Study of Coumarin-Resveratrol Hybrids as Potent Antioxidant Compounds
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Antioxidant Capacity Assays
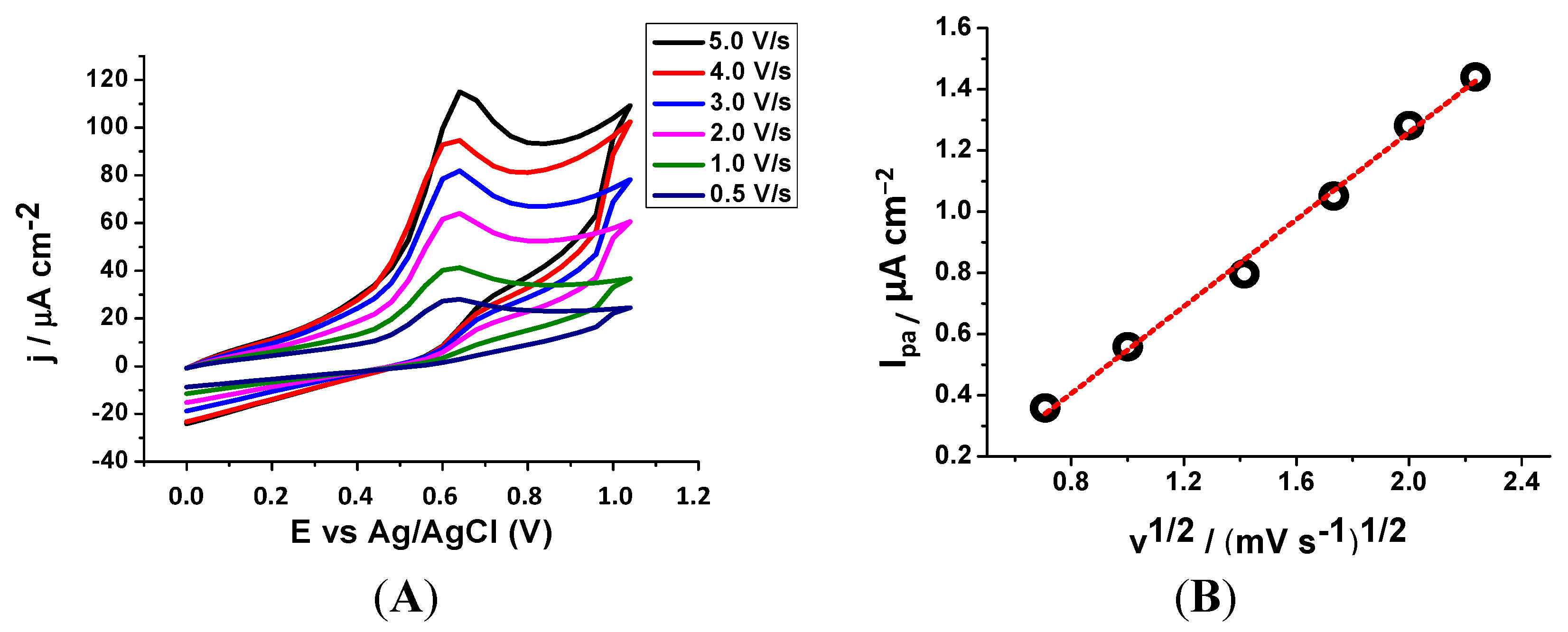
2.2.1. Cyclic Voltammetry (CV)
| Compounds | Epa (V) a |
|---|---|
| 5 | 0.762 |
| 6 | 0.640 |
| 7 | 0.652 |
| 8 | 0.720 |
2.2.2. ORAC-Fluorescein (ORAC-FL)
| Compounds | ORAC-FL Index a |
|---|---|
| 5 | 11.0 ± 2.3 |
| 6 | 9.6 ± 1.3 |
| 7 | 5.7 ± 0.6 |
| 8 | 11.8 ± 1.4 |
| Trolox | 1 |
| Quercetin | 7.28 b |
| 6,7-Dihydroxy-4-methylcoumarin | 3.3 c |

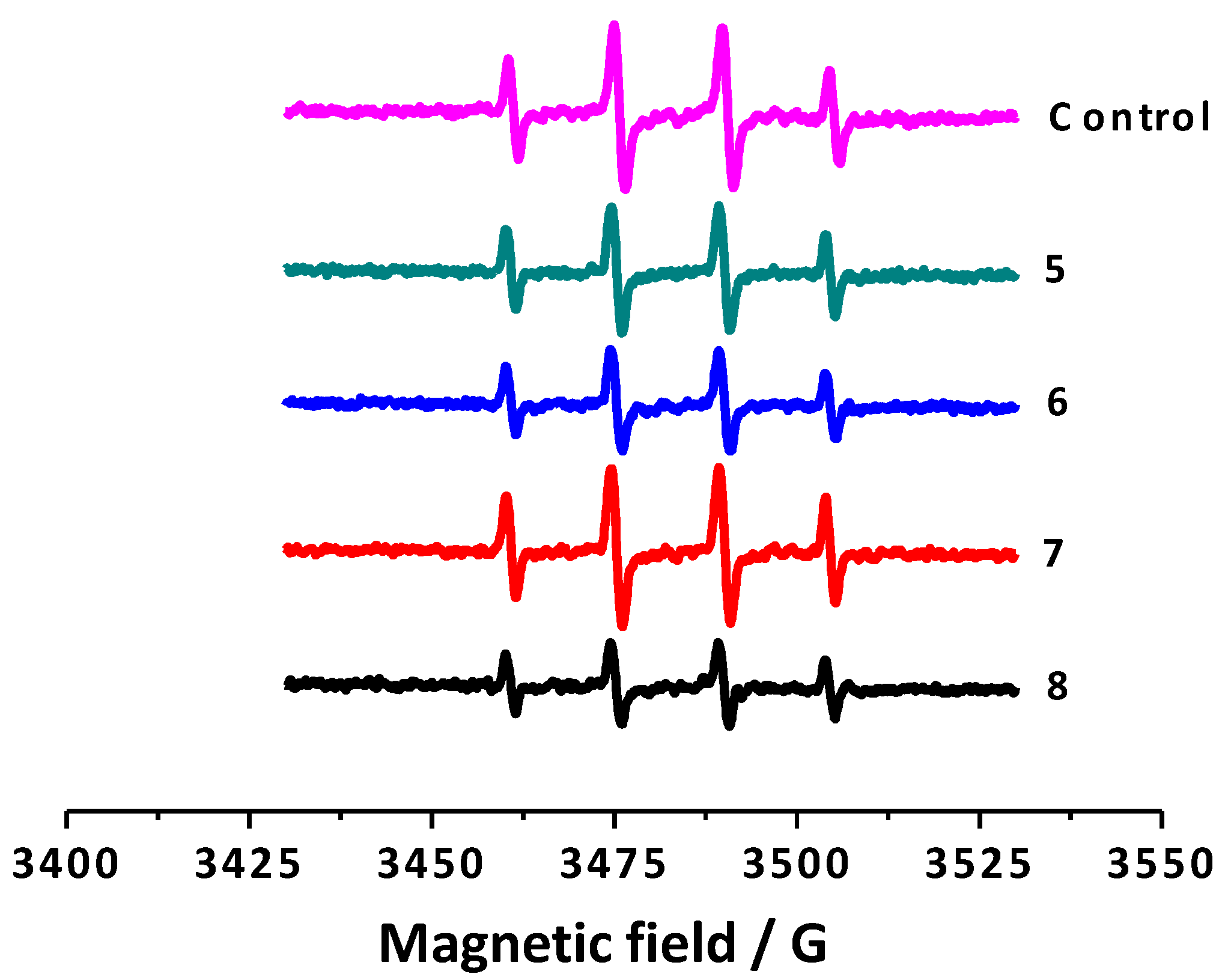
2.2.3. Electron Spin Resonance (ESR)
| Compounds | % Scavenging of Hydroxyl Radicals a | Trolox Index |
|---|---|---|
| 5 | 24.7 ± 6.3 | 2.28 ± 0.05 |
| 6 | 44.7 ± 1.2 | 2.32 ± 0.05 |
| 7 | 10.9 ± 1.1 | 1.23 ± 0.01 |
| 8 | 51.4 ± 4.5 | 2.33 ± 0.05 |
| Trolox | - | 1 |
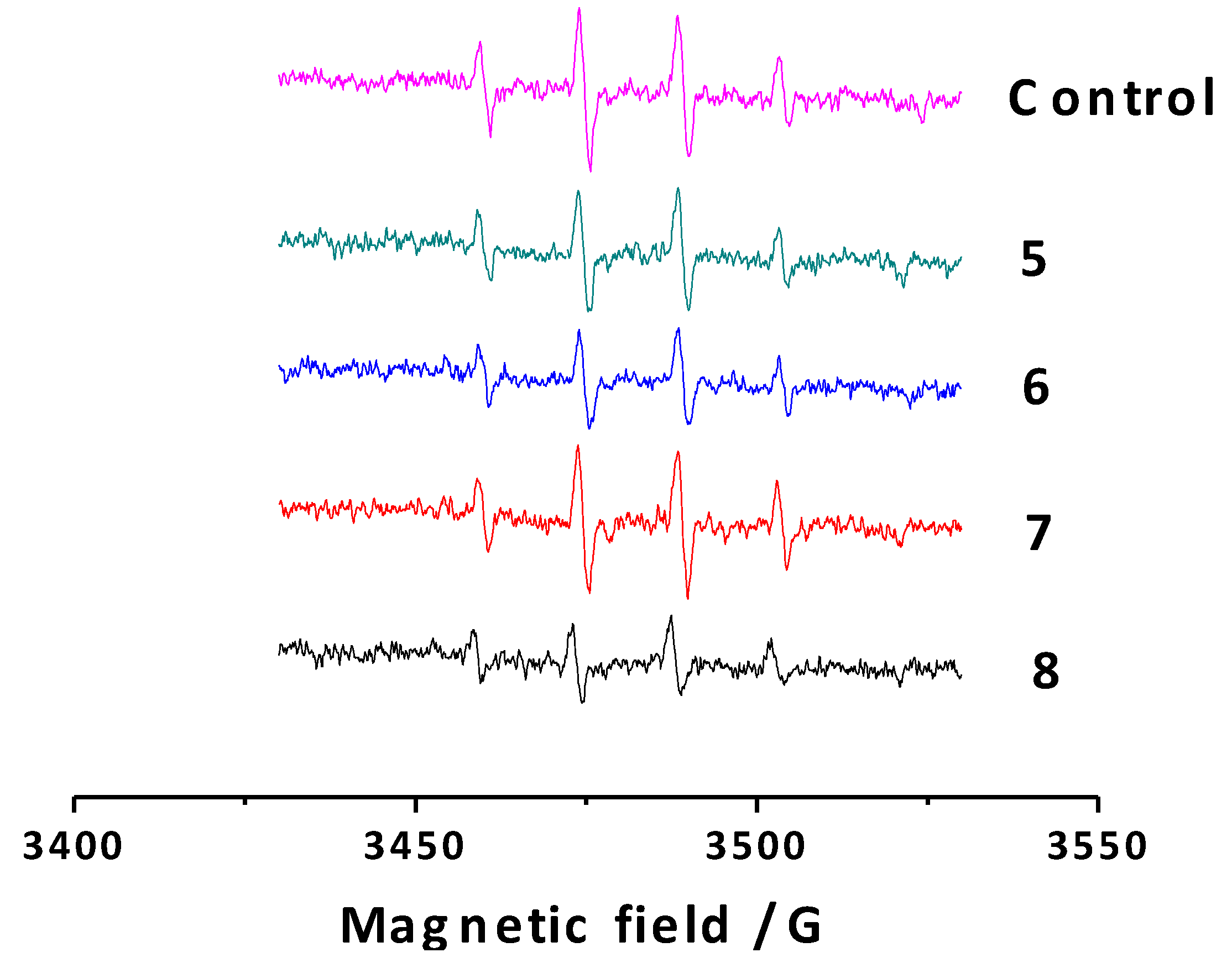
2.2.4. Antioxidant Capacity against Alkoxyl Radicals by ESR
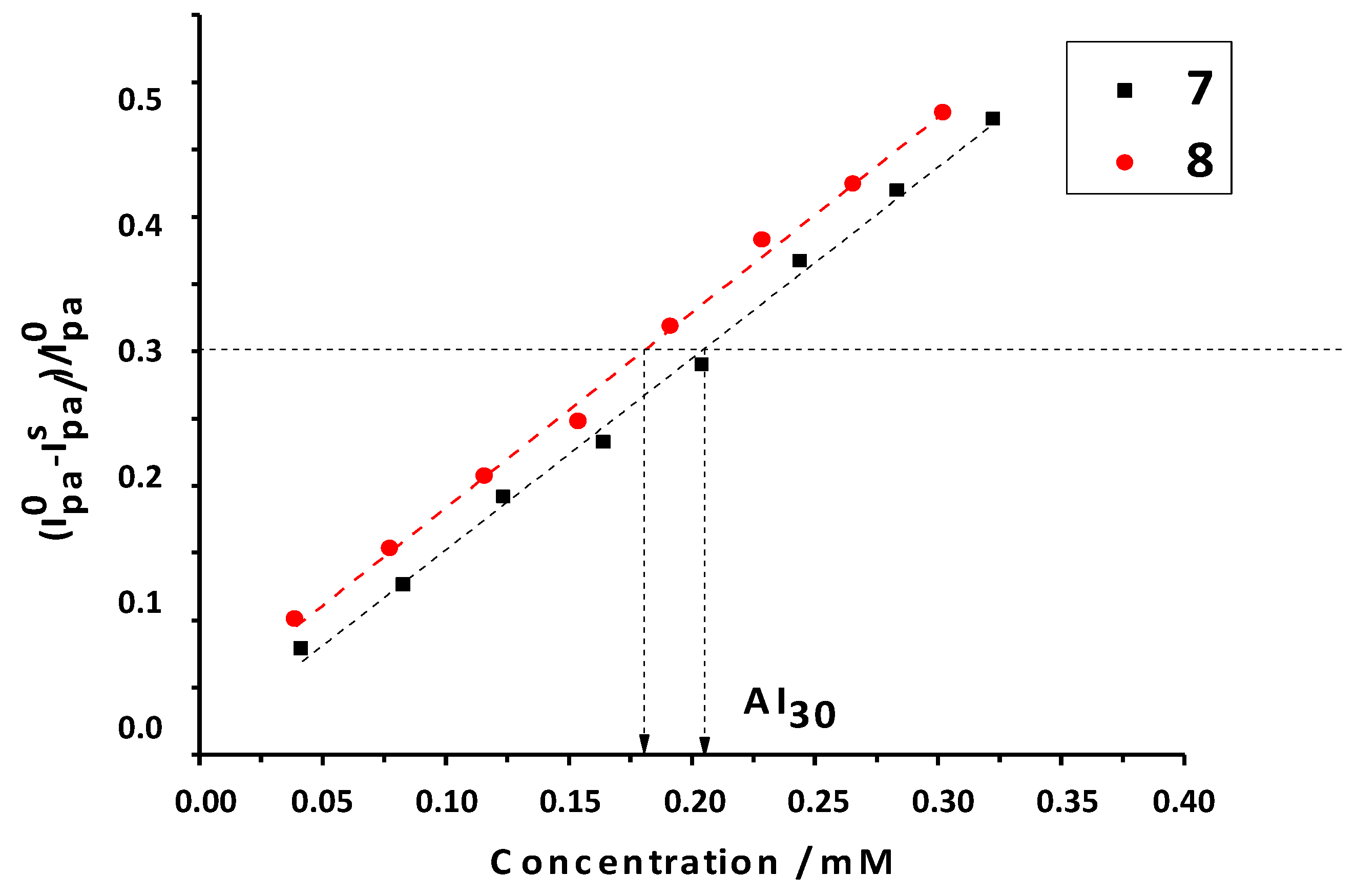
2.2.5. Antioxidant Activity against Superoxide Radicals by CV
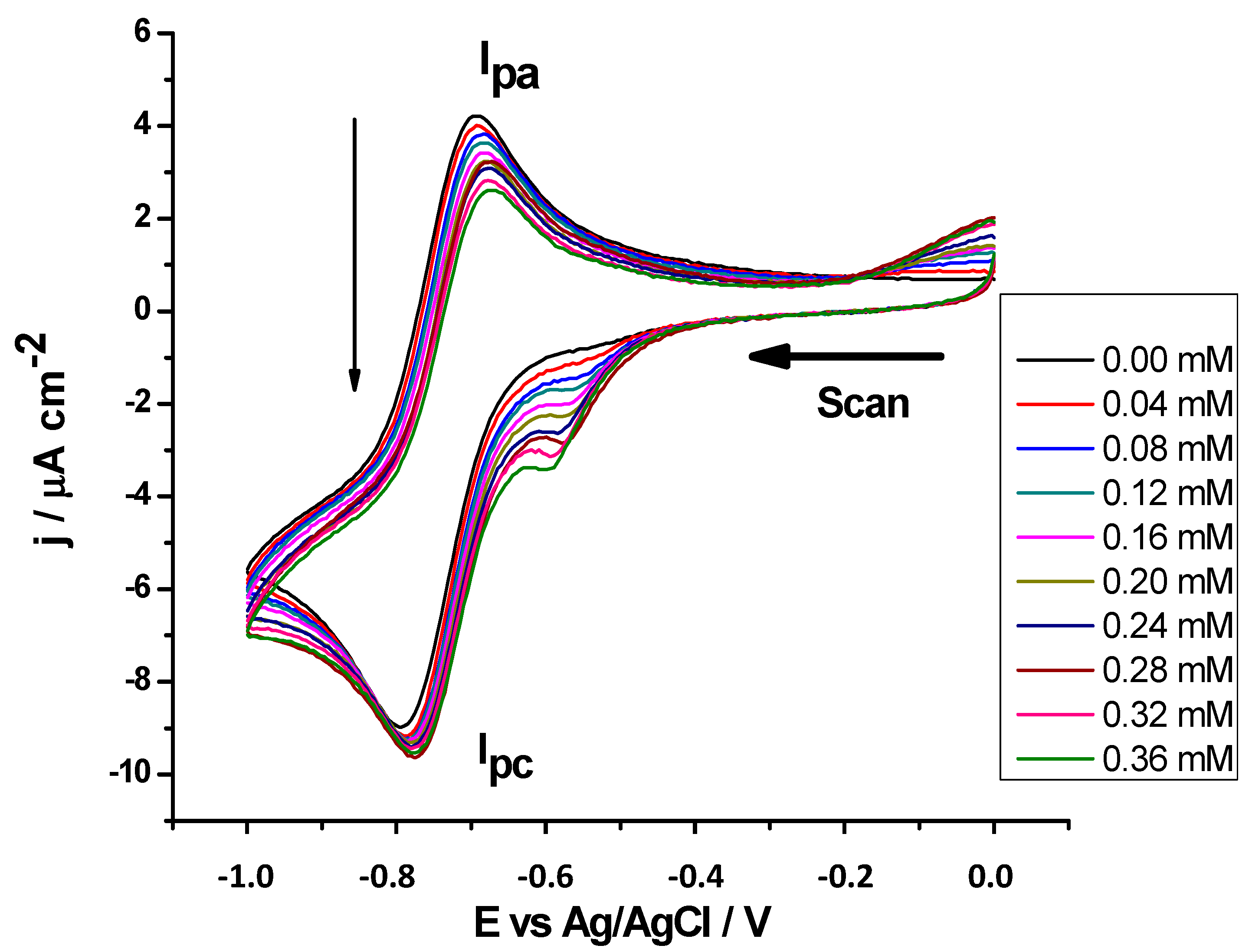
| Compounds | AI30/mM |
|---|---|
| 5 | 0.19 ± 0.02 |
| 6 | 0.23 ± 0.02 |
| 7 | 0.23 ± 0.04 |
| 8 | 0.18 ± 0.04 |
| Trolox | 0.24 ± 0.02 |
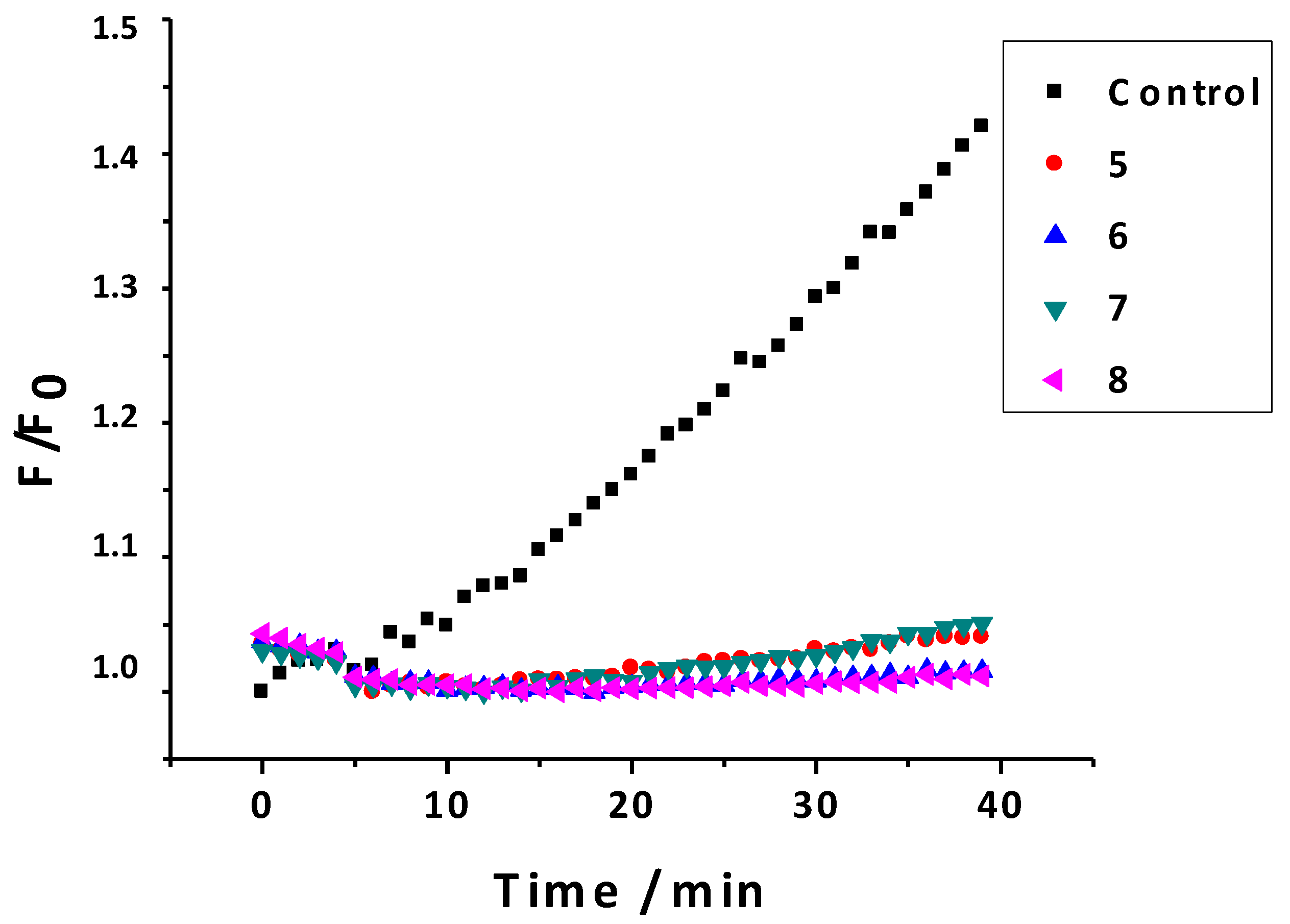
2.2.6. Inhibition of ROS
| Compounds | % Disappearance of Fluorescence |
|---|---|
| 5 | 92.57 ± 0.09 |
| 6 | 92.52 ± 0.03 |
| 7 | 42.88 ± 0.30 |
| 8 | 88.69 ± 0.08 |
| Trolox | 1.48 ± 0.01 |
2.3. ADME Theoretical Properties Calculation
| Compd. | logP | TPSA (Å2) | n-OH Acceptors | n-OHNH Donors | Volume (Å3) |
|---|---|---|---|---|---|
| 5 | 3.23 | 50.44 | 3 | 1 | 208.01 |
| 6 | 3.23 | 50.44 | 3 | 1 | 208.01 |
| 7 | 3.23 | 50.44 | 3 | 1 | 208.01 |
| 8 | 2.73 | 70.67 | 4 | 2 | 216.03 |
3. Experimental Section
3.1. Synthesis
3.2. Antioxidant Assays
3.2.1. Oxidation Potential Determination by Cyclic Voltammetry (CV)
3.2.2. Oxygen Radical Antioxidant Capacity-Fluorescein (ORAC-FL)
3.2.3. Hydroxyl Radical Scavenging Assay Using Electron Spin Resonance (ESR)
3.2.4. Determination of Alcoxyl Radicals Generated by Photolysis of AAPH Assay Using Electron Spin Resonance (ESR)
3.2.5. Superoxide Antioxidant Assay Using Cyclic Voltammetry (CV)
3.3. Statistical Data Analysis
3.4. Inhibition of Radical Oxygen Species (ROS)
3.5. Theoretical Evaluation of ADME Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guardado-Yordi, E.; Pérez-Molina, E.; Matos, M.J.; Uriarte, E. Antioxidant and pro-oxidant effects of polyphenolic compounds and structure-activity relationship evidence. In Nutrition, Well-Being and Health; Bouayed, J., Bohn, T., Eds.; InTech: Rijeka, Croatia, 2012; Chapter 2; pp. 23–48. [Google Scholar]
- Tyagi, Y.K.; Kumar, A.; Raj, H.G.; Vohra, P.; Gupta, G.; Kumari, R.; Kumar, P.; Gupta, R.K. Synthesis of novel amino and acetyl amino-4-methylcoumarins and evaluation of their antioxidant activity. Eur. J. Med. Chem. 2005, 40, 413–420. [Google Scholar] [CrossRef]
- Hamdi, N.; Puerta, C.; Valerga, P. Synthesis, structure, antimicrobial and antioxidant investigations of dicoumarol and related compounds. Eur. J. Med. Chem. 2008, 43, 2541–2548. [Google Scholar] [CrossRef] [PubMed]
- Panteleon, V.; Kostakis, I.K.; Marakos, P.; Pouli, N.; Andreado, I. Synthesis and free radical scavenging activity of some new spiropyranocoumarins. Bioorg. Med. Chem. Lett. 2008, 18, 5781–5784. [Google Scholar] [CrossRef] [PubMed]
- Symeonidis, T.; Chamilos, M.; Hadjipavlou-Litina, D.J.; Kallitsakis, M.; Litinas, K.E. Synthesis of hydroxycoumarins and hydroxybenzo[f]- or [h]coumarins as lipid peroxidation inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 1139–1142. [Google Scholar] [CrossRef]
- Fylaktakidou, K.C.; Hadjipavlou-Litina, D.J.; Litinas, K.; Nicolaides, D.N. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr. Pharm. Des. 2004, 10, 3813–3833. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I. Synthetic and natural coumarins as antioxidants. Mini Rev. Med. Chem. 2006, 6, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Tabart, J.; Kevers, C.; Pincelmail, J.; Defraigne, J.-O.; Dommes, J. Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chem. 2009, 113, 1226–1233. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple coumarins and analogues in medicinal chemistry: Occurrence, synthesis and biological activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef] [PubMed]
- Borges, F.; Roleira, F.; Milhazes, N.; Uriarte, E.; Santana, L. Simple coumarins: Privileged scaffolds in medicinal chemistry. Front. Med. Chem. 2009, 4, 23–85. [Google Scholar]
- Matos, M.J.; Terán, C.; Pérez-Castillo, Y.; Uriarte, E.; Santana, L.; Viña, D. Synthesis and study of a series of 3-arylcoumarins as potent and selective monoamine oxidase B inhibitors. J. Med. Chem. 2011, 54, 7127–7137. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J.; Viña, D.; Vázquez-Rodríguez, S.; Uriarte, E.; Santana, L. Focusing on new monoamine oxidase inhibitors: Differently substituted coumarins as an interesting scaffold. Curr. Top. Med. Chem. 2012, 12, 2210–2239. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cruz, F.; Serra, S.; Delogu, G.; Lapier, M.; Maya, J.D.; Olea-Azar, C.; Santana, L.; Uriarte, E. Antitrypanosomal and antioxidant properties of 4-hydroxycoumarins derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 5569–5573. [Google Scholar] [CrossRef] [PubMed]
- Janeiro, P.; Matos, M.J.; Santana, L.; Uriarte, E.; Oliveira-Brett, A.M. New hydroxylated 3-arylcoumarins, synthesis and electrochemical study. J. Electroanal. Chem. 2013, 689, 243–251. [Google Scholar] [CrossRef]
- Matos, M.J.; Pérez-Cruz, F.; Vazquez-Rodriguez, S.; Uriarte, E.; Santana, L.; Borges, F.; Olea-Azar, C. Remarkable antioxidant properties of a series of hydroxy-3-arylcoumarins. Bioorg. Med. Chem. 2013, 21, 3900–3906. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Cruz, F.; Vazquez-Rodriguez, S.; Matos, M.J.; Herrera-Morales, A.; Villamena, F.A.; Das, A.; Gopalakrishnan, B.; Olea-Azar, C.; Santana, L.; Uriarte, E. Synthesis and electrochemical and biological studies of novel coumarin-chalcone hybrid compounds. J. Med. Chem. 2013, 56, 6136–6145. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Rodriguez, S.; Figueroa-Guíñez, R.; Matos, M.J.; Santana, L.; Uriarte, E.; Lapier, M.; Maya, J.D.; Olea-Azar, C. Synthesis of coumarin-chalcone hybrids and evaluation of their antioxidant and trypanocidal properties. Med. Chem. Commun. 2013, 4, 993–1000. [Google Scholar] [CrossRef]
- Orallo, F. trans-Resveratrol: A magical elixir of eternal youth? Curr. Med. Chem. 2008, 15, 1887–1898. [Google Scholar] [CrossRef] [PubMed]
- Krishnaswamy, N.R.; Seshadri, T.R.; Sharma, B.R. Study of partial demethylation of some polymethoxy-3-phenylcoumarins and preparation of some new members. Indian J. Chem. 1966, 4, 120–126. [Google Scholar]
- Dey, B.B.; Row, K.K. The reactivity of the methylene group in coumarin-4-acetic acids and their esters. Condensation with salicylaldehyde to 4:3'-dicoumaryls. J. Indian Chem. Soc. 1924, 1, 107–124. [Google Scholar]
- Baker, W. 7-Hydroxy-3-phenylcoumarin. J. Chem. Soc. 1927, 2898–2899. [Google Scholar]
- Richtzenhain, H.; Alfredsson, B. cis- and trans-2,3-Dimethoxy-α-phenylcinnamic acid. Acta Chem. Scand. 1953, 7, 1173–1175. [Google Scholar] [CrossRef] [Green Version]
- Walker, G.N. Reduction of phenols. New synthesis of oxyhexahydro-3-oxophenanthrenes by cyclodehydration of 4-(β-arylethyl)-1,3-cyclohexanediones. J. Am. Chem. Soc. 1958, 80, 645–652. [Google Scholar] [CrossRef]
- Kirkiacharian, S.; Lormier, A.T.; Resche-Rigon, M.; Bouchoux, F.; Cerede, E. Synthesis and binding affinity of 3-aryl-7-hydroxycoumarins to human alpha and beta estrogen receptors. Ann. Pharm. Francaises 2003, 61, 51–56. [Google Scholar]
- Kabeya, L.M.; Marchi, A.A.; Kanashiro, A.; Lopes, N.P.; Silva, C.H.T.P.; Pupo, M.T.; Lucisano-Valima, Y.M. Inhibition of horseradish peroxidase catalytic activity by new 3-phenylcoumarin derivatives: Synthesis and structure-activity relationships. Bioorg. Med. Chem. 2007, 15, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Hotta, H.; Sakamoto, H.; Nagano, S.; Osakai, T.; Tsujino, Y. Unusually large numbers of electrons for the oxidation of polyphenolic antioxidants. Biochim. Biophys. Acta 2001, 1526, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic. Biol. Med. 2010, 49, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cruz, F.; Montecinos, R.; Villamena, F.A.; Das, A.; Uriarte, E.; Lopez-Alarcón, C.; Olea-Azar, C. Protective effect of synthetic hydroxycoumarin derivatives on bovine aortic endothelial cells against oxidative stress induced by 3-morpholinosydnonimine and hydrogen peroxide. Free Radic. Biol. Med. 2012, 53, S115. [Google Scholar] [CrossRef]
- Bisby, R.H.; Brooke, R.; Navaratnam, S. Effect of antioxidant oxidation potential in the oxygen radical absorption capacity (ORAC) assay. Food Chem. 2008, 108, 1002–1007. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Inomata, T.; Nakazawa, H.; Kubo, H.; Yamaguchi, F.; Ariga, T. Evaluation of free radical scavenging activities of antioxidants with an H2O2/NaOH/DMSO system by electron spin resonance. J. Agric. Food Chem. 1999, 47, 4653–4656. [Google Scholar] [CrossRef] [PubMed]
- Endo, N.; Oowada, S.; Sueishi, Y.; Shimmei, M.; Makino, K.; Fujii, H.; Kotake, Y. Serum hydroxyl radical scavenging capacity as quantified with iron-free hydroxyl radical source. J. Clin. Biochem. Nutr. 2009, 45, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Matsuda, E.; Masuda, Y.; Sameshima, H.; Ikenoue, T. Characteristics of the spin-trapping reaction of a free radical derived from AAPH: Further development of the ORAC-ESR assay. Anal. Bioanal. Chem. 2012, 403, 1961–1970. [Google Scholar] [CrossRef] [PubMed]
- Sueishi, Y.; Ishikawa, M.; Yoshioka, D.; Endoh, N.; Oowada, S.; Shimmei, M.; Fujii, H.; Kotake, Y. Oxygen radical absorbance capacity (ORAC) of cyclodextrin-solubilized flavonoids, resveratrol and astaxanthin as measured with the ORAC-EPR method. J. Clin. Biochem. Nutr. 2012, 50, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Kohri, S.; Fujii, H.; Oowada, S.; Endoh, N.; Sueishi, Y.; Kusakabe, M.; Shimmei, M.; Kotake, Y. An oxygen radical absorbance capacity-like assay that directly quantifies the antioxidant’s scavenging capacity against AAPH-derived free radicals. Anal. Biochem. 2009, 386, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Kadiiska, M.; Guo, Q.; Mason, R. A novel protocol to identify and quantify all spin trapped free radicals from in vitro/in vivo interaction of HO• and DMSO: LC/ESR, LC/MS, and dual spin trapping combinations. Free Radic. Biol. Med. 2005, 38, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Shakeel, F. Antioxidant activity coefficient, mechanism, and kinetics of different derivatives of flavones and flavanones towards superoxide radical. Czech J. Food Sci. 2012, 30, 153–163. [Google Scholar]
- Le Bourvellec, C.; Hauchard, D.; Darchen, A.; Burgota, J.L.; Abasqa, M.L. Validation of a new method using the reactivity of electrogenerated superoxide radical in the antioxidant capacity determination of flavonoids. Talanta 2008, 75, 1098–1103. [Google Scholar] [CrossRef] [PubMed]
- Korotkova, E.I.; Karbainov, Y.A.; Shevchuk, A.V. Antioxidant activity coefficients for plant aquatic-alcohol extracts. J. Electroanal. Chem. 2002, 518, 56–60. [Google Scholar] [CrossRef]
- Korotkova, E.I.; Karbainov, Y.A.; Avramchik, O.A. Investigation of antioxidant and catalytic properties of some biologically active substances by voltammetry. Anal. Bioanal. Chem. 2003, 375, 465–468. [Google Scholar] [PubMed]
- Criddle, D.N.; Gillies, S.; Baumgartner-Wilson, H.K.; Jaffar, M.; Chinje, E.C.; Passmore, S.; Chvanov, M.; Barrow, S.; Gerasimenko, O.V.; Tepikin, A.V.; et al. Menadione-induced reactive oxygen species generation via redox cycling promotes apoptosis of murine pancreatic acinar cells. J. Biol. Chem. 2006, 281, 40485–40492. [Google Scholar] [CrossRef] [PubMed]
- Molinspiration Cheminformatics. Available online: http://www.molinspiration.com/services/properties.html (accessed on 15 December 2014).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Toward minimalistic modeling of oral drug absorption. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Wardman, P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: Progress, pitfalls, and prospects. Free Rad. Bio. Med. 2007, 43, 995–1022. [Google Scholar] [CrossRef]
- Roginsky, V.; Lissi, E. Review of methods to determine chain-breaking antioxidant activity in food. Food Chem. 2005, 92, 235–254. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef] [PubMed]
- Tsushima, M.; Tokuda, K.; Ohsaka, T. Use of hydrodynamic chronocoulometry for simultaneous determination of diffusion coefficients and concentrations of dioxygen in various media. Anal. Chem. 1994, 66, 4551–4556. [Google Scholar] [CrossRef]
- Ahmed, S.; Shakeel, F. Voltammetric determination of antioxidant character in Berberis lycium Royel, Zanthoxylum armatum and Morus nigra Linn plants. Pak. J. Pharm. Sci. 2012, 25, 501–507. [Google Scholar] [PubMed]
- Alves, C.Q.; David, J.M. Métodos para determinação de atividade antioxidante in vitro em substratos orgânicos. Quim. Nova 2010, 33, 2202–2210. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, M.J.; Mura, F.; Vazquez-Rodriguez, S.; Borges, F.; Santana, L.; Uriarte, E.; Olea-Azar, C. Study of Coumarin-Resveratrol Hybrids as Potent Antioxidant Compounds. Molecules 2015, 20, 3290-3308. https://doi.org/10.3390/molecules20023290
Matos MJ, Mura F, Vazquez-Rodriguez S, Borges F, Santana L, Uriarte E, Olea-Azar C. Study of Coumarin-Resveratrol Hybrids as Potent Antioxidant Compounds. Molecules. 2015; 20(2):3290-3308. https://doi.org/10.3390/molecules20023290
Chicago/Turabian StyleMatos, Maria J., Francisco Mura, Saleta Vazquez-Rodriguez, Fernanda Borges, Lourdes Santana, Eugenio Uriarte, and Claudio Olea-Azar. 2015. "Study of Coumarin-Resveratrol Hybrids as Potent Antioxidant Compounds" Molecules 20, no. 2: 3290-3308. https://doi.org/10.3390/molecules20023290
APA StyleMatos, M. J., Mura, F., Vazquez-Rodriguez, S., Borges, F., Santana, L., Uriarte, E., & Olea-Azar, C. (2015). Study of Coumarin-Resveratrol Hybrids as Potent Antioxidant Compounds. Molecules, 20(2), 3290-3308. https://doi.org/10.3390/molecules20023290









