyl group provided improved activity compared with N-dimethyl groups ((±)-5a versus (±)-5b; 18g versus 19a). On the other hand, compounds (±)-10-16, all possessing bigger and more polar substituents which had been expected to give reduction of PPB affinity, had no or only slight activity.
From the previous finding that 3-acyltetramic acids possessing substituted phenyl groups with a C3-C4 chain length bridge exhibited good antibacterial activity [14], the SAR of 17–19 were studied in detail. It was found that lipophilicity is a crucial factor for cell permeability in the whole-cell antibacterial assay (see below for details). The more lipophilic analogues 17c (compared with 17a,b), 18b (compared with 17a and 18a) and 19a (compared with 18g and 19b) all exhibited better activity. Of particular interest is that di-substituted phenyl 18e (R1 and R4) and 18f,g (R1 and R2) exhibited better activity than di-substituted phenyl 18c (R2 and R3) and 18d (R3 and R4), even though they all possess similar steric effects and lipophilicity. This result indicates that the activity is sensitive to the nature of phenyl substitution (especially at R1 position). In addition, compounds 21–24 possessing polar atoms on the 3-acyl group and/or shorter bridge than 17–19 exhibited poor activity, with the exception of compound 20.
As mentioned above, N-unsubstituted or -monosubstituted 25–28 dropped in activity, even though the 3-acyl functionality of (±)-25b and 26b,d is identical to that of active barbiturates (±)-5a-c and 18g, respectively. On the other hand, alkyl carboxamides 31 and 32 (possessing similar functionality with active 3-acyl barbiturates as well as 3-acyl and 3-carboxamide tetramic acids [13,14,15]) tended to exhibit better activity than aryl carboxamides 29 and 30 (possessing similar functionality with active 3-carboxamide tetramic acids), while 3-enamines 33–37 had no or only weak antibacterial activity, similarly to 3-enamine tetramic acids [19].
2.5. Physicochemical Property-Antibacterial Activity Relationships
Examination of physicochemical property-activity relationships [35], especially in order to understand any trends for bacterial cell permeability, including transportation by efflux pump and PPB affinity, for antibiotic discovery was made. Figure 5 presents a plot of ClogD7.4 against molecular surface area (MSA) of 3-acyl (50 analogues), 3-carboxamide (7 analogues) and 3-enamine (6 analogues) barbituric acids 2–37 along with the corresponding tetramic acids (326 analogues) from our previous reports [13,14,15,19], classified into active (MIC ≤ 4 µg/mL), mild (4 < MIC ≤ 32 µg/mL) and inactive (MIC > 32 µg/mL) analogues. In addition, Supplementary Figures 3–5 in the Supporting Information presents the plots of ClogP, polar surface area (PSA) and relative-PSA (rel-PSA = PSA/MSA), respectively (see also Supplementary Table 1 in Supporting Information for physicochemical properties in detail). As shown in Figure 5A and Supplementary Figures 3A–5A, analogues with a wide range of physicochemical properties permeate Gram-positive bacteria whereas the cell permeability of Gram-negative bacteria is limited to a much narrower range of physicochemical properties (with an especially higher threshhold for lipophilicity). This is clearly indicated by the limited activity against efflux-positive H. influenzae H3 shown in Figure 5B and the inactivity against P. aeruginosa and Escherichia coli. Since analogues with a wider range of physicochemical properties exhibit better antibacterial activity against efflux-negative H. influenzae H3 than the efflux-positive strain (Figure 5B,C), and the fact that tetramic acids exhibited antibacterial activity against TolC negative E. coli and Klebsiella pneumonia in our previous study [13], the main obstacle to Gram-negative bacteria cell permeability appears to transportation by efflux-pumps.
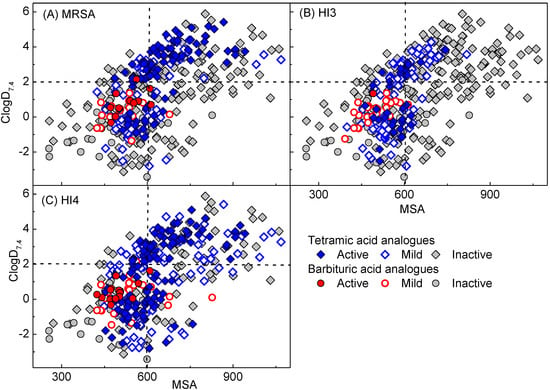
Figure 5.
Plot of ClogD7.4 against MSA of barbituric acids 2–37 along with tetramic acids in our previous reports [13,14,15,19] against (A) MRSA; (B) H. influenzae 3 and (C) efflux-negative H. influenzae 4. Active, mild and inactive mean that the values are MIC ≤ 4 µg/mL, 4 µg/mL < MIC ≤ 32 µg/mL and MIC > 32 µg/mL, respectively.
In this analysis, barbiturates active against MRSA (red-filled circles) and efflux-negative H4 are positioned in the same range of ClogD7.4, ClogP, PSA and MSA for active tetramates (blue-filled circles) and tend to be less lipophilic, have higher PSA and smaller MSA character than active 3-acyltetramic acids [14,19] (Figure 5A,C and Supplementary Figures 3A,C and 4A,C); however, active barbiturates possess slightly higher rel-PSA than active tetramates (Supplementary Figure 5A,C). Of particular interest is that the active barbiturates possess similar physicochemical properties to clinical antibiotics for oral or injectable use (−3.0 < ClogD7.4 < 2.0; 0 < ClogP < 3.0; 60 < PSA < 120 Å2; 10 < rel-PSA < 30% and 270 < MSA < 650 Å3, see below) as well as acceptable molecular weight (<400 Da), rotatable bonds (usually less than 6) and the number of proton-donor (1–2) and -acceptor (4–6) for oral availability in the rule of five (Supplementary Table 1) [36].
In our previous analysis with tetramates [13,14,19] presented as blue-filled circles in Figure 5B and C and Supplementary Figure 3B,C, tetramates possessing less lipophilic (ClogD7.4 < 3.0 and ClogP < 4.0) and smaller (MSA < 620 Å3) characteristics tended to be less easily transported by efflux pumps in H. influenzae. Although the active barbiturates are in this zone of lipophilicity and MSA, they were slightly more easily transported than tetramates. This may be due to the fact that barbiturates possess higher PSA (78 < PSA < 100 Å2) than tetramates (65< PSA < 90 Å2), are in the same range of MSA (420 < MSA < 650 Å3) and this results in higher rel-PSA (15 < rel-PSA < 20% versus 10 < rel-PSA < 15%). This phenomena agrees with the observation that compounds possessing higher topological PSA are more easily transported by multidrug resistance-associated protein 1 (MRP1/ABBC1) [37].
2.6. Physicochemical Property-Plasma Protein Binding Affinity Relationships
In order to investigate physicochemical property-PPB affinity relationships, plots of MIC difference against MSA, PSA, rel-PSA, ClogP and ClogD7.4 of barbiturates (32 analogues) used in this study along with tetramates (208 analogues) from our previous studies [13,14,15,17] were made (Figure 6). In this analysis, the MIC difference is defined as the value from MIC against S. pneumonia P9 in the presence of 2.5% blood divided by MIC without blood (inactive analogues against any one of these panels were not considered). Since PPB affinity is affected by multiple interactions in many proteins such as human serum albumin, lipoprotein, glycoprotein and globulins in blood, there is no linear correlation between the MIC difference and the physicochemical properties in Figure 6. However, this analysis, which uses a larger number of analogues than in our previous analysis [13,14], shows clearer trends (especially against MSA and PSA). As with the ClogP-PPB affinity relationship in the literature [38,39], PPB affinity is more closely related to lipophilicity as represented by rel-PSA, ClogP and ClogD7.4 (Figure 6C–E) than MSA and PSA (Figure 6A,B). It appears that less lipophilic analogues (rel-PSA > 16%, ClogP < 1.5 and ClogD7.4 < −0.69) exhibit higher probability of having lower PPB affinity (MIC difference ≤ 2) while those analogues with high PPB affinity (MIC difference > 30) are positioned in the area of highly lipophilic regions (rel-PSA < 13%, ClogP > 2.9 and ClogD7.4 > 2.9, Figure 6C–E). Although the trends of PPB affinity with steric effect (MSA) and PSA are weaker than lipophilicity, the analogues in the range of MSA between 600 and 900 Å3 and PSA being less than 100 Å2 exhibit a higher probability of possessing high PPB affinity (Figure 6A,B). However, the barbiturate library (red-filled circles) tended to exhibit lower PPB affinity compared to the tetramate library because of their less lipophilic (rel-PSA > 14%, ClogP < 2.0 and ClogD7.4 < 1.0) and smaller MSA (MSA < 650 Å3) character on average.
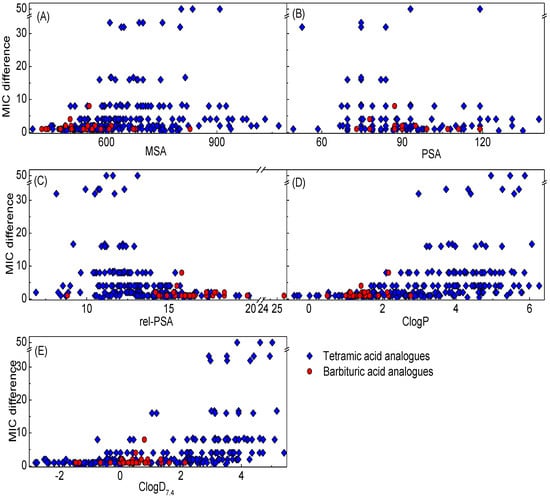
Figure 6.
Plot of MIC difference against (A) MSA; (B) PSA; (C) rel-PSA; (D) ClogP (E) ClogD7.4 of barbituric acids along with tetramic acids in our previous reports [13,14,15,17]. The MIC difference is defined as MIC with 2.5% blood/ MIC without blood against S. pneumonia 9.
In fact, potency enhancements of 3-acyltetramic acids [13,14] arising from improvement of lipophilicity and molecular size (ClogD7.4 >2.0 and MSA >600 Å3) are unlikely to provide both lower PPB affinity as well as efflux pump transport. In contrast, we believe that active barbiturates and 3-carboxamide tetramic acids [13,15] exhibit amenable PPB affinity for in vivo activity because of their similar physicochemical properties with clinical antibiotics used for oral or injectable administration as well as being an anionic microspecies under weakly basic conditions (see below). Indeed, the ability to control PPB affinity by adjustment of physicochemical properties proved to be limited; therefore, although appropriate physicochemical properties might be necessary for overcoming PPB affinity, there appear to be other factors involved. One possible hypothesis is that the main core (tetramic and barbituric acids) with its inherent acidic character might be responsible for binding to serum albumin, the major protein in blood, at the sites for aromatic carboxylic acids such as salicylates and ibuprofen. In order to understand whether this is the case, computational and NMR study of serum albumin affinity with our analogues is under investigation.
2.7. Physicochemical Property-Activity Relationships of Small Molecule Antibacterial Agents
Although physicochemical properties of antibacterial agents have been discussed in detail in the previous literature [1,40], an examination of the desirable properties for small molecule antibacterial agents, especially to understand cell permeability and PPB affinity, was made using cheminformatic analysis. In order to achieve a more reliable comparison with our library (Mw < 650 Da), antibiotic families with a large molecular weight (Mw > 600 Da), such as glycopeptides which act at the peptidoglycan layer and do not therefore require the penetration of a lipid membrane, and macrolides, have been excluded. In this study, 8 bins for each antibiotic family along with a separate bin for topical antibiotic agents but without consideration of their family, were created. The plots of lipophilicity descriptors (ClogD7.4, and ClogP) and polar surface descriptors (PSA and rel-PSA) against steric effect descriptor (MSA) were made (Figure 7, see also Supplementary Table 2–10 in Supporting Information for the physicochemical properties in detail). In this analysis, most antibiotics, with the exception of topical agents, tended to have a higher limit for lipophilicity (ClogD7.4 < 2.0 and ClogP < 3.0) and a lower limit for polar surface area (PSA > 60 Å2 and rel-PSA > 13%). Furthermore, it is noteworthy that the bigger antibiotics (MSA > 650 Å3) tend to have a stricter limit for lipophilicity (ClogD7.4 < −4.0 and ClogP < 0) and polar surface area (PSA > 120 Å2 and rel-PSA > 20%). That these margins might result from PPB affinity is supported by the fact that topical agents, for which PPB affinity is less important, are free from these boundaries, although of course other factors such as intrinsic antibacterial activity and ADMET are clearly important (for example, antibiotics with higher antibacterial activity compensate for higher PPB affinity for oral or injectable use). However, the lipophilicity (ClogD7.4 and ClogP) and the PSA are in inverse proportion to the MSA while the rel-PSA is not affected by the MSA, generally displaying values between 12%–38%. The analogues as shown in Figure 5 and Supplementary Figures 3–5 are opposite to this tendency. Since bigger tetramic acids (MSA > 600 Å3) usually possess more lipophilic character, it would be worth investigating antibacterial activity and PPB affinity of libraries with bigger and less lipophilic character (MSA > 700 Å3, ClogD7.4 < −4.0) in the future.
From a consideration of their physicochemical properties, on the other hand, the 8 bins for oral or injectable antibiotics can be classified into 3 sub-types. The first one is aminoglycosides populating the most hydrophilic (lowest ClogP and ClogD7.4 and highest PSA and rel-PSA) and the biggest (MSA > 600 Å3) regions. Due to this hydrophilicity (ClogD7.4 < −12), their oral administration has been generally limited as a result of problems with absorption. The second class includes Gram-negative active β-lactams and tetracyclines, and this class of antibiotics possesses lipophilicity and MSA between aminoglycosides and the third class of antibiotics. The third class includes antibiotics of natural origin such as Gram-positive only active penicillins and amphenicols as well as synthetic origin antibiotics such as fluoroquinolones, sulfa drugs and oxazolidinones, possessing higher lipophilic character and smaller MSA than both of the other classes. Of particular interest is that the third class displays a narrow zone of physicochemical properties (−3.0 < ClogD7.4 < 2.0; 0 < ClogP < 3.0; 60 < PSA < 120 Å2, 270 < MSA < 650 Å3) with the exception of rel-PSA, while the two other classes populate a wider range of the physicochemical space, with the preference for lower lipophilicity and bigger MSA than the third class. The physicochemical space populated by the third class is amenable to general drug design, and appears to be the most suitable for antibiotic development. As shown in Figure 5 and Supplementary Figures 3–5, our active barbiturates in this study and 3-carboxamide tetramic acids from the previous study [13,15] also satisfy these properties, in contrast to active 3-acyltetramic acids which generally possess higher ClogP and ClogD7.4 values [13,14]. From a consideration of MSA of the third class of antibacterial agents (MSA < 650) as well as from the fact that smaller molecules (MSA < 620 Å3) tend to be less easily transported by the efflux pump in H. influenzae as described above (Figure 5B,C), and that analogues with 600 < MSA < 900 Å3 exhibit a higher probability of higher PPB affinity (Figure 6A), both lipophilicity and molecular size might be crucial factors for antibiotic discovery in which smaller analogues (MSA < 600 Å3) are likely to have a benefit for both PPB affinity and cell permeability.
Figure 7.
Plot of (A) ClogD7.4, (B) ClogP, (C) PSA and (D) rel-PSA against MSA of small molecule antibacterial agents.
2.8. Ionic State of Small Molecule Antibacterial Agents
Since for bacterial cell permeability (including the transportation by efflux pump), ionic state (related to pKa) might be expected to be a major factor, the major microspecies at pH 7.4 were calculated (data not shown, see Supplementary Figures 6–14 for the structures of antibacterial agents). Of particular interest is that Gram-negative active agents favoured anionic(s) and zwitterionic(s) microspecies, while aminoglycosides exist as cationic forms, generally as a result of the presence of the amine functionality. Fluoroquinolones, tetracyclines and Gram-negative active β-lactams exist as various forms of zwitterionic and anionic microspecies. This might result from the fact that their key skeletons include an acidic carboxylic acid (fluoroquinolones and β-lactams) or two acidic enols and a basic amine group (tetracyclines), which make them easily able to form anionic or zwitterionic species under weakly basic conditions (pH = 7.4). In addition, sulfa drugs mainly exist as an anionic form on the nitrogen in the sulfone amide group, in some cases with a minor amount of the uncharged form. In this family, sulfaguanidine (used in the treatment of gastrointestinal infections) exceptionally exists as a neutral microspecies under weakly basic conditions but is cationic in acidic conditions (pH = 1.0). In addition, amphenicols also exist as a mixture of a neutral and an anionic (on the nitrogen of amide functionality) microspecies. On the other hand, Gram-positive only active antibiotics generally exist as an anionic (rather than a zwitterionic, Gram-positive only β-lactams), neutral (oxazolidinones) or a mixture of a neutral and cationic (lincosamides and 2,4-diaminopyrimidines) microspecies, and tend to be less charged state than Gram-negative active agents. In general, Gram-negative antibacterial agents tend to be more charged than the Gram-positive only agents to penetrate outer membrane via porins as well as prevent efflux.
3. Experimental Section
3.1. General
All starting materials and 3-acyl 2a were commercially available. Melting points were checked by STUART SCIENTIFIC SMP1. The 1H-, 13C- and HMBC-NMR spectra were obtained using a Bruker AVB500 (500 MHz for 1H-NMR and 126 MHz for 13C-NMR) or DPX400 (400 MHz 1H-NMR and 101 MHz for 13C-NMR). Mass spectra (MS) and high resolution MS were obtained with Micro Mass LCT and GCT spectrometers under the conditions of electrospray ionization (ESI) and chemical ionization (CI) respectively, and values were reported as a ratio of mass to charge in Daltons. The energies at ground state were computed with Density Functional B3LYP (6-31G*) in Spartan 02 in each enol form and the physicochemical properties were calculated with MarvinSketch version 5.3.8. in which VG method for ClogP and ClogD7.4, van der Waals method for MSA were selected and sulfur atoms were excluded in the calculation of PSA. In vitro antibacterial activity was performed using standard methodology as described in our previous paper [13].
3.2. Synthesis of Barbituric Acid Templates 40a–c
3.2.1. Synthesis of Compounds 40c and 2c
Synthesis of Urea 39c
To the solution of 2-methoxy ethylamine 38 (3.2 g, 42.2 mmol) in dichloromethane (50 mL) was slowly added ethyl isocyanate (3.0 g, 42.2 mmol) at 0 °C under nitrogen atmosphere and the mixture was stirred for 30 min. Concentration in vacuo followed by precipitation in ethyl acetate and petrol solution gave urea 30c (5.9 g, 40.2 mmol, 95% yield) as a solid (M.P.; 40.0 °C).
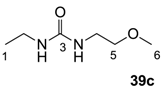
1H-NMR (400 MHz, CDCl3); 5.56 (brs, 1H, NH), 5.46 (brs, 1H, NH), 3.40 (t, 2H, J = 5.2 Hz, C5), 3.32–3.28 (m, 5H, C4 and C6), 3.17–1.10 (m, 2H, C2), 1.06 (t, 3H, J = 7.2 Hz, C1). 13C-NMR (100 MHz, CDCl3); 159.0 (C3), 72.2 (C5), 58.5 (C6), 39.9 (C4), 34.8 (C2), 15.3 (C1). MS (ES+); 169.08 (M+Na); HRMS (M+Na); calcd for C6H14N2Na1O2; 169.0947; found; 169.0950.
Synthesis of Barbituric Acid 40c and 3-acetylbarbituric Acid 2c
To the solution of urea 39c (5.9 g, 40.15 mmol) in acetic acid (80 mL) was added malonic acid (3.9 g, 40.15 mmol) at room temperature. The mixture was slowly added acetic anhydride (60 mL) at 60 °C for 30 min and the mixture was stirred at 90 °C for 5 h. Concentration in vacuo followed by flash column chromatography gave barbituric acid 40c (6.1 g, 28.5 mmol, 71%) as oil and 3-acetylbarbituric acid 2c (1.0 g, 3.90 mmol, 10%) as oil.
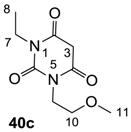 Compound 40c; 1H-NMR (400 MHz, CDCl3); 4.01 (t, 2H, J = 5.6 Hz, C9), 3.84 (q, 2H, J = 6.8 Hz, C7), 3.59 (s, 2H, C3), 4.01 (t, 2H, J = 5.6 Hz, C10), 3.24 (s, 3H, C11), 1.11 (t, 3H, J = 6.8 Hz, C8). 13C NMR (100 MHz, CDCl3); 164.6 (C=O), 164.3 (C=O), 151.1 (C6), 68.9 (C10), 58.4 (C11), 40.3 (C9), 39.4 (C3), 37.0 (C7), 12.9 (C8). MS (ES−); 213.08 (M−H); MS (ES+); 237.09 (M+Na); HRMS (M+Na); calcd for C9H14N2Na1O4; 237.0846; found; 237.0848.
Compound 40c; 1H-NMR (400 MHz, CDCl3); 4.01 (t, 2H, J = 5.6 Hz, C9), 3.84 (q, 2H, J = 6.8 Hz, C7), 3.59 (s, 2H, C3), 4.01 (t, 2H, J = 5.6 Hz, C10), 3.24 (s, 3H, C11), 1.11 (t, 3H, J = 6.8 Hz, C8). 13C NMR (100 MHz, CDCl3); 164.6 (C=O), 164.3 (C=O), 151.1 (C6), 68.9 (C10), 58.4 (C11), 40.3 (C9), 39.4 (C3), 37.0 (C7), 12.9 (C8). MS (ES−); 213.08 (M−H); MS (ES+); 237.09 (M+Na); HRMS (M+Na); calcd for C9H14N2Na1O4; 237.0846; found; 237.0848.
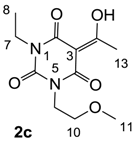 Compound 2c; 1H-NMR (400 MHz, CDCl3); 4.17–4.12 (m, 2H, C9), 4.01–3.93 (m, 2H, C7), 3.62–3.56 (m, 2H, C10), 3.33 and 3.32 (2 of s, 3H, C11), 2.69 and 2.68 (2 of s, 3H, C13), 1.24–1.17 (m, 3H, C8). 13C-NMR (100 MHz, CDCl3); 196.3 and 196.1 (C12), 169.4 and 169.2 (C2), 160.9 and 160.7 (C4), 149.8 and 149.7 (C6), 96.0 and 95.9 (C3), 69.3 and 69.2 (C10), 58.7 and 58.6 (C11), 40.1 and 40.0 (C9), 36.7 and 36.6 (C7), 24.7 and 24.6 (C13), 13.1 (C8). MS (ES−); 255.11 (M−H); MS (ES+); 279.10 (M+Na); HRMS (M+Na); calcd for C11H16N2Na1O5; 279.0951; found; 279.0946.
Compound 2c; 1H-NMR (400 MHz, CDCl3); 4.17–4.12 (m, 2H, C9), 4.01–3.93 (m, 2H, C7), 3.62–3.56 (m, 2H, C10), 3.33 and 3.32 (2 of s, 3H, C11), 2.69 and 2.68 (2 of s, 3H, C13), 1.24–1.17 (m, 3H, C8). 13C-NMR (100 MHz, CDCl3); 196.3 and 196.1 (C12), 169.4 and 169.2 (C2), 160.9 and 160.7 (C4), 149.8 and 149.7 (C6), 96.0 and 95.9 (C3), 69.3 and 69.2 (C10), 58.7 and 58.6 (C11), 40.1 and 40.0 (C9), 36.7 and 36.6 (C7), 24.7 and 24.6 (C13), 13.1 (C8). MS (ES−); 255.11 (M−H); MS (ES+); 279.10 (M+Na); HRMS (M+Na); calcd for C11H16N2Na1O5; 279.0951; found; 279.0946.


3.2.2. Synthesis of Barbituric Acid 40b and 3-acetylbarbituric Acid 2b
Barbituric acid 40b (3.9 g, 21.2 mmol, 82%) as oil and 3-acetylbarbituric acid 2b (660 mg, 2.93 mmol, 11%) as a solid (M.P.; 68 °C) were obtained from 1,3-diethyl urea 39b (3.0 g, 25.8 mmol) and malonic acid (2.7 g, 25.8 mmol) as the same method with synthesis of compounds 40c and 2c.
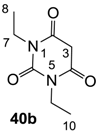 Compound 40b; 1H-NMR (400 MHz, CDCl3); 3.87 (q, 4H, J = 7.2 Hz, C7 and C9), 3.60 (s, 2H, C3), 1.15 (t, 6H, J = 7.2 Hz, C8 and C10). 13C-NMR (100 MHz, CDCl3); 164.4 (C2 and C4), 150.9 (C6), 39.5 (C3), 37.0 (C7 and C9), 13.0 (C8 and C10). MS (ES−); 183.06 (M−H); HRMS (M−H); calcd for C8H11N2O3; 183.0775; found; 183.0771.
Compound 40b; 1H-NMR (400 MHz, CDCl3); 3.87 (q, 4H, J = 7.2 Hz, C7 and C9), 3.60 (s, 2H, C3), 1.15 (t, 6H, J = 7.2 Hz, C8 and C10). 13C-NMR (100 MHz, CDCl3); 164.4 (C2 and C4), 150.9 (C6), 39.5 (C3), 37.0 (C7 and C9), 13.0 (C8 and C10). MS (ES−); 183.06 (M−H); HRMS (M−H); calcd for C8H11N2O3; 183.0775; found; 183.0771.
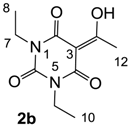 Compound 2b; 1H-NMR (400 MHz, CDCl3); 4.00–3.92 (m, 4H, C7 and C9), 2.67 (s, 3H, C12), 1.23–1.16 (m, 6H, C8 and C10). 13C-NMR (100 MHz, CDCl3); 196.1 (C11), 169.2 (C2), 160.7 (C4), 149.5 (C6), 95.9 (C3), 36.5 (N-CH2), 36.4 (N-CH2), 24.7 (C12), 13.1 (C8 and C10). MS (ES−); 225.08 (M−H); HRMS (M−H); calcd for C10H13N2O4; 225.0881; found; 225.0882.
Compound 2b; 1H-NMR (400 MHz, CDCl3); 4.00–3.92 (m, 4H, C7 and C9), 2.67 (s, 3H, C12), 1.23–1.16 (m, 6H, C8 and C10). 13C-NMR (100 MHz, CDCl3); 196.1 (C11), 169.2 (C2), 160.7 (C4), 149.5 (C6), 95.9 (C3), 36.5 (N-CH2), 36.4 (N-CH2), 24.7 (C12), 13.1 (C8 and C10). MS (ES−); 225.08 (M−H); HRMS (M−H); calcd for C10H13N2O4; 225.0881; found; 225.0882.


3.2.3. Synthesis of Barbituric Acid 40a
To the solution of N-allylurea 39a (2.0 g, 20.0 mmol) in acetic acid (60 mL) was added malonic acid (2.1 g, 20.0 mmol) at room temperature. The mixture was slowly added acetic anhydride (40 mL) at 60 °C for 30 min and the mixture was stirred at 90 °C for 5 h. Concentration in vacuo followed by precipitation in ethyl acetate gave barbituric acid 40a (960 mg, 5.71 mmol, 30%) as a solid (M.P.; 158 °C).
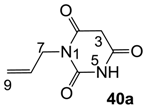
1H-NMR (400 MHz, DMSO, keto form); 11.35 (brs, 1H, NH), 5.82–5.72 (m, 1H, C8), 5.17 (dd, 1H, J1 = 17.6 Hz, J2 = 1.6 Hz, C9), 5.08 (dd, 1H, J1 = 10.4 Hz, J2 = 1.6 Hz, C9), 4.27 (d, 2H, J = 4.8 Hz, C7), 3.64 (s, 2H, C3). 13C-NMR (100 MHz, DMSO, keto form); 166.5 (C2 and C4), 151.4 (C6), 132.5 (C8), 116.2 (C9), 41.9 (C7), 39.9 (C3). 1H-NMR (400 MHz, CD3OD, enol form); 5.89–5.79 (m, 1H, C8), 5.23 (dd, 1H, J1 = 17.2 Hz, J2 = 1.2 Hz, C9), 5.14 (dd, 1H, J1 = 10.0 Hz, J2 = 1.2 Hz, C9), 4.84 (brs, 2H, NH, OH and C3), 4.40 (d, 2H, J = 5.6 Hz, C7). 13C-NMR (100 MHz, CD3OD, enol form, due to deuterium exchange, C3 signal is not appeared); 168.2 (C2 and C4), 152.9 (C6), 133.4 (C8), 118.0 (C9), 43.9 (C7). MS (ES−); 167.04 (M−H), HRMS (M−H); calcd for C7H7N2O3; 167.0462; found; 167.0454.
3.3. Synthesis of 3-alkoxycarbonyl Barbituric Acid Templates 41a–d
General procedure; To the solution of barbituric acid (1 eq) and DMAP (2.2 eq) in dichloromethane was slowly added butyl chloroformate (1.2 eq) at 0 °C under nitrogen atmosphere, and the mixture was stirred overnight at room temperature under nitrogen atmosphere. Then the mixture was washed with 2 M HCl. The organic layer was dried over MgSO4 and evaporated in vacuo to give 3-alkoxycarbonyl barbituric acid templates 41a–d contained about 10%–20% impurity. The impure 3-alkoxycarbonyl barbituric acid template was used for next step without further purification.
3.3.1. Synthesis of Compound 41a
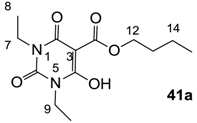
Yield; 85%; M.P. 33 °C; 1H-NMR (400 MHz, CDCl3); 4.33 (t, 2H, J = 6.8 Hz, C12), 4.03–3.92 (m, 4H, C7 and C9), 1.78–1.71 (m, 2H, C13), 1.48–1.39 (m, 2H, C14), 1.25 (t, 3H, J = 6.8 Hz, CH3), 1.17 (t, 3H, J = 6.8 Hz, CH3), 0.92 (t, 3H, J = 7.2 Hz, C15). 13C-NMR (100 MHz, CDCl3); 173.5 (C11), 168.7 (C2), 158.2 (C4), 148.9 (C6), 82.9 (C3), 66.5 (C12), 37.7 (N-CH2), 36.4 (N-CH2), 30.4 (C13), 18.9 (C14), 13.6 (C15), 13.3 (CH3), 12.9 (CH3). MS (ES−); 283.14 (M−H); MS (ES+); 307.13 (M+Na); HRMS (M+Na); calcd for C13H20N2Na1O5; 307.1264; found; 307.1256.
3.3.2. Synthesis of Compound 41b
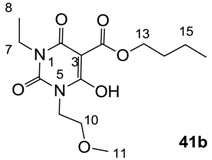
Yield; 65% (oil); 1H-NMR (400 MHz, CDCl3); 4.34 (t, 2H, J = 6.8 Hz, C13), 4.17–3.90 (m, 4H, C7 and C9), 3.61–3.54 (m, 2H, C10), 3.30 (s, 3H, C11), 1.78–1.70 (m, 2H, C14), 1.45–1.40 (m, 2H, C15), 1.25–1.14 (m, 3H, C8), 0.92 (t, 3H, J = 7.2 Hz, C16). 13C-NMR (100 MHz, CDCl3); 173.5 and 173.4 (C12), 169.0 and 168.8 (C2), 158.3 and 158.0 (C4), 149.3 and 149.1 (C6), 82.8 (C3), 69.1 (C10), 66.5 (C13), 58.5 (C11), 41.2 and 40.0 (C9), 37.8 and 36.5 (C7), 30.4 (C14), 18.9 (C15), 13.5 (C16), 13.2 (C8). MS (ES−); 313.15 (M−H); MS (ES+); 315.16 (M+H), 337.14 (M+Na); HRMS (M+Na); calcd for C14H22N2Na1O6; 337.1370; found; 337.1369.
3.3.3. Synthesis of Compound 41c
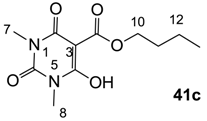
Yield; 90%; M.P.; 59 °C; 1H-NMR (500 MHz, CDCl3); 4.29 (t, 2H, J = 5.5 Hz, C10), 3.28 (brs, 6H, C7 and C8), 1.73–1.67 (m, 2H, C11), 1.44–1.37 (m, 2H, C12), 0.88 (t, 3H, J = 7.5 Hz, C13). 13C-NMR (125 MHz, CDCl3); 173.2 (C9), 168.7 (C2), 158.3 (C4), 149.6 (C6), 82.6 (C3), 66.4 (C10), 30.2 (C11), 28.4 (N-CH3), 27.8 (N-CH3), 18.8 (C12), 13.4 (C13). MS (ES−); 255.09 (M−H); HRMS (M−H); calcd for C11H15N2O5; 255.0986; found; 255.0987.
3.3.4. Synthesis of Compound 41d
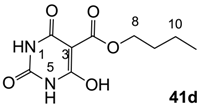
Yield; 56 %; M.P.; 259 °C; 1H-NMR (400 MHz, DMSO); 11.58 (brs, 2H, NH), 4.23 (t, 2H, J = 6.4 Hz, C8), 1.65–1.58 (m, 2H, C9), 1.40–1.35 (m, 2H, C10), 0.89 (t, 3H, J = 7.2 Hz, C11). 13C-NMR (125 MHz, DMSO); 171.9 (C7), 167.9 (C2), 165.0 (C4), 148.8 (C6), 82.2 (C3), 65.2 (C8), 30.2 (C9), 18.6 (C10), 13.6 (C11). MS (ES−); 227.07 (M−H); MS (ES+); 251.07 (M+Na); HRMS (M+Na); calcd for C9H12N2Na1O5; 251.0638; found; 251.0640.
3.4. Direct acylation of Barbituric Acid Templates
General procedure: To a solution of carboxilic acid (1.0 eq) in dichloromethane were added DCC (1.1 eq), barbituric acid (1.0 eq) and DMAP (1.2 eq) at room temperature. The mixture was stirred overnight at room temperature. The crude reaction mixture was filtered with dichloromethane. Concentration in vacuo followed by flash column chromatography gave metal-chelated barbituric acid. The crude product was dissolved in dichloromethane and washed with 1M HCl. The organic layer was dried with MgSO4 and concentrated in vacuo to give 3-acylbarbituric acid.
3.4.1. Synthesis of Compound 3
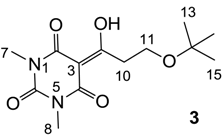
Yield; 51%; M.P.; 92 °C; 1H-NMR (400 MHz, CDCl3); 3.73 (t, 2H, J = 6.4 Hz, C11), 3.36 (t, 2H, J = 6.4 Hz, C10), 3.35 (s, 3H, C7), 3.31 (s, 3H, C8), 1.16 (s, 9H, C13–C15). 13C-NMR (100 MHz, CDCl3); 197.5 (C9), 169.7 (C2), 160.8 (C4), 150.3 (C6), 95.7 (C3), 73.2 (C12), 57.5 (C11), 38.3 (C10), 28.0 (N-CH3), 27.8 (N-CH3), 27.4 (C13–C15). MS (ES−); 283.14 (M−H), MS (ES+); 307.14 (M+Na), HRMS (M+Na); calcd for C13H20N2Na1O5; 307.1264; found; 307.1269.
3.4.2. Synthesis of Compound 4
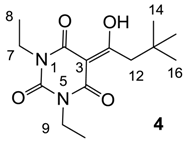
Yield; 57% (oil); 1H-NMR (400 MHz, CDCl3); 3.98–3.89 (m, 4H, C7 and C9), 3.14 (s, 2H, C12), 1.22–1.13 (m, 6H, C8 and C10), 1.02 (s, 9H, C14–C16). 13C-NMR (100 MHz, CDCl3); 198.5 (C11), 169.5 (C2), 160.6 (C4), 149.3 (C6), 96.8 (C3), 46.8 (C12), 36.5 (C7 and C9), 33.4 (C13), 29.9 (C14–C16), 13.1 (C8 and C10). MS (ES−); 281.15 (M−H); HRMS (M−H); calcd for C14H21N2O4; 281.1507; found; 281.1505.
3.4.3. Synthesis of Compound (±)-5b
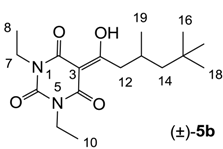
Yield; 71% (oil); 1H-NMR (400 MHz, CDCl3); 4.03–3.94 (m, 4H, C7 and C9), 3.08 (d, 2H, J = 7.2 Hz, C12), 2.22–2.12 (m, 1H, C13), 1.35 (dd, 1H, J1 = 14.0 Hz, J2 = 3.2 Hz, C14), 1.26–1.15 (m, 7H, C8, C10 and C14), 1.01 (d, 3H, J = 6.8 Hz, C19), 0.90 (s, 9H, C16-C18). 13C-NMR (100 MHz, CDCl3); 199.3 (C11), 169.6 (C2), 160.5 (C4), 149.7 (C6), 96.0 (C3), 50.7 (C14), 45.5 (C12), 36.6 (NCH2), 36.5 (NCH2), 31.1 (C15), 29.9 (C16–C18), 27.7 (C13), 22.7 (C19), 13.2 (C8 and C10). MS (ES−); 323.20 (M−H); HRMS (M−H); calcd for C17H27N2O4; 323.1976; found; 323.1980.
3.4.4. Synthesis of Compound (±)-5c
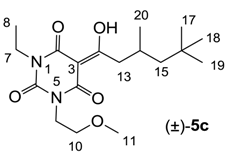
Yield; 42% (oil); 1H-NMR (400 MHz, CDCl3); 4.17–4.12 (m, 2H, C9), 4.00–3.92 (m, 2H, C7), 3.62–3.55 (m, 2H, C10), 3.33 and 3.32 (2 of s, 3H, C11), 3.06 (d, 2H, J = 7.2 Hz, C13), 2.19–2.12 (m, 1H, C14), 1.35–1.30 (m, 1H, C15), 1.24–1.12 (m, 4H, C8 and C15), 0.99 and 0.98 (2 of d, 3H, J = 6.8 Hz, C20), 0.87 (s, 9H, C17–C19). 13C-NMR (100 MHz, CDCl3); 199.3 and 199.2 (C12), 169.7 and 169.5 (C2), 160.6 and 160.3 (C4), 149.7 and 149.6 (C6), 95.9 and 95.8 (C3), 69.3 and 69.2 (C10), 58.7 and 58.6 (C11), 50.6 (C15), 45.4 and 45.3 (C13), 40.1 and 40.0 (C9), 36.7 and 36.6 (C7), 31.0 (C16), 29.8 (C17–C19), 27.7 (C14), 22.7 (C20), 13.1 (C8). MS (ES−); 353.22 (M−H); MS (ES+); 355.23 (M+H), 377.20 (M+Na); HRMS (M+Na); calcd for C18H30N2Na1O5; 377.2047; found; 377.2040.
3.4.5. Synthesis of Compound 6a
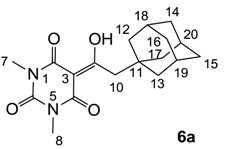
Yield; 90%; M.P.; 133 °C; 1H-NMR (500 MHz, CDCl3); 3.35 (s, 3H, C7), 3.31 (s, 3H, C8), 3.06 (s, 2H, C10), 1.99 (brs, 3H, C18–C20), 1.65–1.34 (m, 12H, C12–C17). 13C-NMR (125 MHz, CDCl3); 197.8 (C9), 169.7 (C2), 161.1 (C4), 150.2 (C6), 97.1 (C3), 47.9 (C10), 42.6 (CH2), 36.6 (CH2), 36.3 (C11), 28.8 (N-CH3), 28.1 (CH), 27.8 (CH). MS (ES−); 331.18 (M−H), HRMS (M−H); calcd for C18H23N2O4; 331.1663; found; 331.1657.
3.4.6. Synthesis of Compound 6b
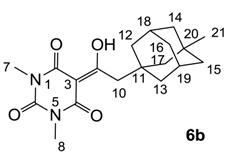
Yield; 54% (oil); 1H-NMR (400 MHz, CDCl3); 3.36 (s, 3H, C7), 3.31 (s, 3H, C8), 3.08 (s, 2H, C10), 1.99 (brs, 2H, C18 and C19), 1.65–1.34 (m, 12H, C12-C17), 0.78 (s, 3H, C21). 13C-NMR (100 MHz, CDCl3); 197.8 (C9), 169.7 (C2), 161.1 (C4), 150.2 (C6), 97.1 (C3), 49.6 (CH2), 47.6 (C10), 43.6 (CH2), 41.8 (CH2), 37.0 (C11), 35.8 (CH2), 30.9 (C21), 30.8 (C20), 29.3 (C18 and C19), 28.1 (N-CH3), 27.8 (N-CH3). MS (ES−); 345.20 (M−H), HRMS (M−H); calcd for C19H25N2O4; 345.1820; found; 345.1811.
3.4.7. Synthesis of Compound 6c
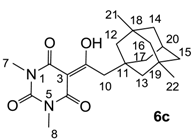
Yield; 39%; M.P.; 77 °C; 1H-NMR (400 MHz, CDCl3); 3.37 (s, 3H, C7), 3.32 (s, 3H, C8), 3.10 (s, 2H, C10), 2.05–2.02 (m, 1H, C20), 1.52–1.07 (m, 12H, C12–C17), 0.79 (s, 6H, C21 and C22). 13C-NMR (100 MHz, CDCl3); 197.8 (C9), 169.7 (C2), 161.1 (C4), 150.2 (C6), 97.1 (C3), 50.8 (CH2), 48.9 (CH2), 47.3 (C10), 42.9 (CH2), 41.0 (CH2), 37.7 (C18 and C19), 31.5 (C11), 30.5 (C21 and C22), 29.8 (C20), 28.2 (N-CH3), 27.8 (N-CH3). MS (ES−); 359.18 (M−H), HRMS (M-H); calcd for C20H27N2O4; 359.1976; found; 359.1965.
3.4.8. Synthesis of Compound 7a
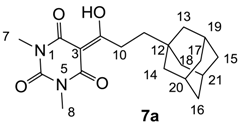
Yield; 50%; M.P.; 130 °C; 1H-NMR (400 MHz, CDCl3); 3.36 (s, 3H, C7), 3.33 (s, 3H, C8), 3.11–3.07 (m, 2H, C10), 1.98 (brs, 3H, C19–C21), 1.73–1.54 (m, 12H, C13–C18), 1.45–1.41 (m, 2H, C11). 13C-NMR (100 MHz, CDCl3); 201.2 (C9), 169.7 (C2), 160.8 (C4), 150.4 (C6), 95.2 (C3), 42.0 (C10), 40.0 (CH2), 37.0 (CH2), 32.4 (C12), 30.8 (CH2), 28.6 (N-CH3), 28.0 (CH), 27.8 (CH). MS (ES−); 345.19 (M−H), HRMS (M−H); calcd for C19H25N2O4; 345.1820; found; 345.1815.
3.4.9. Synthesis of Compound 7b
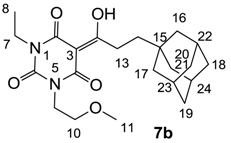
Yield; 44% (oil); 1H-NMR (400 MHz, CDCl3); 4.18–4.14 (m, 2H, C9), 4.02–3.95 (m, 2H, C7), 3.63–3.57 (m, 2H, C10), 3.34 and 3.33 (2 of s, 3H, C11), 3.11–3.07 (m, 2H, C13), 1.96 (brs, 3H, C22–C24), 1.71–1.61 (m, 6H, C16, C17 and C20), 1.52 (brs, 6H, C18, C19 and C21), 1.44–1.40 (m, 2H, C14), 1.26–1.18 (m, 3H, C8). 13C-NMR (100 MHz, CDCl3); 201.3 and 201.2 (C12), 169.7 and 169.5 (C2), 160.5 and 160.3 (C4), 149.8 and 149.7 (C6), 95.3 and 95.2 (C3), 69.4 and 69.2 (C10), 58.7 and 58.6 (C11), 42.0 (C16, C17 and C20), 40.1 and 40.0 (C9), 39.8 and 39.7 (C13), 37.0 (C18, C19 and C21), 36.7 and 36.6 (C7), 32.3 (C15), 30.8 and 30.7 (C14), 28.6 (C22–C24), 13.2 and 13.1 (C8). MS (ES−); 403.23 (M-H); MS (ES+); 427.21 (M+Na); HRMS (M+Na); calcd for C22H32N2Na1O5; 427.2203; found; 427.2191.
3.4.10. Synthesis of Compound 8a
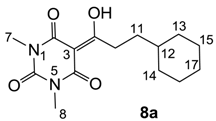
Yield; 25%; M.P.; 55 °C; 1H-NMR (400 MHz, CDCl3); 3.34 (s, 3H, C7), 3.31 (s, 3H, C8), 3.14–3.10 (m, 2H, C10), 1.76–1.62 (m, 5H, CH2), 1.58–1.52 (m, 2H, C11), 1.37–1.27 (m, 1H, C12), 1.23–1.11 (m, 3H, CH2), 0.98–0.89 (m, 2H, CH2). 13C-NMR (100 MHz, CDCl3); 200.3 (C9), 169.7 (C2), 160.7 (C4), 150.3 (C6), 95.1 (C3), 37.6 (C12), 34.4 (C10), 33.1 (C11), 32.9 (CH2), 28.0 (N-CH3), 27.7 (N-CH3), 26.5 (CH2), 26.2 (CH2). MS (ES−); 293.16 (M−H), HRMS (M−H); calcd for C15H21N2O4; 293.1507; found; 293.1510.
3.4.11. Synthesis of Compound 8b
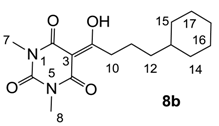
Yield; 53% (oil); 1H-NMR (400 MHz, CDCl3); 3.34 (s, 3H, C7), 3.30 (s, 3H, C8), 3.09 (t, 2H, J = 7.6 Hz, C10), 1.71–1.60 (m, 7H, C13 and CH2), 1.30–1.09 (m, 6H, CH2), 0.91–0.81 (m, 2H, CH2). 13C-NMR (100 MHz, CDCl3); 199.9 (C9), 169.7 (C2), 160.7 (C4), 150.3 (C6), 95.1 (C3), 37.3 (C13), 37.1 (CH2), 36.9 (CH2), 33.1 (CH2), 27.9 (N-CH3), 27.7 (N-CH3), 26.6 (CH2), 26.2 (CH2), 23.1 (CH2). MS (ES−); 307.16 (M−H); HRMS (M−H); calcd for C16H23N2O4; 307.1663; found; 307.1661.
3.4.12. Synthesis of Compound 9
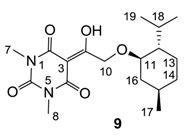
Yield; 84%; M.P.; 67 °C; 1H-NMR (400 MHz, CDCl3); 5.00 (d, 1H, J = 20.4 Hz, C10), 4.87 (d, 1H, J = 20.4 Hz, C10), 3.33 (s, 3H, C7), 3.27 (s, 3H, C8), 3.21–3.15 (m, 1H, C11), 2.29–2.21 (m, 1H, C15), 2.09–2.05 (m, 1H, C16), 1.62–1.57 (m, 2H, CH2), 1.35–1.27 (m, 2H, C12 and C18), 1.00–0.80 (m, 11H, C13, C14, C16, C19 and C20), 0.74 (d, 3H, J = 6.8 Hz, C17). 13C-NMR (100 MHz, CDCl3); 197.1 (C9), 169.4 (C2), 160.5 (C4), 150.1 (C6), 93.5 (C3), 80.5 (C11), 69.3 (C10), 47.9 (C12), 39.9 (C16), 34.3 (C14), 31.4 (N-CH3), 27.7 (N-CH3), 25.5 (C15), 23.2 (C13), 22.1 (C18), 20.8 (C19 and C20), 16.2 (C17). MS (ES−); 351.19 (M-H); MS (ES+); 375.22 (M+H); HRMS (M−H); calcd for C18H27N2O5; 351.1925; found; 351.1922.
3.4.13. Synthesis of Compound (±)-10
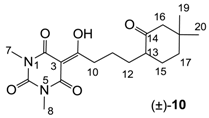
Yield; 51% (oil); 1H-NMR (500 MHz, CDCl3); 3.32 (s, 3H, C7), 3.28 (s, 3H, C8), 3.15–3.05 (m, 2H, C10), 2.25–2.21 (m, 1H, C13), 2.18 (d, 1H, J = 12.5 Hz, C16), 2.07 (d, 1H, J = 12.5 Hz, C16), 2.02–1.97 (m, 1H, C17), 1.88–1.81 (m, 1H, C12), 1.69–1.63 (m, 2H, C11), 1.62–1.43 (m, 3H, C15 and C17), 1.30–1.21 (m, 1H, C12), 1.01 (s, 3H, CH3), 0.83 (s, 3H, CH3). 13C-NMR (125 MHz, CDCl3); 212.4 (C14), 199.3 (C9), 169.6 (C2), 160.7 (C4), 150.3 (C6), 95.1 (C3), 54.8 (C16), 49.1 (C13), 37.9 (C10), 36.9 (C17), 36.6 (C18), 31.3 (CH3), 29.4 (C12 or C15), 28.8 (C12 or C15), 27.9 (N-CH3), 27.7 (N-CH3), 25.5 (CH3), 23.3 (C11). MS (ES−); 349.19 (M−H); MS (ES+); 351.21 (M+H), 373.19 (M+Na); HRMS (M+Na); calcd for C18H26N2Na1O5; 373.1734; found; 373.1723.
3.4.14. Synthesis of Compound cis-11
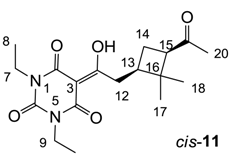
Yield; 46 % (oil); 1H-NMR (400 MHz, CDCl3); 3.99–3.91 (m, 4H, C7 and C9), 3.20 (dd, 1H, J1 = 15.2 Hz, J2 = 6.4 Hz, C12), 3.05 (dd, 1H, J1 = 15.2 Hz, J2 = 8.0 Hz, C12), 2.84 (dd, 1H, J1 = 10.0 Hz, J2 = 7.6 Hz, C15), 2.43–2.34 (m, 1H, C13), 2.11–2.03 (m, 1H, C14), 2.01 (s, 3H, C20), 1.92–1.85 (m, 1H, C14), 1.31 (s, 3H, C17), 1.23–1.16 (m, 6H, C8 and C10), 0.90 (s, 3H, C17). 13C NMR (100 MHz, CDCl3); 207.3 (C20), 198.8 (C11), 169.5 (C2), 160.4 (C4), 149.4 (C6), 95.2 (C3), 54.2 (C15), 43.7 (C16), 38.5 (C13), 37.3 (C12), 36.6 (NCH2), 36.5 (NCH2), 30.0 (C18 and C20), 23.2 (C14), 17.5 (C17), 13.1 (C8 and C10). MS (ES−); 349.18 (M−H); MS (ES+); 373.18 (M+Na); HRMS (M+Na); calcd for C18H26Na1N2O5; 373.1734; found; 373.1728.
3.4.15. Synthesis of Compound (±)-12
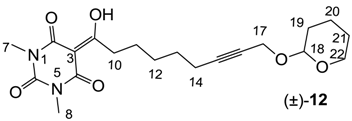
Yield; 35% (oil); 1H-NMR (400 MHz, CDCl3); 4.73 (brs, 1H, C18), 4.21 (d, 1H, J = 15.6 Hz, C17), 4.12 (d, 1H, J = 15.6 Hz, C17), 3.79–3.74 (m, 1H, C22), 3.47–3.43 (m, 1H, C22), 3.30 (s, 3H, C7), 3.25 (s, 3H, C8), 3.07 (t, 2H, J = 7.2 Hz, C10), 2.28–2.16 (m, 2H, C14), 1.78–1.45 (m, 10H, CH2). 13C-NMR (100 MHz, CDCl3); 199.3 (C9), 169.6 (C2), 160.6 (C4), 150.1 (C6), 96.3 (C18), 95.0 (C3), 86.0 (C15), 75.9 (C16), 61.7 (C22), 54.4 (C17), 36.4 (C10), 30.1 (CH2), 28.4 (CH2), 28.0 (CH2), 27.8 (N-CH3), 27.6 (N-CH3), 25.2 (CH2), 25.1 (CH2), 18.9 (CH2), 18.5 (CH2). MS (ES−); 391.19 (M−H); HRMS (M+Na); calcd for C20H28N2Na1O6; 415.1840; found; 415.1821.
3.4.16. Synthesis of Compound 13
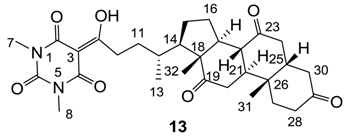
Yield; 87%; M.P.; 245 °C (decomposed); 1H-NMR (400 MHz, CDCl3); 3.35 (s, 3H, C7), 3.32 (s, 3H, C8), 3.20–3.05 (m, 2H), 2.94–2.81 (m, 3H), 2.37–1.81 (m, 15H), 1.70–1.57 (m, 2H), 1.39 (s, 3H, C32), 1.33–1.24 (m, 2H), 1.07 (s, 3H, C31), 0.92 (d, 3H, J = 6.4 Hz, C13). 13C-NMR (100 MHz, CDCl3); 211.8 (C=O), 209.0 (C=O), 208.7 (C=O), 200.1 (C=O), 169.7 (C2), 160.7 (C4), 150.3 (C6), 95.2 (C3), 56.9 (C18), 51.7 (CH), 48.9 (CH), 46.8 (CH), 45.7 (CH), 45.5 (CH), 44.9 (CH2), 42.7 (CH2), 38.6 (CH2), 36.4 (CH2), 36.3 (C12), 35.9 (C26), 35.2 (CH2), 34.3 (CH2), 33.9 (CH2), 31.5 (CH2), 28.0 (N-CH3), 27.8 (N-CH3), 27.6 (CH2), 25.6 (CH2), 25.1 (CH2), 24.9 (CH2), 21.8 (C13), 18.7 (C32), 11.8 (C31). MS (ES−); 539.30 (M−H), HRMS (M-H); calcd for C30H39N2O7; 539.2763; found; 539.2763.
3.4.17. Synthesis of Compound 14
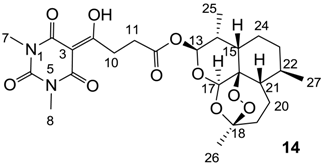
Yield; 66%; M.P. 96 °C; 1H-NMR (500 MHz, CDCl3); 5.79 (d, 1H, J = 10.0 Hz, C13), 5.42 (s, 1H, C17), 3.62–3.56 (m, 1H, C11), 3.50–3.44 (m, 1H, C11), 3.36 (s, 3H, C7), 3.32 (s, 3H, C8), 2.81 (t, 2H, J = 6.5 Hz, C10), 2.59–2.52 (m, 1H, C14), 2.36 (td, 1H, J1 = 13.5 Hz, J2 = 3.5 Hz, CH2), 2.05–2.00 (m, 1H, CH2), 1.91–1.85 (m, 1H, CH2), 1.79–1.69 (m, 2H, C24 and CH2), 1.63–1.59 (m, 1H, C15), 1.51–1.40 (m, 1H, CH2), 1.43 (s, 3H, C26), 1.38–1.24 (m, 3H, C21, C22 and C24), 1.04–0.99 (m, 1H, CH2), 0.95 (d, 3H, J = 6.0 Hz, C27), 0.85 (d, 3H, J = 7.0 Hz, C25). 13C-NMR (125 MHz, CDCl3); 197.4 (C9), 171.0 (C12), 169.7 (C2), 160.7 (C4), 150.2 (C6), 104.4 (C18), 95.2 (C3), 92.2 (C13), 91.4 (C17), 80.0 (C16), 51.5 (C21), 45.2 (C15), 37.2 (C22), 36.1 (CH2), 34.0 (CH2), 31.9 (C11), 31.8 (C14), 28.6 (C10), 27.9 (N-CH3), 27.8 (N-CH3), 25.9 (C26), 24.5 (CH2), 21.9 (C24), 20.2 (C27), 12.0 (C25). MS (ES−); 521.22 (M−H); HRMS (M−H); calcd for C25H33N2O10; 521.2141; found; 521.2148.
3.4.18. Synthesis of Compound 15a
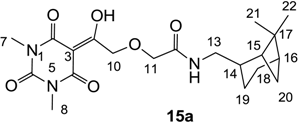
Yield; 27%; M.P.; 118 °C; 1H-NMR (400 MHz, CDCl3); 6.83 (brs, 1H, NH), 4.98 (s, 2H, CH2), 4.07 (s, 2H, CH2), 3.38 (s, 3H, C7), 3.34–3.28 (m, 5H, C8 and C13), 2.38–2.32 (m, 1H, C18), 2.16–2.18 (m, 1H, C14), 1.98–1.81 (m, 5H, C15, C16, C19 and C20), 1.54–1.44 (m, 1H, C19), 1.18 (s, 3H, C21), 1.03 (s, 3H, C22), 0.89 (d, 1H, J = 9.6 Hz, C18). 13C-NMR (100 MHz, CDCl3); 195.2 (C9), 169.5 (C12), 168.6 (C2), 160.4 (C4), 150.0 (C6), 93.9 (C3), 72.1 (CH2), 71.3 (CH2), 44.4 (C13), 43.7 (CH), 41.2 (CH), 41.2 (CH), 38.6 (C17), 33.1 (CH2), 28.0 (CH3), 27.9 (CH3), 27.8 (CH3), 25.9 (CH2), 23.1 (CH3), 19.7 (CH2). MS (ES−); 406.20 (M−H); HRMS (M−H); calcd for C20H28N3O6; 406.1984; found; 406.1986.
3.4.19. Synthesis of Compound 15b
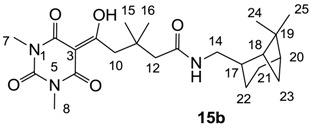
Yield; 43% (oil); 1H-NMR (500 MHz, CDCl3); 5.95 (brs, 1H, NH), 3.34–3.16 (m, 10H, C7, C8, C10 and C14), 2.30–2.27 (m, 1H, C21), 2.25 (s, 2H, C12), 2.17–2.10 (m, 1H, C17), 1.92–1.77 (m, 5H, C18, C20, C22 and C23), 1.47–1.41 (m, 1H, C22), 1.12 (s, 9H, CH3), 0.98 (s, 3H, CH3), 0.84 (d, 1H, J = 9.5 Hz, C21). 13C-NMR (125 MHz, CDCl3); 198.4 (C9), 170.7 (C13), 169.9 (C2), 160.8 (C4), 149.9 (C6), 96.8 (C3), 48.3 (CH2), 45.0 (CH2), 44.5 (CH2), 43.8 (CH), 41.3 (CH), 41.2 (CH), 38.5 (C19), 35.4 (C11), 33.0 (CH2), 28.1 (CH3), 28.1 (CH3), 27.8 (CH3), 25.8 (CH2), 23.1 (CH3), 19.8 (CH2). MS (ES−); 432.24 (M−H); MS (ES+); 434.29 (M+H), 456.27 (M+Na); HRMS (M+H); calcd for C23H36N3O5; 434.2649; found; 434.2634.
3.4.20. Synthesis of Compound 16
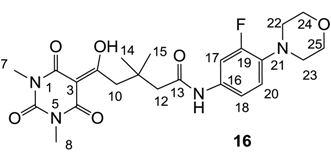
Yield; 49%; M.P.; 102 °C; 1H-NMR (500 MHz, CDCl3); 8.39 (brs, 1H, ArH), 7.39 (brs, 1H, ArH), 7.14 (d, 1H, J = 7.5 Hz, C20), 3.98 (brs, 4H, C24 and C25), 3.43 (s, 2H, C10), 3.37 (s, 3H, C7), 3.31 (s, 3H, C8), 3.21 (brs, 4H, C22 and C23), 2.43 (s, 2H, C12), 1.22 (s, 6H, C14 and C15). 13C-NMR (125 MHz, CDCl3); 198.2 (C9), 170.0 (C2), 162.7 (C13), 162.0 (C4), 155.5 (d, JC-F = 245 Hz, C19), 149.9 (C6), 135.6 (C21), 120.3 (C16), 115.5 (Ar-tert C), 115.5 (Ar-tert C), 109.0 (d, JC-F = 25.5 Hz, C17), 97.0 (C3), 66.0 (C24 and C25), 51.8 (C22 and C23), 48.2 (CH2), 44.4 (CH2), 36.0 (C11), 28.3 (C14 and C15), 28.2 (N-CH3), 28.0 (N-CH3). MS (ES−); 475.20 (M−H), MS (ES+); 499.23 (M+Na), HRMS (M+Na); calcd for C23H29F1N4Na1O6; 499.1963; found; 499.1964.
3.4.21. Synthesis of Compound 17a
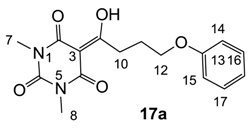
Yield; 84%; M.P.; 95 °C; 1H-NMR (500 MHz, CDCl3); 7.27–7.23 (m, 2H, C16 and C17), 6.91 (t, 1H, J = 7.0 Hz, C18), 6.85 (d, 2H, J = 8.0 Hz, C14 and C15), 4.05 (t, 2H, J = 6.0 Hz, C12), 3.37–3.34 (m, 5H, C10 and C7), 3.29 (s, 3H, C8), 2.23–2.17 (m, 2H, C11). 13C-NMR (125 MHz, CDCl3); 198.8 (C9), 169.6 (C2), 160.7 (C4), 158.6 (C13), 150.1 (C6), 129.2 (C16 and C17), 120.6 (C18), 114.3 (C14 and C15), 95.3 (C3), 66.7 (C12), 33.6 (C10), 27.8 (N-CH3), 27.7 (N-CH3), 25.1 (C11). MS (ES−); 317.13 (M−H), HRMS (M−H); calcd for C16H17N2O5; 317.1143; found; 317.1141.
3.4.22. Synthesis of Compound 17b
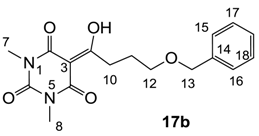
Yield; 63%; M.P.; 84 °C; 1H-NMR (400 MHz, CDCl3); 7.34–7.23 (m, 5H, C15-C19), 4.49 (s, 2H, C13), 3.58 (t, 2H, J = 6.0 Hz, C12), 3.33 (s, 3H, C7), 3.31 (s, 3H, C8), 3.27 (t, 2H, J = 6.0 Hz, C10), 2.07–2.00 (m, 2H, C11). 13C-NMR (100 MHz, CDCl3); 199.4 (C9), 169.6 (C2), 160.8 (C4), 150.2 (C6), 138.3 (C14), 128.2 (C17 and C18), 127.5 (C15 and C16), 127.4 (C19), 95.2 (C3), 72.8 (C13), 69.4 (C12), 33.8 (C10), 27.9 (N-CH3), 27.7 (N-CH3), 25.7 (C11). MS (ES−); 331.13 (M−H); MS (ES+); 355.14 (M+Na); HRMS (M+Na); calcd for C17H20N2Na1O5; 355.1264; found; 355.1262.
3.4.23. Synthesis of Compound 17c
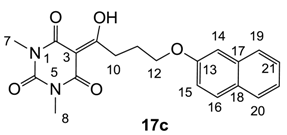
Yield; 70%; M.P.; 142 °C; 1H-NMR (400 MHz, CDCl3); 7.77–7.70 (m, 3H, C16, C19 and C20), 7.44 (dd, 1H, J1=J2 = 6.8 Hz, C21), 7.33 (dd, 1H, J1=J2 = 6.8 Hz, C22), 7.12–7.11 (m, 2H, C14 and C15), 4.20 (t, 2H, J = 5.6 Hz, C12), 3.41 (t, 2H, J = 5.6 Hz, C10), 3.36 (s, 3H, C7), 3.29 (s, 3H, C8), 2.31–2.25 (m, 2H, C11). 13C-NMR (100 MHz, CDCl3); 198.9 (C9), 169.7 (C2), 160.8 (C4), 156.6 (C13), 150.2 (C6), 134.5 (quart C), 129.3 (tert C), 128.9 (quart C), 127.6 (tert C), 126.6 (tert C), 126.3 (tert C), 123.6 (tert C), 118.8 (tert C), 95.4 (C3), 66.9 (C12), 33.7 (C10), 27.9 (N-CH3), 27.8 (N-CH3), 25.2 (C11). MS (ES−); 367.14 (M−H); MS (ES+); 369.19 (M+H), 391.14 (M+Na); HRMS (M+Na); calcd for C20H20N2Na1O5; 391.1264; found; 391.1261.
3.4.24. Synthesis of Compound 18a
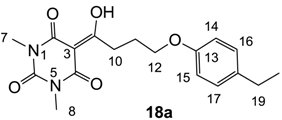
Yield; 75%; M.P.; 87 °C; 1H-NMR (400 MHz, CDCl3); 7.09 (d, 2H, J = 8.0 Hz, C16 and C17), 6.80 (d, 2H, J = 8.0 Hz, C14 and C15), 4.05 (t, 2H, J = 6.0 Hz, C12), 3.38–3.34 (m, 5H, C7 and C10), 3.31 (s, 3H, C8), 2.58 (q, 2H, J = 7.6 Hz, C19), 2.23–2.17 (m, 2H, C11), 1.21 (t, 3H, J = 7.6 Hz, C20). 13C-NMR (100 MHz, CDCl3); 199.0 (C9), 169.7 (C2), 160.8 (C4), 156.7 (C13), 150.3 (C6), 130.5 (C18), 128.6 (C16 and C17), 114.3 (C14 and C15), 95.4 (C3), 66.9 (C12), 33.7 (C10), 28.0 (N-CH3), 27.9 (C19), 27.8 (N-CH3), 25.3 (C11), 15.8 (C20). MS (ES−); 345.15 (M−H); MS (ES+); 369.16 (M+Na); HRMS (M+Na); calcd for C18H22N2Na1O5; 369.1421; found; 369.1412.
3.4.25. Synthesis of Compound 18b
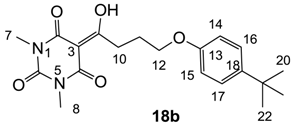
Yield; 58%; M.P.; 92 °C; 1H-NMR (400 MHz, CDCl3); 7.29 (d, 2H, J = 8.8 Hz, C16 and C17), 6.81 (d, 2H, J = 8.8 Hz, C14 and C15), 4.06 (t, 2H, J = 6.4 Hz, C12), 3.39–3.35 (m, 5H, C7 and C10), 3.31 (s, 3H, C8), 2.24–2.17 (m, 2H, C11), 1.30 (s, 9H, C20–C22). 13C-NMR (100 MHz, CDCl3); 199.0 (C9), 169.7 (C2), 160.8 (C4), 156.4 (C13), 150.2 (C6), 143.4 (C18), 126.1 (C16 and C17), 113.4 (C14 and C15), 95.4 (C3), 66.9 (C12), 34.0 (C19), 33.7 (C10), 31.5 (C20–C22), 27.9 (N-CH3), 27.8 (N-CH3), 25.3 (C11). MS (ES−); 373.19 (M−H); MS (ES+); 397.20 (M+Na); HRMS (M+Na); calcd for C20H26N2Na1O5; 397.1734; found; 397.1740.
3.4.26. Synthesis of Compound 18c
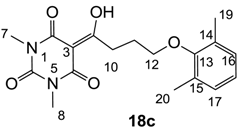
Yield; 67%; M.P.; 150 °C; 1H-NMR (400 MHz, CDCl3); 7.00 (d, 2H, J = 7.6 Hz, C16 and C17), 6.91 (dd, 1H, J1 = 7.6 Hz, J2= 7.6 Hz, C18), 3.87 (t, 2H, J = 6.4 Hz, C12), 3.44 (t, 2H, J = 6.4 Hz, C10), 3.39 (s, 3H, C7), 3.35 (s, 3H, C8), 2.29 (s, 6H, C19 and C20), 2.25–2.18 (m, 2H, C11). 13C-NMR (100 MHz, CDCl3); 199.1 (C9), 169.7 (C2), 160.8 (C4), 155.7 (C13), 150.3 (C6), 130.8 (C14 or C15), 128.8 (C16 and C17), 123.7 (C18), 95.3 (C3), 70.9 (C12), 33.7 (C10), 28.0 (N-CH3), 27.8 (N-CH3), 26.1 (C11), 16.3 (C19 and C20). MS (ES−); 345.16 (M−H); MS (ES+); 347.20 (M+H), 369.17 (M+Na); HRMS (M+Na); calcd for C18H22N2Na1O5; 369.1421; found; 369.1415.
3.4.27. Synthesis of Compound 18d
Yield; 72%; M.P.; 139 °C; 1H-NMR (400 MHz, CDCl3); 7.00 (d, 1H, J = 7.2 Hz, C16), 6.67 (d, 1H, J = 7.2 Hz, C18), 6.63 (s, 1H, C15), 4.07 (t, 2H, J = 6.0 Hz, C12), 3.42–3.38 (m, 5H, C7 and C10), 3.33 (s, 3H, C8), 2.32 (s, 6H, C20), 2.27–2.20 (m, 2H, C11), 2.17 (s, 6H, C19). 13C-NMR (100 MHz, CDCl3); 199.0 (C9), 169.7 (C2), 160.8 (C4), 156.6 (C13), 150.3 (C6), 136.4 (C17), 130.3 (C16), 123.5 (C14), 120.8 (C18), 111.8 (C15), 95.3 (C3), 66.7 (C12), 33.7 (C10), 27.9 (N-CH3), 27.8 (N-CH3), 25.3 (C11), 21.3 (C20), 15.7 (C19). MS (ES−); 345.15 (M−H); MS (ES+); 347.18 (M+H), 369.16 (M+Na); HRMS (M+Na); calcd for C18H22N2Na1O5; 369.1421; found; 369.1407.
3.4.28. Synthesis of Compound 18e
Yield; 43%; M.P.; 127 °C; 1H-NMR (400 MHz, CDCl3); 7.17 (d, 1H, J = 8.8 Hz, C16), 6.72 (d, 1H, J = 2.8 Hz, C15), 6.62 (dd, 1H, J1 = 8.8 Hz, J2= 2.8 Hz, C14), 4.01 (t, 2H, J = 6.0 Hz, C12), 3.36–3.29 (m, 8H, C7, C8 and C10), 2.30 (s, 3H, C19), 2.21–2.14 (m, 2H, C11). 13C-NMR (100 MHz, CDCl3); 198.7 (C9), 169.7 (C2), 160.7 (C4), 157.2 (C13), 150.2 (C6), 136.8 (C17 or C18), 129.4 (C16), 125.7 (C17 or C18), 116.9 (C14 or C15), 112.9 (C14 or C15), 95.3 (C3), 67.1 (C12), 35.6 (C10), 27.9 (N-CH3), 27.8 (N-CH3), 25.1 (C11), 20.2 (C19). MS (ES−); 365.11 (M−H), HRMS (M+Na); calcd for C17H19Cl1N2Na1O5; 389.0875; found; 389.0863.
3.4.29. Synthesis of Compound 18f
Yield; 70%; M.P.; 123 °C; 1H-NMR (400 MHz, CDCl3); 7.31 (s, 1H, C17), 7.14 (d, 1H, J = 8.8 Hz, C16), 6.81 (d, 1H, J = 8.8 Hz, C14), 4.09 (t, 2H, J = 6.0 Hz, C12), 3.39–3.35 (m, 5H, C10 and C7), 3.29 (s, 3H, C8), 2.28–2.21 (m, 2H, C11). 13C-NMR (100 MHz, CDCl3); 198.5 (C9), 169.7 (C2), 160.8 (C4), 153.0 (C13), 150.2 (C6), 129.8 (C17), 127.4 (C16), 125.6 (C18), 123.6 (C15), 113.8 (C14), 95.4 (C3), 68.4 (C12), 33.5 (C10), 27.9 (N-CH3), 27.8 (N-CH3), 24.9 (C11). MS (ES−); 385.06 (M−H), HRMS (M−H); calcd for C16H15Cl2N2O5; 385.0364; found; 385.0368.
3.4.30. Synthesis of Compound 18g
Yield; 56%; M.P.; 148 °C; 1H-NMR (400 MHz, CDCl3); 7.09–7.07 (m, 2H, C16 and C17), 6.70 (d, 1H, J = 8.0 Hz, C14), 4.04 (t, 2H, J = 6.0 Hz, C12), 3.40–3.36 (m, 5H, C10 and C7), 3.33 (s, 3H, C8), 2.26–2.20 (m, 2H, C11), 2.18 (s, 3H, C19). 13C-NMR (100 MHz, CDCl3); 198.8 (C9), 169.8 (C2), 160.8 (C4), 155.4 (C13), 150.3 (C6), 130.3 (C17), 128.7 (C15), 126.2 (C16), 125.0 (C18), 111.8 (C14), 95.4 (C3), 67.2 (C12), 33.7 (C10), 28.0 (N-CH3), 27.8 (N-CH3), 25.2 (C11), 16.0 (C19). MS (ES−); 365.10 (M−H), HRMS (M−H); calcd for C17H18Cl1N2O5; 365.0910; found; 365.0901.
3.4.31. Synthesis of Compound 19a
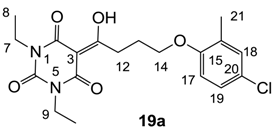
Yield; 37%; M.P. 77 °C; 1H-NMR (400 MHz, CDCl3); 7.07–7.05 (m, 2H, C18 and C19), 6.69 (d, 1H, J = 8.4 Hz, C17), 4.04–3.94 (m, 6H, C7, C9 and C14), 3.37 (t, 2H, J = 7.2 Hz, C12), 2.25–2.18 (m, 2H, C13), 2.17 (s, 3H, C21), 1.25 (t, 3H, J = 7.6 Hz, CH3), 1.20 (t, 3H, J = 7.6 Hz, CH3). 13C-NMR (100 MHz, CDCl3); 198.9 (C11), 169.5 (C2), 160.4 (C4), 155.4 (C15), 149.3 (C6), 130.3 (C18), 128.6 (quart C), 126.2 (C19), 124.9 (quart C), 111.7 (C17), 95.4 (C3), 67.1 (C14), 36.7 (N-CH2), 36.5 (N-CH2), 33.7 (C12), 25.0 (C13), 16.0 (C21), 13.2 (CH3), 13.1 (CH3). MS (ES−); 393.12 (M−H); HRMS (M−H); calcd for C19H22Cl1N2O5; 393.1223; found; 393.1215.
3.4.32. Synthesis of Compound 19b
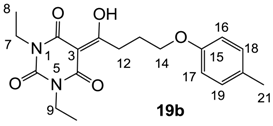
Yield; 40% (oil); 1H-NMR (400 MHz, CDCl3); 7.04 (d, 2H, J = 8.4 Hz, C18 and C19), 6.76 (d, 2H, J = 8.4 Hz, C17 and C16), 4.05–3.93 (m, 6H, C7, C9 and C14), 3.35 (t, 2H, J = 7.2 Hz, C12), 2.27 (s, 3H, C21), 2.23–2.16 (m, 2H, C13), 1.28–1.17 (m, 6H, C8 and C10). 13C-NMR (100 MHz, CDCl3); 199.1 (C11), 169.5 (C2), 160.4 (C4), 156.5 (C15), 149.3 (C6), 129.8 (C20), 129.7 (C18 and C19), 114.1 (C16 and C17), 95.4 (C3), 66.9 (C14), 36.6 (N-CH2), 36.5 (N-CH2), 33.7 (C12), 25.2 (C13), 20.3 (C21), 13.1 (CH3), 13.0 (CH3). MS (ES−); 359.17 (M−H); HRMS (M−H); calcd for C19H23N2O5; 359.1612; found; 359.1611.
3.4.33. Synthesis of Compound 20
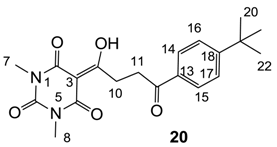
Yield; 15% (oil); 1H-NMR (400 MHz, CDCl3); 7.93 (d, 2H, J = 8.4 Hz, C16 and C17), 7.49 (d, 2H, J = 8.4 Hz, C14 and C15), 3.63 (t, 2H, J = 6.0 Hz, C11), 3.41 (t, 2H, J = 6.0 Hz, C10), 3.37 (s, 3H, C7), 3.34 (s, 3H, C8), 1.34 (s, 9H, C20–C22). 13C-NMR (100 MHz, CDCl3); 198.6 (C9), 197.7 (C12), 169.7 (C2), 160.9 (C4), 157.0 (C18), 150.3 (C6), 133.7 (C13), 128.0 (C14 and C15), 125.5 (C16 and C17), 95.2 (C3), 35.1 (C19), 32.9 (CH2), 31.3 (CH2), 31.0 (C20-C22), 28.0 (N-CH3), 27.8 (N-CH3). MS (ES−); 371.17 (M−H), HRMS (M+Na); calcd for C20H24N2Na1O5; 395.1577; found; 395.1567.
3.4.34. Synthesis of Compound 21
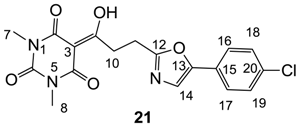
Yield; 70%; M.P.; 173 °C; 1H-NMR (400 MHz, CDCl3); 7.52 (d, 2H, J = 8.0 Hz, C16 and C17), 7.36 (d, 2H, J = 8.0 Hz, C18 and C19), 7.20 (s, 1H, C14), 3.73 (t, 2H, J = 7.2 Hz, C11), 3.37 (s, 3H, C7), 3.32 (s, 3H, C8), 3.24 (t, 2H, J = 7.2 Hz, C10). 13C-NMR (100 MHz, CDCl3); 196.9 (C9), 169.7 (C2), 162.6 (quart C), 160.7 (C4), 150.3 (C6), 150.2 (quart C), 133.9 (C20), 129.1 (C18 and C19), 126.5 (C15), 125.2 (C16 and C17), 122.3 (C14), 95.4 (C3), 33.9 (C10), 28.0 (N-CH3), 27.9 (N-CH3), 23.2 (C11). MS (ES−); 388.08 (M−H), HRMS (M−H); calcd for C18H15Cl1N3O5; 388.0706; found; 388.0689.
3.4.35. Synthesis of Compound 22
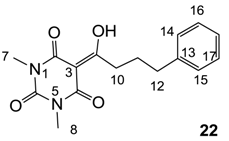
Yield; 69% (oil); 1H-NMR (400 MHz, CDCl3); 7.31–7.27 (m, 2H, C16 and C17), 7.22–7.18 (m, 3H, C14, C15 and C18), 3.37 (s, 3H, C7), 3.34 (s, 3H, C8), 3.20 (t, 2H, J = 8.0 Hz, C10), 2.75 (t, 2H, J = 8.0 Hz, C10), 2.08–2.01 (m, 2H, C11). 13C-NMR (100 MHz, CDCl3); 199.3 (C9), 169.7 (C2), 160.8 (C4), 150.3 (C6), 141.3 (C13), 128.4 (C16 and C17), 128.3 (C14 and C15), 126.0 (C18), 95.3 (C3), 36.3 (C10), 35.5 (C12), 28.0 (N-CH3), 27.8 (N-CH3), 27.2 (C11). MS (ES−); 301.13 (M−H), HRMS (M−H); calcd for C16H17N2O4; 301.1194; found; 301.1193.
3.4.36. Synthesis of Compound 23a
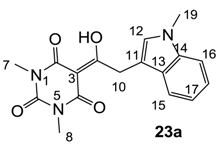
Yield; 20%; M.P.; 156 °C; 1H-NMR (400 MHz, CDCl3); 7.77 (d, 1H, J = 8.0 Hz, C15), 7.31–7.15 (m, 4H, C12 and C16-C18), 4.62 (s, 2H, C10), 3.77 (s, 3H, C19), 3.39 (s, 3H, C7), 3.33 (s, 3H, C8). 13C-NMR (100 MHz, CDCl3); 196.7 (C9), 169.8 (C2), 160.7 (C4), 150.2 (C6), 136.7 (C14), 128.6 (C12), 127.8 (C13), 121.7 (C18), 119.3 (C15 or C17), 119.2 (C15 or C17), 109.2 (C16), 106.5 (C11), 94.6 (C3), 32.6 (C19), 32.2 (C10), 28.0 (N-CH3), 27.7 (N-CH3). MS (ES−); 326.13 (M−H), HRMS (M+Na); calcd for C17H17N3Na1O4; 350.1111; found; 350.1099.
3.4.37. Synthesis of Compound 23b
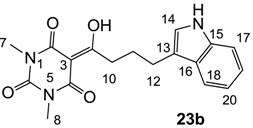
Yield; 57%; M.P.; 182 °C; 1H-NMR (400 MHz, CDCl3); 7.97 (brs, 1H, NH), 7.62 (d, 1H, J = 8.0 Hz, C18), 7.35 (d, 1H, J = 8.4 Hz, C17), 7.19 (dd, 1H, J1 = J2 = 8.0 Hz, C19 or C20), 7.12 (dd, 1H, J1 = J2 = 8.0 Hz, C19 or C20), 7.04 (s, 1H, C14), 3.36 (s, 3H, C7), 3.33 (s, 3H, C8), 3.26 (t, 2H, J = 7.6 Hz, C10), 2.91 (t, 2H, J = 7.6 Hz, C12), 2.19–2.12 (m, 2H, C11). 13C-NMR (100 MHz, CDCl3); 199.7 (C9), 169.7 (C2), 160.8 (C4), 150.4 (C6), 136.3 (C15), 127.4 (C16), 121.9 (C19 or C20), 121.5 (C19 or C20), 119.2 (C17 or C18), 118.9 (C17 or C18), 115.6 (C13), 111.0 (C14), 95.3 (C3), 36.6 (C10), 28.0 (N-CH3), 27.8 (N-CH3), 26.0 (C12), 24.9 (C11). MS (ES−); 340.14 (M−H), HRMS (M−H); calcd for C18H18N3O4; 340.1303; found; 340.1295.
3.4.38. Synthesis of Compound 24
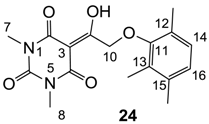
Yield; 77%; M.P.; 184 °C; 1H-NMR (400 MHz, CDCl3); 6.91 (d, 1H, J = 7.6 Hz, C14), 6.86 (d, 1H, J = 7.6 Hz, C16), 5.24 (s, 2H, C10), 3.42 (s, 3H, C7), 3.30 (s, 3H, C8), 2.26 (s, 3H, CH3), 2.23 (s, 3H, CH3), 2.20 (s, 3H, CH3). 13C-NMR (100 MHz, CDCl3); 194.7 (C9), 169.6 (C2), 160.5 (C4), 155.0 (C11), 150.1 (C6), 136.0 (quart C), 129.2 (quart C), 127.9 (C14), 127.8 (quart C), 125.8 (C16), 93.6 (C3), 72.5 (C10), 27.9 (N-CH3), 27.8 (N-CH3), 19.8 (CH3), 16.1 (CH3), 12.3 (CH3). MS (ES−); 331.15 (M−H), HRMS (M+Na); calcd for C17H20N2Na1O5; 355.1264; found; 355.1271.
3.4.39. Synthesis of Compound 25a
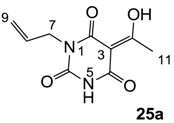
Yield; 58%; M.P.; 181 °C; Mixture of two exo-enol tautomers (E1: E2 = 40: 60); 1H-NMR (500 MHz, CDCl3); 8.84 (brs, 1H, NH E1), 8.54 (brs, 1H, NH E2), 5.92–5.83 (m, 1H, C8), 5.32–5.20 (m, 2H, C9), 4.53–4.50 (m, 2H, C7), 2.73 (s, 3H, C11). 13C-NMR (125 MHz, CDCl3); 196.9 (C10 E1), 196.4 (C10 E2), 170.1 (C2 E2), 169.0 (C4 E1), 161.2 (C2 E1), 161.0 (C4 E2), 149.0 (C6 E1), 148.8 (C6 E2), 131.5 (C8 E1), 130.8 (C8 E2), 118.8 (C9 E2), 118.1 (C9 E1), 95.7 (C3 E2), 95.6 (C3 E1), 42.8 (C7 E1), 42.7 (C7 E2), 24.5 (C11 E1), 24.4 (C11 E2). MS (ES−); 209.07 (M−H); HRMS (M−H); calcd for C9H9N2O4; 209.0568; found; 209.0570.
3.4.40. Synthesis of Compound (±)-25b
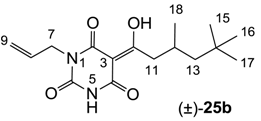
Yield; 40%; M.P.; 76 °C; Mixture of two exo-enol tautomers (E1:E2 = 40:60); 1H-NMR (400 MHz, CDCl3); 9.94 (brs, 1H, NH E1), 9.39 (brs, 1H, NH E2), 5.92–5.81 (m, 1H, C8), 5.30–5.16 (m, 2H, C9), 4.53–4.50 (m, 2H, C7), 3.09–3.07 (m, 2H, C11), 2.11–2.13 (m, 1H, C12), 1.36 (dd, 1H, J1 = 6.4 Hz, J2 = 6.0 Hz, C13 E1), 1.33 (dd, 1H, J1 = 6.0 Hz, J2 = 4.0 Hz, C13 E2), 1.19 (dd, 1H, J1 = 6.0 Hz, J2 = 4.0 Hz, C13 E2), 1.16 (dd, 1H, J1 = 6.4 Hz, J2 = 6.0 Hz, C13 E1), 1.02–1.00 (m, 3H, C18), 0.89 (s, 9H, C15-C17). 13C-NMR (125 MHz, CDCl3); 200.1 (C10 E1), 199.5 (C10 E2), 170.4 (C2 E2), 169.7 (C4 E1), 161.2 (C2 E1), 160.9 (C4 E2), 149.6 (C6 E1), 149.0 (C6 E2), 131.6 (C8 E1), 130.9 (C8 E2), 118.6 (C9 E2), 117.8 (C9 E1), 95.7 (C3 E2), 95.6 (C3 E1), 50.6 (CH2 E2), 50.5 (CH2 E1), 45.2 (CH2 E2), 45.1 (CH2 E1), 42.6 (C7 E1), 42.5 (C7 E2), 31.0 (C14), 29.9 (C15-C17), 28.1 (CH3 E2), 27.8 (CH3 E1), 22.7 (CH3 E1), 22.6 (CH3 E2). MS (ES−); 307.19 (M−H); HRMS (M−H); calcd for C16H23N2O4; 307.1663; found; 307.1657.
3.4.41. Synthesis of Compound 26a
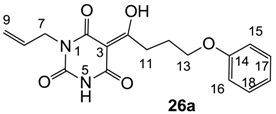
Yield; 56%; M.P.; 131 °C; Mixture of two exo-enol tautomers (E1: E2 = 40: 60); 1H-NMR (500 MHz, CDCl3); 9.36 (brs, 1H, NH E1), 8.90 (brs, 1H, NH E2), 7.29–7.25 (m, 2H, C17 and C18), 6.96–6.92 (m, 1H, C19), 6.87 (d, 2H, J = 8.0 Hz, C15 and C16), 5.93–5.80 (m, 1H, C8), 5.33–5.18 (m, 2H, C9), 4.53 (d, 2H, J = 6.0 Hz, C7 E2), 4.48 (d, 2H, J = 6.0 Hz, C7 E1), 4.09–4.06 (m, 2H, C13), 3.38 (t, 2H, J = 7.5 Hz, C11), 2.25–2.20 (m, 2H, C12). 13C-NMR (125 MHz, CDCl3); 199.9 (C10 E1), 199.3 (C10 E2), 170.4 (C2 E2), 169.5 (C4 E1), 160.9 (C4 E2 and C2 E1), 158.6 (C14), 149.2 (C6 E1), 148.8 (C6 E2), 131.5 (C8 E1), 130.8 (C8 E2), 129.4 (C17 and C18 E1), 129.4 (C17 and C18 E2), 120.7 (C19), 118.8 (C9 E2), 118.0 (C9 E1), 114.4 (C15 and C16), 95.2 (C3 E2), 95.2 (C3 E1), 66.7 (C13), 42.7 (C7 E2), 42.7 (C7 E1), 33.6 (C11 E1), 33.5 (C11 E2), 25.3 (C12 E2), 25.1 (C12 E1). MS (ES−); 329.11 (M−H); HRMS (M−H); calcd for C17H17N2O5; 329.1143; found; 329.1143.
3.4.42. Synthesis of Compound 26b
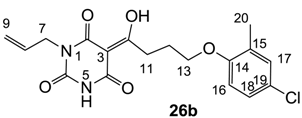
Yield; 59%; M.P.; 128 °C; Mixture of two exo-enol tautomers (E1: E2 = 40: 60); 1H-NMR (400 MHz, CDCl3); 9.56 (brs, 1H, NH E1), 9.10 (brs, 1H, NH E2), 7.09–7.07 (m, 2H, C17 and C18), 6.71 (d, 1H, J = 8.4 Hz, C16), 5.93–5.80 (m, 1H, C8), 5.32–5.18 (m, 2H, C9), 4.53 (d, 2H, J = 5.6 Hz, C7 E2), 4.50 (d, 2H, J = 5.6 Hz, C7 E1), 4.04 (t, 2H, J = 6.0 Hz, C11), 3.38 (t, 2H, J = 7.6 Hz, C13), 2.26–2.19 (m, 2H, C12), 2.18 (s, 3H, C20 E1), 2.17 (s, 3H, C20 E2). 13C-NMR (100 MHz, CDCl3); 199.7 (C10 E1), 199.1 (C10 E2), 170.4 (C2 E2), 169.5 (C4 E1), 161.1 (C4 E2), 160.9 (C2 E1), 155.4 (C14), 149.3 (C6 E1), 148.8 (C6 E2), 131.5 (C8 E1), 130.8 (C8 E2), 130.3 (C17), 128.6 (quart C), 126.2 (C18), 125.0 (quart C), 118.9 (C9 E2), 118.1 (C9 E1), 111.8 (C16), 95.2 (C3), 66.1 (C13 E2), 67.1 (C13 E1), 42.7 (C7), 33.6 (C11 E1), 33.5 (C11 E2), 25.2 (C12 E2), 24.9 (C12 E1), 16.0 (C20). MS (ES−); 377.11 (M−H); HRMS (M−H); calcd for C18H18Cl1N2O5; 377.0910; found; 377.0908.
3.4.43. Synthesis of Compound 26c
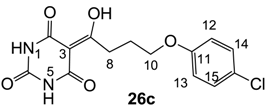
Yield; 37%; M.P.; 201 °C; 1H-NMR (500 MHz, DMSO); 11.86 (brs, 1H, NH), 11.05 (brs, 1H, NH), 7.30 (d, 2H, J = 9.0 Hz, C14 and C15), 6.93 (d, 2H, J = 9.0 Hz, C12 and C13), 4.02 (t, 2H, J = 6.5 Hz, C10), 3.20 (t, 2H, J = 6.5 Hz, C8), 2.07–2.01 (m, 2H, C9). 13C-NMR (125 MHz, DMSO); 197.9 (C7), 157.3 (C11), 149.0 (C2, C4 and C6), 129.2 (C14 and C15), 124.2 (C16), 116.2 (C12 and C13), 94.9 (C3), 67.2 (C10), 32.8 (C8), 24.4 (C9). MS (ES−); 323.07 (M−H); HRMS (M−H); calcd for C14H12N2O5; 323.0440; found; 323.0436.
3.4.44. Synthesis of Compound 26d
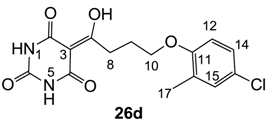
Yield; 44%; M.P.; 207 °C; 1H-NMR (400 MHz, DMSO); 11.86 (brs, 1H, NH), 11.06 (brs, 1H, NH), 7.17–7.14 (m, 2H, C14 and C15), 6.91 (d, 1H, J = 8.8 Hz, C12), 4.01 (t, 2H, J = 6.0 Hz, C10), 3.24 (t, 2H, J = 6.0 Hz, C8), 2.12–2.03 (m, 5H, C9 and C17). 13C-NMR (125 MHz, DMSO); 198.0 (C7), 155.4 (C11), 149.1 (C2, C4 and C6), 129.9 (C15), 128.3 (tert-C), 126.5 (C14), 123.7 (tert-C), 112.7 (C12), 94.9 (C3), 67.2 (C10), 32.9 (C8), 24.5 (C9), 15.6 (C17). MS (ES−); 337.08 (M−H); HRMS (M−H); calcd for C15H14N2O5; 337.0597; found; 337.0587.
3.4.45. Synthesis of Compound 27
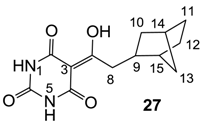
Yield; 50%; M.P.; over 260 °C; 1H-NMR (500 MHz, mixture of CDCl3 and CD3OD); 2.90 (dd, 1H, J1 = 14.0 Hz, J2 = 8.0 Hz, C8), 2.84 (dd, 1H, J1 = 14.0 Hz, J2 = 8.0 Hz, C8), 2.07 (brs, 1H, C14), 1.87 (brs, 1H, C15), 1.83–1.78 (m, 1H, C9), 1.39–1.25 (m, 4H, CH2), 1.06–0.95 (m, 4H, CH2). 13C-NMR (125 MHz, mixture of CDCl3 and CD3OD); 199.1 (C7), 171.8 (C2), 163.1 (C4), 150.0 (C6), 95.8 (C3), 42.8 (C8), 41.5 (C15), 39.6 (C9), 38.1 (CH2), 37.2 (C14), 35.7 (CH2), 30.1 (CH2), 29.0 (CH2). MS (ES−); 263.12 (M−H), HRMS (M−H); calcd for C13H15N2O4; 263.1037; found; 263.1040.
3.4.46. Synthesis of Compound cis-28
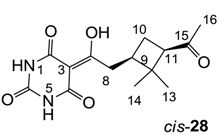
Yield; 61%; M.P.; 192 °C; 1H-NMR (500 MHz, CD3OD); 3.20 (dd, 1H, J1 = 14.0 Hz, J2 = 6.5 Hz, C11), 3.06–2.97 (m, 2H, C8), 2.45–2.40 (m, 1H, C9), 2.09–2.03 (m, 4H, C10 and C16), 1.91–1.86 (m, 1H, C10), 1.34 (s, 3H, C13), 0.92 (s, 3H, C14). 13C-NMR (125 MHz, CD3OD); 210.5 (C15), 199.3 (C7), 173.2 (C2), 164.3 (C4), 151.1 (C6), 96.2 (C3), 55.4 (C11), 45.1 (C12), 40.4 (C9), 37.7 (C8), 30.4 (C16), 30.3 (C13), 24.4 (C10), 18.0 (C14). MS (ES−); 293.12 (M−H); MS (ES+); 317.13 (M+Na); HRMS (M+Na); calcd for C14H18N2Na1O5; 317.1108; found; 317.1099.
3.5. Synthesis of Compound 5a Via O-Acylation Followed by Acyl Migration
3.5.1. Synthesis of Compound 41
To the mixture of barbituric acid 40d (1.0 g, 6.40 mmol) and triethylamine (0.78 g, 7.70 mmol) in dichloromethane (50 mL) was slowly added 3,5,5-trimethylhexanoyl chloride (1.20 g, 6.72 mmol) at 0 °C under nitrogen atmosphere and the mixture was stirred for 3 h at room temperature. After completion of the reaction, the mixture was washed with 2 M HCl. The organic layer was dried over MgSO4 and evaporated in vacuo to give crude product. Further purification was carried out by recrystallization in the mixture of ethyl acetates and petrol giving pure compound 41 (1.56 g, 5.37 mmol, 84% yield) as a solid (M.P.; 49 °C)
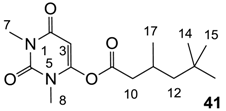
1H-NMR (400 MHz, CDCl3); 5.56 (s, 1H, C3), 3.27 (s, 3H, C8), 3.25 (s, 3H, C7), 2.57 (dd, 1H, J1 = 15.6, J2 = 6.0, C10), 2.37 (dd, 1H, J1 = 15.6, J2 = 8.0, C10), 2.14–2.01 (m, 1H, C11), 1.02 (d, 3H, J = 6.4 Hz, C12), 0.88–0.85 (m, 12H, C14–C17). 13C-NMR (100 MHz, CDCl3); 167.5 (C9), 162.3 (C2), 153.4 (C4), 151.1 (C6), 91.3 (C3), 50.2 (C12), 43.2 (C10), 30.9 (C13), 29.7 (C14-C16), 29.5 (C7), 28.0 (C8), 26.7 (C11), 22.3 (C17). MS (ES−); 295.18 (M−H), MS (ES+); 297.21 (M+H), 319.19 (M+Na), HRMS (M+Na); calcd for C15H24N2Na1O4; 319.1628; found; 319.1621.
3.5.2. Synthesis of Compound (±)-5a
To the solution of compound 41 (500 mg, 1.69 mmol) in dichloromethane (30 mL) was added DMAP (250 mg, 2.02 mmol) at room temperature and the mixture was stirred overnight. After completion of the reaction, the mixture was washed with 2 M HCl and the organic layer was evaporated in vacuo. Short flash column chromatography of the crude material gave 3-acylbarbituric acid 5a (245 mg, 0.845 mmol, 49 %) as oil.
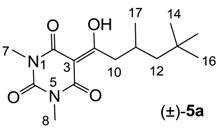
1H-NMR (400 MHz, CDCl3); 3.33 (s, 3H, C7), 3.29 (s, 3H, C8), 3.05 (d, 2H, J = 7.6 Hz, C10), 2.19–2.11 (m, 1H, C11), 1.32 (dd, 1H, J1 = 14.0 Hz, J2 = 3.6 Hz, C12), 1.15 (dd, 1H, J1 = 14.0 Hz, J2 = 6.8 Hz, C12), 0.98 (d, 3H, J = 6.4 Hz, C17), 0.87 (s, 9H, C14-C16). 13C-NMR (100 MHz, CDCl3); 199.1 (C9), 169.7 (C2), 160.7 (C4), 150.3 (C6), 95.8 (C3), 50.6 (C12), 45.2 (C10), 31.0 (C13), 29.8 (C14-C16), 27.9 (N-CH3), 27.8 (N-CH3), 27.7 (C11), 22.7 (C17). MS (ES−); 295.18 (M−H), HRMS (M−H); calcd for C15H23N2O4; 295.1663; found; 295.1678.
3.6. Synthesis of 3-Carboxamide an 3-Enamine Barbituric Acids
General procedure: To the solution of 3-alkoxycarbonyl or 3-acylbarbituric acid (1.0 equivalent) in toluene (or methanol for compound 36) was added primary amine (1.0 equivalent) and the mixture was refluxed for 4–24 h checking TLC. After completion of the reaction, the solution was evaporated and column chromatography (or precipitation in methanol for compound 36) gave metal-chelated 3-carboxamide tetramic acid or pure 3-enamine tetramic acid. In case of 3-carboxamide tetramic acid, the compound was dissolved in dichloromethane (50 mL) and washed with 1 N HCl (20 mL) to make metal free form. The organic layer was dried with MgSO4 and concentrated in vacuo to give metal free 3-carboxamide tetramic acid.
3.6.1. Synthesis of Compound 29a
Yield; 63%; M.P.: >250 °C; 1H-NMR (400 MHz, DMSO); 11.54 (brs, 2H, NH), 11.37 (s, 1H, NH), 7.32 (d, 2H, J = 8.4 Hz, C9 and C10), 6.93 (d, 2H, J = 8.4 Hz, C11 and C12), 3.11 (brs, 4H, C14 and C15), 1.60 (brs, 4H, C16 and C17), 1.53 (brs, 2H, C18). 13C-NMR; not determined because of peak broading and solubility problems. MS (ES−); 329.13 (M−H); MS (ES+); 331.15 (M+H), 353.13 (M+Na); HRMS (M+H); calcd for C16H19N4O4; 331.1401; found; 331.1392.
3.6.2. Synthesis of Compound 29b
Yield; 77%; M.P.; 180 °C; 1H-NMR (500 MHz, CD3OD); 7.78 (d, 2H, J = 9.5 Hz, C11 and C12), 7.68 (d, 2H, J = 9.5 Hz, C13 and C14), 3.62 (t, 4H, J = 6.0 Hz, C16 and C17), 3.34 (s, 6H, C7 and C8), 2.07–2.03 (m, 4H, C18 and C19), 1.81 (brs, 2H, C20). 13C-NMR; not determined because of peak broading and solubility problems. MS (ES−); 357.16 (M−H); HRMS (M−H); calcd for C18H21N4O4; 357.1568; found; 357.1568.
3.6.3. Synthesis of Compound 29c
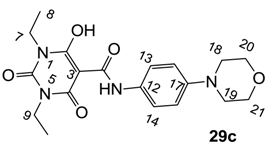
Yield; 48%; M.P.: 167 °C; 1H-NMR (400 MHz, CDCl3); 11.82 (brs, 1H, NH or OH), 7.41 (d, 2H, J = 8.8 Hz, C13 and C14), 6.91 (d, 2H, J = 8.8 Hz, C15 and C16), 4.02 (q, 4H, J = 6.8 Hz, C7 and C9), 3.86 (t, 4H, J = 4.8 Hz, C20 and C21), 3.16 (t, 4H, J = 4.8 Hz, C18 and C19), 1.29–1.24 (m, 6H, C8 and C9). 13C-NMR (100 MHz, CDCl3); 168.9 (C11), 168.4 (C4), 163.0 (C2), 149.4 (C6), 149.2 (C17), 127.8 (C12), 122.9 (C13 and C14), 115.9 (C15 and C16), 80.5 (C3), 66.7 (C20 and C21), 49.1 (C18 and C19), 36.6 (N-CH2), 36.3 (N-CH2), 13.3 (CH3), 13.2 (CH3). MS (ES−); 387.17 (M−H); HRMS (M+Na); calcd for C19H24N4Na1O5; 411.1639; found; 411.1627.
3.6.4. Synthesis of Compound 30
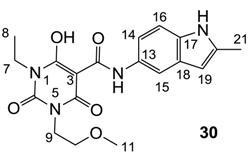
Yield; 53%; M.P. 160 °C; 1H-NMR (400 MHz, DMSO); 11.79 (s, 1H, NH or OH), 11.04 (s, 1H, indole NH), 7.55 (s, 1H, C15), 7.27 (d, 1H, J = 8.8 Hz, C14), 7.04 (dd, 1H, J1 = 8.8 Hz, J2 = 2.0 Hz, C16), 6.12 (s, 1H, C19), 3.99 (t, 2H, J = 6.0 Hz, C9), 3.85 (q, 2H, J = 6.8 Hz, C7), 3.50 (t, 2H, J = 6.0 Hz, C10), 3.25 (s, 3H, C11), 2.37 (s, 3H, C21), 1.13 (t, 3H, J = 6.8 Hz, C8). 13C-NMR (100 MHz, DMSO); 168.3 (C2, C4 and C12), 149.1 (C6), 137.3 (Ar quart-C), 134.2 (Ar quart-C), 128.7 (Ar quart-C), 126.7 (Ar quart-C), 115.1 (Ar tert-C), 112.5 (Ar tert-C), 110.8 (Ar tert-C), 99.5 (C19), 79.7 (C3), 68.4 (C10), 58.0 (C11), 39.5 (C9, overlapping with DMSO peak, confirmed in DEPT NMR), 35.9 (C7), 13.4 (CH3), 13.0 (CH3). MS (ES−); 385.16 (M−H); HRMS (M+Na); calcd for C19H22N4Na1O5; 409.1482; found; 409.1474.
3.6.5. Synthesis of Compound 31a
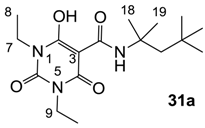
Yield; 67%; M.P.: 92 °C; 1H-NMR (400 MHz, CDCl3); 10.16 (brs, 1H, NH or OH), 3.97–3.90 (m, 4H, C7 and C9), 1.78 (s, 2H, C13), 1.37 (s, 6H, C18 and C19), 1.22–1.16 (m, 6H, C8 and C10), 0.98 (s, 9H, C15-C17). 13C-NMR (100 MHz, CDCl3); 170.4 (C11), 168.2 (C4), 163.1 (C2), 149.6 (C6), 80.1 (C3), 56.6 (C12), 51.7 (C13), 36.2 (N-CH2), 36.0 (N-CH2), 31.6 (C14), 31.2 (C15-C17), 29.5 (C18 and C19), 13.3 (CH3), 13.2 (CH3). MS (ES−); 338.22 (M−H); MS (ES+); 340.21 (M+H), 362.21 (M+Na); HRMS (M+Na); calcd for C17H29N3Na1O4; 362.2050; found; 362.2037.
3.6.6. Synthesis of Compound 31b
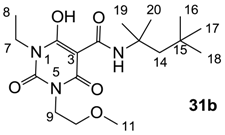
Yield; 68 % (oil); 1H-NMR (400 MHz, CDCl3); 10.21 and 10.14 (2 of s, 1H, NH or OH), 4.15–4.11 (m, 2H, C9), 3.98–3.92 (m, 2H, C7), 3.63–3.57 (m, 2H, C10), 3.35 and 3.34 (2 of s, 3H, C11), 1.79 (s, 2H, C14), 1.49 (s, 6H, C19 and C20), 1.24–1.18 (m, 3H, C8), 0.99 (s, 9H, C16-C18). 13C-NMR (100 MHz, CDCl3); 170.4 and 170.3 (C12), 168.4 and 168.2 (C4), 163.2 and 163.1 (C2), 149.9 and 149.8 (C6), 80.0 (C3), 69.5 and 69.4 (C10), 58.7 (C11), 56.7 and 56.6 (C13), 51.7 and 51.6 (C14), 39.9 and 39.8 (C9), 36.3 and 36.1 (C7), 31.6 (C15), 31.2 (C16-C18), 29.4 (C19 and C20), 13.3 and 13.2 (C8). MS (ES−); 368.23 (M−H); MS (ES+); 370.24 (M+H), 392.21 (M+Na); HRMS (M+Na); calcd for C18H31N3 Na1O5; 392.2156; found; 392.2141.
3.6.7. Synthesis of Compound 32
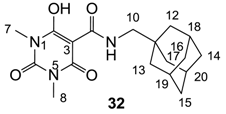
Yield; 74%; M.P.: 132 °C; 1H-NMR (500 MHz, CDCl3); 10.09 (brs, 1H, NH or OH), 3.33–3.32 (m, 6H, C7 and C8), 3.09 (d, 2H, J = 6.5 Hz, C10), 2.00 (brs, 3H, C18-C20), 1.73–1.62 (m, 6H, CH2), 1.53 (brs, 6H, CH2). 13C-NMR (125 MHz, CDCl3); 170.8 (C9), 168.2 (C4), 163.4 (C2), 150.6 (C6), 79.7 (C3), 51.6 (C10), 40.0 (C12, C13 and C17), 36.6 (C14–C16), 33.6 (C11), 28.0 (C18–C20), 27.5 (CH3), 27.4 (CH3). MS (ES−); 346.17 (M−H); HRMS (M−H); calcd for C18H24N3O4; 346.1772; found; 346.1772.
3.6.8. Synthesis of Compound 33a
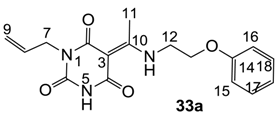
Yield; 96%; M.P.; 142 °C; Mixture of two exo-enol tautomers (E1: E2 = 45: 55); 1H-NMR (400 MHz, CDCl3); 12.77 (brs, 1H, NH E2), 12.71 (brs, 1H, NH E1), 8.68 (brs, 1H, NH), 7.33–7.27 (m, 2H, C17 and C18), 7.02–6.97 (m, 1H, C19), 6.92 (d, 2H, J = 8.8 Hz, C15 and C16), 5.94–5.84 (m, 1H, C8), 5.22 (d, 1H, J = 17.2 Hz, C9), 5.16 (d, 1H, J = 10.4 Hz, C9), 4.50 (d, 2H, J = 5.2 Hz, C7), 4.20 (t, 2H, J = 5.2 Hz, C13), 3.89–3.85 (m, 2H, C12), 2.79 (s, 3H, C11 E2), 2.77 (s, 3H, C11 E1). 13C-NMR (100 MHz, CDCl3); 175.2 (C10 E1), 174.7 (C10 E2), 167.0 (C2 E2), 166.3 (C4 E1), 163.2 (C2 E1), 163.1 (C4 E2), 157.9 (C14), 149.8 (C6), 132.6 (C8 E1), 132.4 (C8 E2), 129.6 (C17 and C18), 121.7 (C19), 116.9 (C9 E2), 116.7 (C9 E1), 114.6 (C15 and C16), 90.3 (C3), 65.7 (C13), 43.2 (C12 E1), 43.1 (C12 E2), 42.4 (C7 E1), 42.2 (C7 E2), 17.8 (C11). MS (ES−); 328.15 (M−H); MS (ES+); 352.15 (M+Na); HRMS (M+Na); calcd for C17H19N3Na1O4; 352.1268; found; 352.1254.
3.6.9. Synthesis of Compound 33b
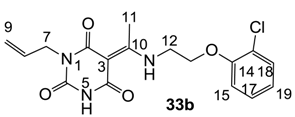
Yield; 84%; M.P.; 173 °C; Mixture of two exo-enol tautomers (E1: E2 = 45: 55); 1H-NMR (500 MHz, CDCl3); 12.82 (brs, 1H, NH E2), 12.73 (brs, 1H, NH E1), 8.34 (brs, 1H, NH E1), 8.25 (brs, 1H, NH E2), 7.39–7.37 (m, 1H, C18), 7.24–7.20 (m, 1H, C17), 6.98–6.92 (m, 2H, C15 and C19), 5.93–5.85 (m, 1H, C8), 5.24–5.14 (m, 2H, C9), 4.50 (d, 2H, J = 5.5 Hz, C7), 4.24 (t, 2H, J = 5.0 Hz, C13), 3.97–3.93 (m, 2H, C12), 2.85 (s, 3H, C11 E2), 2.83 (s, 3H, C11 E1). 13C-NMR (125 MHz, CDCl3); 175.7 (C10 E1), 175.1 (C10 E2), 167.1 (C2 E2), 166.2 (C4 E1), 163.2 (C2 E1), 162.8 (C4 E2), 153.6 (C14), 149.8 (C6 E1), 149.7 (C6 E2), 132.6 (C8 E1), 132.3 (C8 E2), 130.6 (C18), 127.8 (C17 E1), 127.6 (C17 E2), 123.4 (C16 E2), 123.3 (C16 E1), 122.6 (C19 E2), 122.6 (C19 E1), 117.0 (C9 E2), 116.8 (C9 E1), 113.9 (C15 E2), 113.9 (C15 E1), 90.4 (C3), 67.4 (C13 E1), 67.3 (C13 E2), 43.1 (C12 E1), 43.0 (C12 E2), 42.4 (C7 E1), 42.2 (C7 E2), 17.8 (C11 E1), 17.8 (C11 E2). MS (ES−); 362.11 (M−H); MS (ES+); 386.11 (M+Na); HRMS (M+Na); calcd for C17H18Cl1N3Na1O4; 386.0878; found; 386.0876.
3.6.10. Synthesis of Compound 34
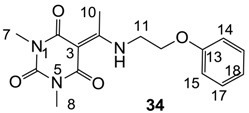
Yield; 63%; M.P.; 129 °C; 1H-NMR (400 MHz, CDCl3); 12.90 (brs, 1H, NH), 7.29 (dd, 2H, J1 = J2 = 7.6 Hz, C16 and C17), 6.99 (t, 1H, J = 7.6 Hz, C18), 6.92 (d, 2H, J = 7.6 Hz, C14 and C15), 4.19 (t, 2H, J = 5.2 Hz, C12), 3.87–3.83 (m, 2H, C11), 3.30 (s, 6H, C7 and C8), 2.76 (s, 3H, C10). 13C-NMR (100 MHz, CDCl3); 174.5 (C9), 166.3 (C4), 162.9 (C2), 157.9 (C13), 151.3 (C6), 129.5 (C16 and C17), 121.6 (C18), 114.6 (C14 and C15), 90.7 (C3), 65.7 (C12), 43.1 (C11), 27.8 (NCH3), 27.5 (NCH3), 17.9 (C10). MS (ES−); 316.15 (M−H); MS (ES+); 318.17 (M+H), 340.15 (M+Na); HRMS (M+Na); calcd for C16H19N3Na1O4; 340.1268; found; 340.1256.
3.6.11. Synthesis of Compound 35
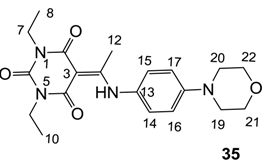
Yield; 66%; M.P.; 209 °C; 1H-NMR (400 MHz, CDCl3); 7.03 (d, 2H, J = 8.8 Hz, C16 and C17), 6.90 (d, 2H, J = 8.8 Hz, C14 and C15), 4.01–3.93 (m, 4H, C7 and C9), 3.84–3.82 (m, 4H, C21 and C22), 3.17–3.15 (m, 4H, C19 and C20), 2.59 (s, 3H, C12), 1.23–1.17 (m, 6H, C8 and C10). 13C-NMR (100 MHz, CDCl3); 173.3 (C11), 166.0 (C4), 162.5 (C2), 150.5 (C18), 150.4 (C6), 127.9 (C13), 126.5 (C14 and C15), 115.6 (C16 and C17), 91.3 (C3), 66.6 (C21 and C22), 48.6 (C19 and C20), 36.1 (N-CH2), 36.0 (N-CH2), 20.1 (C12), 13.35 (CH3), 13.33 (CH3). MS (ES-); 385.19 (M−H); HRMS (M−H); calcd for C20H25N4O4; 385.1881; found; 385.1888.
3.6.12. Synthesis of Compound 36
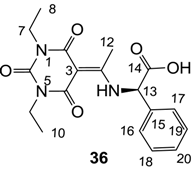
Yield; 60%; M.P.; 203 °C; 1H-NMR (400 MHz, CDCl3); 13.55 (d, 1H, J = 6.4 Hz, NH), 9.04 (brs, 1H, OH), 7.41–7.38 (m, 5H, C16-C20), 5.44 (d, 1H, J = 6.4 Hz, C13), 4.01 (q, 2H, J = 6.8 Hz, NCH2), 3.93 (q, 2H, J = 6.8 Hz, NCH2), 2.55 (s, 3H, C12), 1.21 (t, 3H, J = 6.8 Hz, CH3), 1.14 (t, 3H, J = 6.8 Hz, CH3). 13C-NMR (100 MHz, CDCl3); 173.8 (C11), 171.8 (C14), 165.9 (C4), 162.8 (C2), 150.5 (C6), 134.9 (C15), 129.5 (C16 and C17), 129.3 (C20), 127.0 (C18 and C19), 92.0 (C3), 60.5 (C13), 36.4 (N-CH2), 36.3 (N-CH2), 19.1 (C12), 13.4 (CH3), 13.3 (CH3).
3.6.13. Synthesis of Compound (±)-37
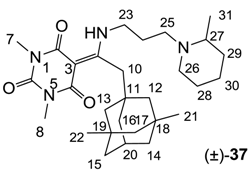
Yield; 82% (oil); 1H-NMR (400 MHz, CDCl3); 4.54–4.51 (m, 1H, NH), 3.65 (brs, 1H, C23), 3.43 (brs, 1H, C23), 3.29 (s, 3H, C7), 3.27 (s, 3H, C8), 2.75–2.68 (m, 2H, C10), 2.27 (brs, 3H, C26 and C27), 2.07–1.97 (m, 2H, C25), 1.77–1.75 (m, 2H, CH2), 1.61–0.99 (m, 21H, C12-C17, C20 and C24, 6H of CH2), 0.74 (s, 6H, C21 and C22). 13C-NMR (100 MHz, CDCl3); 175.0 (C9), 166.3 (C2), 163.2 (C4), 151.2 (C6), 91.2 (C3), 55.9 (C27), 51.6 (CH2), 50.6 (CH2), 50.4 (CH2), 50.1 (CH2), 48.9 (CH2), 43.1 (CH2), 42.7 (CH2), 40.6 (CH2), 39.4 (CH2), 38.7 (C18 and C19), 34.4 (CH2), 31.5 (C11), 30.5 (C21 and C22), 29.7 (C20), 28.0 (N-CH3), 27.6 (N-CH3), 26.6 (CH2), 26.1 (CH2), 23.4 (CH2), 18.4 (C31). MS (ES−); 497.38 (M−H); MS (ES+); 499.36 (M+H); HRMS (M+H); calcd for C29H47N4O3; 499.3643; found; 499.3629.
4. Conclusions
In this paper, we report the synthesis, tautomerism and antibacterial activity with SAR study of novel barbiturates inspired from antibacterial tetramates. Efficient synthetic methodologies leading to 3-acyl, 3-carboxamide and 3-enamine barbiturates permitted a wide range of substituent diversity on the core heterocycle. Computational studies of the favoured tautomeric forms reveals that all of 3-acyl, 3-alkoxycarbonyl, 3-carboxamido and 3-enamine barbituric acids in solution are favored to be the enol-tautomeric form rather than the keto-form, in which the exo-enol form for 3-acyl, 3-carboxamide and 3-enamine and endo-enol form for 3-alkoxycarbonyl barbiturate are more stable than the alternative enol-form. It was found that SAR of substituted barbiturates is similar to tetramates. Thus, 3-acyl and 3-carboxamide barbiturates depending on their substituents (N-disubstitution preferred), along with the equivalent tetramates, exhibited antibacterial activity, especially against resistant and susceptible Gram-positive strains, such as MRSA, VSE, VRE and MDRSP. Of particular interest is that the active barbiturates possess amenable molecular weight, rotatable bonds and the number of proton-donors/acceptors for drug design as well as less lipophilic character, and which are similar to antibiotic agents for oral and injectable use. In addition, it was found that not only lipophilicity and MSA, as discussed in our previous papers [13,14,15,19], but also PSA affected the efficiency of transportation by efflux pump in H. influenzae. Barbiturates tended to be more easily transported than tetramates because of their higher PSA. As expected, replacement of the tetramic acid by barbituric acid gave a positive effect for PPB affinity. Unfortunately, the reduction of PPB affinity by the barbituric core is not sufficient to achieve activity in vivo, although the physicochemical properties and ionic state are similar to antibiotic agents for oral and injectable use. Further optimization to reduce PPB affinity or elevate antibiotic potency is therefore required. On the other hand, physicochemical property-activity relationship analysis of clinical antibiotics shows that oral and injectable antibiotics may have a higher margin for lipophilicity depending on molecular size (MSA) in order to achieve low PPB affinity, while topical antibiotics are free from such constraints. In addition, Gram-negative active antibiotics tended to exist as more ionized microspecies than Gram-positive antibiotics to penetrate outer membrane. We believe that these systems offer unusual opportunities for antibiotic drug discovery, and these results suggest that a strategy based upon the use of natural products is viable [41,42], and highlights the importance of continuing development of methodologies to access tetramate-like systems [43]. The work contained here-in, and another recently reported study [44], indicate that barbiturates offer a core template of promise and worthy of further investigation.
Acknowledgments
We are particularly grateful for valuable input by Phil Dudfield, John Lowther, and for funding by Galapagos SASU (France).
Author Contributions
Y-CJ and MGM designed research; Y-CJ performed research and analyzed the data; Y-CJ and MGM wrote the paper. All authors read and approved the final manuscript.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/20/03/3582/s1.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Silver, L.L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef] [PubMed]
- Royles, B.J. Naturally occurring tetramic acids: Structure, isolation, and synthesis. Chem. Rev. 1995, 95, 1981–2001. [Google Scholar] [CrossRef]
- Schobert, R.; Schlenk, A. Tetramic and tetronic acids: An update on new derivatives and biological aspects. Bioorg. Med. Chem. 2008, 16, 4203–4221. [Google Scholar] [CrossRef] [PubMed]
- Gänzle, M.G. Reutericyclin: Biological activity, mode of action, and potential applications. Appl. Microbiol. Biotechnol. 2004, 64, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Tuske, S.; Sarafianos, S.G.; Wang, X.; Hudson, B.; Sineva, E.; Mukhopadhyay, J.; Birktoft, J.J.; Leroy, O.; Ismail, S.; Clark, A.D.; et al. Inhibition of bacterial RNA polymerase by streptolydigin: Stabilization of a straight-bridge-helix active-center conformation. Cell 2005, 122, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.W.; Goetz, M.A.; Smith, S.K.; Zink, D.L.; Polishook, J.; Onishi, R.; Salowe, S.; Wiltsie, J.; Allocco, J.; Sigmund, J.; et al. Discovery of kibdelomycin, a potent new class of bacterial type II topoisomerase inhibitor by chemical-genetic profiling in Staphylococcus aureus. Chem. Biol. 2011, 18, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Igarashi, M.; Okajima, T.; Ishii, E.; Kino, H.; Hatano, M.; Sawa, R.; Umekita, M.; Kimura, T.; Okamoto, S.; et al. Isolation and characterization of signermycin B, an antibiotic that targets the dimerization domain of histidine kinase WalK. Antimicrob. Agents Chemother. 2012, 56, 3657–3663. [Google Scholar] [CrossRef] [PubMed]
- Pronin, S.V.; Martinez, A.; Kuznedelov, K.; Severinov, K.; Shuman, H.A.; Kozmin, S.A. Chemical synthesis enables biochemical and antibacterial evaluation of streptolydigin antibiotics. J. Am. Chem. Soc. 2011, 133, 12172–12184. [Google Scholar] [CrossRef] [PubMed]
- Ueda, C.; Tateda, K.; Horikawa, M.; Kimura, S.; Ishii, Y.; Nomura, K.; Yamada, K.; Suematsu, T.; Inoue, Y.; Ishiguro, M.; et al. Anti-Clostridium difficile potential of tetramic acid derivatives from Pseudomonas aeruginosa quorum-sensing autoinducers. Antimicrob. Agents Chemother. 2010, 54, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Hurdle, J.G.; Yendapally, R.; Sun, D.; Lee, R.E. Evaluation of analogs of reutericyclin as prospective candidates for treatment of staphylococcal skin infections. Antimicrob. Agents Chemother. 2009, 53, 4028–4031. [Google Scholar] [CrossRef] [PubMed]
- Lowery, C.A.; Park, J.; Gloeckner, C.; Meijler, M.M.; Mueller, R.S.; Boshoff, H.; Ulrich, R.L.; Barry, C.E.; Barlett, D.H.; Kravchenko, V.V.; et al. Defining the mode of action of tetramic acid antibacterials derived from Pseudomonas aeruginosa quorum sensing signals. J. Am. Chem. Soc. 2009, 131, 14473–14479. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-C.; Anwar, M.; Moloney, M.G.; Bikadi, Z.; Hazai, E. Natural product inspired antibacterial tetramic acid libraries with dual enzyme target activity. Chem. Sci. 2013, 4, 1008–1015. [Google Scholar] [CrossRef]
- Jeong, Y.-C.; Moloney, M.G.; Bikadi, Z.; Hazai, E. A detailed study of antibiotic 3-acyltetramic acids and 3-acylpiperidine-2,4-diones. ChemMedChem 2014, 9, 1826–1837. [Google Scholar]
- Jeong, Y.-C.; Moloney, M.G. Synthesis and antibacterial activity of monocyclic 3-carboxamidotetramic acids. Beilstein J. Org. Chem. 2013, 9, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Peukert, S.; Sun, Y.; Zhang, R.; Hurley, B.; Sabio, M.; Shen, X.; Gray, C.; Dzink-Fox, J.; Tao, J.; Cebula, R.; et al. Design and structure–activity relationships of potent and selective inhibitors of undecaprenyl pyrophosphate synthase (UPPS): Tetramic, tetronic acids and dihydropyridin-2-ones. Bioorg. Med. Chem. Lett. 2008, 18, 1840–1844. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.V.; Granda, B.; Dean, K.; Tao, J.; Liu, E.; Zhang, R.; Peukert, S.; Wattanasin, S.; Xie, X.; Ryder, N.S.; et al. Biophysical investigation of the mode of inhibition of tetramic acids, the allosteric inhibitors of undecaprenyl pyrophosphate synthase. Biochemistry 2010, 49, 5366–5376. [Google Scholar] [CrossRef] [PubMed]
- Sinko, W.; Oliveira, C.; Williams, S.; Wynsberghe, A.V.; Durrant, J.D.; Cao, R.; Oldfield, E.; McCammon, J.A. Applying molecular dynamics simulations to identify rarely sampled ligand-bound conformational states of undecaprenyl pyrophosphate synthase, an antibacterial target. Chem. Biol. Drug Des. 2011, 77, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-C.; Anwar, M.; Moloney, M.G.; Bikadi, Z.; Hazai, E. Antibiotic activity and structure-activity relationship study of some 3-enaminetetramic acids. Biorg. Med. Chem. Lett. 2014, 24, 1901–1906. [Google Scholar] [CrossRef]
- Faidallah, H.M.; Khan, K.A. Synthesis and biological evaluation of new barbituric and thiobarbituric acid fluoro analogs of benzenesulfonamides as antidiabetic and antibacterial agents. J. Fluorine Chem. 2012, 142, 96–104. [Google Scholar] [CrossRef]
- Siddiqui, Z.N.; Musthafa, T.N.M.; Ahmad, A.; Khan, A.U. Thermal solvent-free synthesis of novel pyrazolyl chalcones and pyrazolines as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 2011, 21, 2860–2865. [Google Scholar] [CrossRef] [PubMed]
- Laxmi, S.V.; Reddy, Y.T.; Kuarm, B.S.; Reddy, P.N.; Crooks, P.A.; Rajitha, B. Synthesis and evaluation of chromenyl barbiturates and thiobarbiturates as potential antitubercular agents. Bioorg. Med. Chem. Lett. 2011, 21, 4329–4331. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Cao, R.; Chen, W.Y.Z.; Wen, H.; Ma, L.; Song, H. Inhibitory effects of 5-benzylidene barbiturate derivatives on mushroom tyrosinase and their antibacterial activities. Eur. J. Med. Chem. 2009, 44, 4235–4243. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Benmohamed, R.; Kim, J.; Arvanites, A.C.; Morimoto, R.I.; Ferrante, R.J.; Kirsch, D.R.; Silverman, R.B. Pyrimidine-2,4,6-trione derivatives and their inhibition of mutant SOD1-dependent protein aggregation. Toward a treatment for amyotrophic lateral sclerosis. J. Med. Chem. 2011, 54, 2409–2421. [Google Scholar] [CrossRef] [PubMed]
- Jursic, B.S.; Neumann, D.M. Preparation of 5-formyl- and 5-acetylbarbituric acids, including the corresponding Schiff bases and phenylhydrazones. Tetrahedron Lett. 2001, 42, 8435–8439. [Google Scholar] [CrossRef]
- Denoyelle, S.; Chen, T.; Chen, L.; Wang, Y.; Klosi, E.; Haiperin, J.A.; Aktas, B.H.; Chorev, M. In vitro inhibition of translation initiation by N,N’-diarylureas-potential anti-cancer agents. Bioorg. Med. Chem. Lett. 2012, 22, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-C.; Moloney, M.G. Synthesis of and tautomerism in 3-acyltetramic acids. J. Org. Chem. 2011, 76, 1342–1354. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-C.; Anwar, M.; Minh Nguyen, T.; Song Wei Tan, B.; Li Lin Chai, C.; Moloney, M. G. Control of chemoselectivity in Dieckmann ring closures leading to tetramic acids. Org. Biomol. Chem. 2011, 9, 6663–6669. [Google Scholar]
- Jursic, B.S.; Neumann, D.M.; Bowdy, K.L.; Stevens, E.D. Simple, efficient, high yield syntheses of substituted and unsubstituted 5-benzoylbarbituric acids, and their corresponding schiff base phenylhydrazones. J. Heterocycl. Chem. 2004, 41, 233–246. [Google Scholar] [CrossRef]
- Zoorob, H.; Ismail, M.A. Synthesis of 5-(functionalized acyl)-1,3-dialkyl-substituted barbituric and 2-thiobarbituric acids. J. Heterocycl. Chem. 2001, 38, 359–363. [Google Scholar] [CrossRef]
- Singh, P.; Kaur, J.; Bhardwaj, A. Synthesis of highly functionalized barbituric acids and study of their interactions with p-glycoprotein and Mg2+—Potential candidates for multi drug resistance modulation. Eur. J. Med. Chem. 2010, 45, 1256–1262. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.T.; Lima, E.L. Reaction of 1,3-dimethyl-5-acetyl-barbituric acid (DAB) with primary amines. Access to intermediates for selectively protected spermidines. Tetrahedron Lett. 2003, 44, 3621–3624. [Google Scholar] [CrossRef]
- Morgan, L.R.; Jursic, B.S.; Hooper, C.L.; Neumann, D.M.; Thangaraj, K.; LeBlanc, B. Anticancer activity for 4,4’-dihydroxybenzophenone-2,4-dinitrophenylhydrazone (A-007) analogues and their abilities to interact with lymphoendothelial cell surface markers. Bioorg. Med. Chem. Lett. 2002, 12, 3407–3411. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-C.; Moloney, M.G. Tetramic acids as scaffolds: Synthesis, tautomeric and antibacterial behavior. Synlett 2009, 2487–2491. [Google Scholar]
- Holloway, C.A.; Matthews, C.J.; Jeong, Y.-C.; Moloney, M.G.; Roberts, C.F.; Yaqoob, M. Novel chiral skeletons for drug discovery: Antibacterial tetramic acids. Chem. Biol. Drug Des. 2011, 78, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development setting. Adv. Drug Del. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Fernandes, J.; Gattass, R. Topological polar surface area defines substrate transport by multidrug resistance associated protein 1 (MRP1/ABCC1). J. Med. Chem. 2009, 52, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Thorarensen, A.; Li, J.; Wakefield, B.D.; Romero, D.L.; Marotti, K.R.; Sweeney, M.T.; Zurenko, G.E.; Sarver, R.W. Preparation of novel anthranilic acids as antibacterial agents: Extensive evaluation of structural and physical properties on antibacterial activity and human serum albumin affinity. Bioorg. Med. Chem. Lett. 2007, 17, 3113–3116. [Google Scholar]
- Colmenarejo, G.; Pedraglio, A.A.; Lavabdera, J.-L. Cheminformatic models to predict binding affinities to human serum albumin. J. Med. Chem. 2001, 44, 4370–4378. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, R.; Moser, H.E. Physicochemical properties of antibacterial compounds: Implications for drug discovery. J. Med. Chem. 2008, 51, 2871–2878. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Lister, T.; May-Dracka, T.L. Natural products as leads for antibacterial drug discovery. Biorg. Med. Chem. Lett. 2014, 24, 413–418. [Google Scholar] [CrossRef]
- Singh, S.B. Confronting the challenges of discovery of novel antibacterial agents. Biorg. Med. Chem. Lett. 2014, 24, 3683–3689. [Google Scholar] [CrossRef]
- Hofferberth, M.L.; Brückner, R. α- and β-Lipomycin: Total Syntheses by Sequential Stille Couplings and Assignment of the Absolute Configuration of All Stereogenic Centers. Angew. Chem. Int. Ed. 2014, 53, 7328–7334. [Google Scholar] [CrossRef]
- Sokmen, B.B.; Ugras, S.; Sarikaya, H.Y.; Ugras, H.I.; Yanardag, R. Antibacterial, Antiurease, and Antioxidant Activities of Some Arylidene Barbiturates. Appl. Biochem. Biotechnol. 2013, 171, 2030–2039. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).