Antimicrobial and Seasonal Evaluation of the Carvacrol-Chemotype Oil from Lippia origanoides Kunth.
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Seasonal Variation in Oil Yield
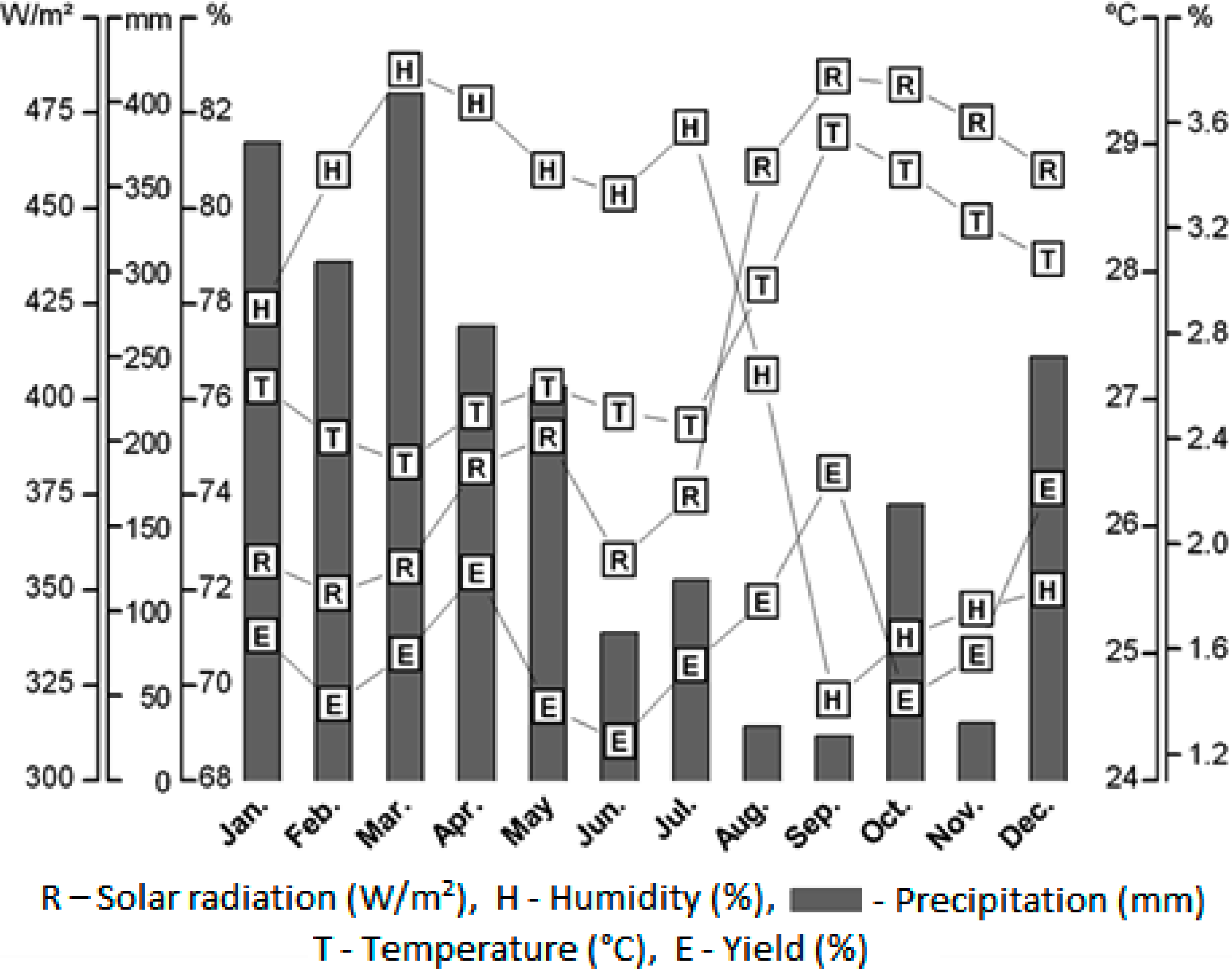
| Environmental Factors | Correlation Coefficient (r2) |
|---|---|
| Temperature (°C) | 0.49 * |
| Air relative humidity (%) | −0.49 * |
| Radiation (W/m²) | 0.51 ** |
2.2. Effect of Seasonal Variation in Oil Composition
| Month/Oil Yield | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1, 7 * | 1, 4 * | 1, 6 * | 1, 9 * | 1, 4 * | 1, 3 * | 1, 5 | 1, 8 | 2, 3 | 1, 4 | 1, 6 * | 2, 2 * | |||
| Constituents | RICalc. | RILit. | Oil % | |||||||||||
| (Z)-Hexen-3-ol | 854 | 859 | - | 0.2 | 0.4 | - | 0.2 | 0.2 | 0.2 | 0.3 | 0.3 | - | - | 0.3 |
| α-Thujene | 926 | 924 | 0.7 | 0.6 | 0.7 | 0.7 | 0.5 | 0.6 | 0.8 | 0.7 | 0.7 | 0.8 | 0.9 | 0.6 |
| α-Pinene | 934 | 932 | 0.6 | 0.4 | 0.4 | 0.4 | 0.3 | 0.4 | 0.4 | 0.5 | 0.5 | 0.6 | 0.7 | 0.5 |
| 1-Octen-3-ol | 976 | 974 | 0.1 | 0.2 | 0.3 | 0.1 | 0.3 | 0.1 | 0.2 | 0.2 | - | - | - | - |
| β-Pinene | 978 | 976 | 0.2 | 0.2 | 0.1 | - | 0.1 | - | - | 0.2 | 0.2 | 0.2 | 0.3 | 0.2 |
| Myrcene | 990 | 988 | 1.3 | 1.2 | 1.3 | 1.3 | 1.0 | 1.1 | 1.2 | 1.1 | 1.2 | 1.5 | 1.5 | 1.1 |
| α-Terpinene | 1016 | 1014 | 0.7 | 0.8 | 0.9 | 0.9 | 0.7 | 0.5 | 0.7 | 0.7 | 0.5 | 0.7 | 0.8 | 0.7 |
| p-Cymene | 1025 | 1020 | 11.2 | 9.6 | 10.5 | 10.2 | 8.5 | 9.7 | 9.6 | 7.9 | 9.5 | 11.8 | 11.5 | 8.9 |
| Limonene | 1026 | 1024 | 0.3 | 0.2 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 |
| 1,8-Cineol | 1032 | 1026 | 0.9 | 0.9 | 0.9 | 0.7 | 0.8 | 1.3 | 0.7 | 0.6 | 1.0 | 0.8 | 1.1 | 0.9 |
| γ-Terpinene | 1056 | 1054 | 1.3 | 2.0 | 1.6 | 1.7 | 1.7 | 0.2 | 1.7 | 1.5 | 0.1 | 1.9 | 1.1 | 0.8 |
| Linalool | 1098 | 1095 | 3.9 | 3.8 | 3.5 | 2.8 | 3.7 | 2.9 | 2.8 | 2.9 | 2.5 | 2.4 | 3.1 | 2.6 |
| Ipsdienol | 1144 | 1140 | 0.2 | 0.2 | - | - | 0.1 | - | - | 0.1 | - | - | - | - |
| Umbellulone | 1169 | 1167 | 0.1 | 0.3 | 0.2 | 0.3 | 0.3 | 0.3 | 0.4 | 0.5 | 0.1 | 0.3 | 0.3 | |
| Terpinen-4-ol | 1176 | 1174 | 1.1 | 1.1 | 1.3 | 0.9 | 1.1 | 1 | 0.9 | 0.9 | 0.8 | 1.0 | 0.9 | 0.7 |
| Thymol methyl ether | 1234 | 1232 | 2.2 | 2 | 1.8 | 1.4 | 1.7 | 1.3 | 1.6 | 2.0 | 1.4 | 2.1 | 1.8 | 1.3 |
| Thymol isomer (MW = 150) | 1282 | - | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | - | - | - | - | - |
| Thymol | 1292 | 1289 | 9.0 | 10.3 | 12.5 | 11.7 | 11.7 | 12.8 | 11.8 | 11.5 | 9.8 | 9.3 | 8.2 | 9.2 |
| Carvacrol | 1299 | 1298 | 40.7 | 43.4 | 46.4 | 46.6 | 45.5 | 47.2 | 46.1 | 40.5 | 37.7 | 41.2 | 35.9 | 38.3 |
| Thymol acetate | 1351 | 1349 | 0.8 | 0.8 | 0.4 | 0.3 | 0.5 | 0.4 | 0.6 | 0.7 | 0.6 | 0.6 | 0.9 | 0.6 |
| Carvacrol acetate | 1372 | 1370 | 1.7 | 1.6 | 0.6 | 0.6 | 0.8 | 0.6 | 1.1 | 1.3 | 0.9 | 1.1 | 1.8 | 1.1 |
| Geranyl acetate | 1382 | 1379 | 0.4 | 0.4 | 0.3 | 0.3 | 0.4 | 0.6 | 0.4 | 0.6 | 0.6 | 0.5 | 0.7 | 0.8 |
| (E)-Caryophyllene | 1418 | 1416 | 3.4 | 3.5 | 2.5 | 2.6 | 3.4 | 2.3 | 2.1 | 2.8 | 2.7 | 2.9 | 4.8 | 4.6 |
| trans-α-Bergamotene | 1434 | 1432 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.1 | 0.2 | 0.2 | - | 0.2 | 0.2 |
| α-Humulene | 1455 | 1452 | 0.4 | 0.4 | 0.3 | 0.3 | 0.4 | 0.3 | 0.2 | 0.3 | 0.3 | 0.3 | 0.5 | 0.5 |
| p-Methoxythymol | 1487 | 1484 | 8.8 | 8.4 | 7.3 | 10.1 | 8.8 | 7.4 | 8.4 | 10.1 | 12.8 | 10.4 | 13.3 | 14.1 |
| β-Bisabolene | 1506 | 1505 | 0.3 | 0.4 | 0.3 | 0.3 | 0.4 | 0.3 | 0.2 | 0.4 | 0.4 | 0.3 | 0.4 | 0.5 |
| (Z)-α-Bisabolene | 1508 | 1506 | - | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | - | 0.3 | 0.4 | 0.6 | 0.5 | 0.3 |
| δ-Cadinene | 1524 | 1522 | - | - | - | - | 0.1 | - | - | - | - | - | 0.2 | 0.2 |
| trans-γ-Bisabolene | 1531 | 1529 | 0.4 | 0.5 | 0.4 | 0.5 | 0.6 | - | 0.5 | - | - | - | - | 0.7 |
| p-Methoxycarvacrol (tent.) | 1555 | - | 2.8 | 2.5 | 1.1 | 1.2 | 1.6 | 1.3 | 1.6 | 2.3 | 2.7 | 2 | 3.7 | 3.1 |
| Caryophyllene oxide | 1584 | 1582 | 3 | 1.8 | 1.3 | 1.6 | 1.7 | 1.6 | 2.2 | 2.8 | 2.8 | 3.6 | 2 | 1.9 |
| 2-phenylethyl tyglate | 1587 | 1584 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.3 | 0.3 | 0.2 | 0.3 | - |
| Humulene epoxide II | 1611 | 1608 | - | - | - | - | 0.2 | 0.2 | 0.2 | 0.3 | 0.3 | - | - | - |
| α-Cadinol | 1655 | 1652 | 0.2 | - | - | - | 0.1 | - | - | 0.1 | 0.1 | - | 0.2 | 0.2 |
| α-Eudesmol | 1656 | 1653 | - | - | - | - | 0.1 | 0.1 | - | 0.1 | 0.2 | - | 0.2 | 0.2 |
| α-Bisabolol | 1686 | 1685 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | - | 0.2 | 0.3 |
| Unidentified sesquiterpenes | - | - | 2.1 | 0.5 | 0.8 | 0.5 | 0.7 | 1.2 | 1.3 | 3.7 | 3.8 | 2.1 | 1.4 | 2.5 |
| Total | - | - | 97.8 | 99.0 | 98.8 | 99.0 | 98.7 | 96.1 | 97.7 | 93.1 | 92.0 | 96.1 | 98.2 | 95.9 |
2.3. Antimicrobial Activity of the Oil
| Bacteria | Concentration (µL/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Set | Oct | Nov | Dec | ||
| MIC | Staphylococcus aureus | 1.25 | 1.25 | 2.25 | 1.25 | 2.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 |
| Escherichia coli | 0.31 | 0.31 | 0.15 | 0.31 | 0.15 | 0.15 | 0.15 | 0.31 | 0.15 | 0.15 | 0.31 | 0.31 | |
| MBC | Staphylococcus aureus | 2.25 | 2.25 | 2.25 | 2.25 | 2.25 | 2.25 | 2.25 | 2.25 | 2.25 | 2.25 | 2.25 | 2.25 |
| Escherichia coli | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | |
| Bacteria (MIC) | Correlation Coefficient (r2) | |
|---|---|---|
| Carvacrol (%) | Thymol (%) | |
| Escherichia coli | −0.411 * | −0.455 * |
| Staphylococcus aureus | 0.413 * | 0.442 * |
3. Experimental Section
3.1. Plant Material
3.2. Climatic Data
3.3. Plant Processing and Extraction of the Essential Oils
3.4. Oil-Composition Analysis
3.5. Antimicrobial Bioassay
3.5.1. Microorganisms and Inoculum Standardization
3.5.2. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pascual, M.E.; Slowing, K.; Carretero, E.; Mata, D.S.; Villar, A. Lippia: Traditional uses, chemistry and pharmacology: A review. J. Ethnopharmacol. 2001, 76, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.J.B.; Lopes, J.A.D.; Cito, A.M.G.L.; Oliveira, E.H.; Lima, S.G.; Reis, F.A.M. Composition and biological activity of essential oil from Lippia origanoides HBK. J. Essent. Oil Res. 2004, 16, 504–506. [Google Scholar] [CrossRef]
- Oliveira, D.R.; Leitão, G.G.; Bizzo, H.R.; Lopes, D.; Alviano, D.S.; Alviano, C.S.; Leitão, S.G. Chemical and antimicrobial analyses of essential oil of Lippia origanoides H.B.K. Food Chem. 2007, 101, 236–240. [Google Scholar] [CrossRef]
- Viljoen, A.; van Vuuren, S.; Ernst, E.; Klepser, M.; Demirci, B.; Başer, H.; van Wyk, B.-E. Osmitopsis asteriscoides (Asteraceae)—The antimicrobial activity and essential oil composition of a Cape-Dutch remedy. J. Ethnopharmacol. 2003, 88, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.H.A.; Alves, C.N.; Guimarães, E.F.; Carreira, L.M.M.; Maia, J.G.S. Variability in essential oil composition of Piper dilatatum L.C. Rich. Biochem. Syst. Ecol. 2011, 39, 669–675. [Google Scholar]
- Hussain, A.I.; Anwar, F.; Nigam, P.S.; Ashraf, M.; Gilani, A.H. Seasonal variation in content, chemical composition and antimicrobial and cytotoxic activities of essential oils from four Mentha species. J. Sci. Food Agric. 2010, 90, 1827–1836. [Google Scholar] [PubMed]
- Stashenko, E.E.; Martínez, J.R.; Ruíz, C.A.; Arias, G.; Durán, C.; Salgar, W.; Cala, M. Lippia origanoides chemotype differentiation based on essential oil GC-MS and principal component analysis. J. Sep. Sci. 2010, 33, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.; Morales, A.; Pascuale, S.; Márquez, A.; Rondon, R.; Mathé, I.; Verés, K. Comparative study of the chemical composition of the essential oil of Lippia oreganoides collected in two different seasons of the year in Venezuela. Nat. Prod. Commun. 2006, 1, 205–207. [Google Scholar]
- Silva, N.A.; da Silva, J.K.R.; Andrade, E.H.A.; Carreira, L.M.M.; Sousa, P.J.C.; Maia, J.G.S. Essential oil composition and antioxidant capacity of Lippia schomburgkiana. Nat. Prod. Commun. 2009, 4, 1281–1286. [Google Scholar] [PubMed]
- Ribeiro, A.F.; Andrade, E.H.A.; Salimena, F.R.G.; Maia, J.G.S. Circadian and seasonal study of the cynnamate chemotype from Lippia origanoides Kunth. Biochem. Syst. Ecol. 2014, 55, 249–259. [Google Scholar]
- Velasco, J.; Rojas, J.; Salazar, P.; Rodríguez, M.; Díaz, T.; Morales, A.; Rondón, M. Antibacterial activity of the essential oil of Lippia oreganoides against multiresistant bacterial strains of nosocomial origin. Nat. Prod. Commun. 2007, 2, 85–88. [Google Scholar]
- Tangarife-Castaňo, V.; Correa-Royero, J.; Zapata-Londoňo, B.; Durán, C.; Stashenko, E.E.; Mesa-Arango, A.C. Anti-Candida albicans activity, cytotoxicity and interaction with antifungal drugs of essential oils and extracts from aromatic and medicinal plants. Infectio 2011, 15, 160–167. [Google Scholar] [CrossRef]
- Betancur-Galvis, L.; Zapata, B.; Baena, A.; Bueno, J.; Ruíz-Nova, C.A.; Stashenko, E.E.; Mesa-Arango, A.C. Antifungal, cytotoxic and chemical analyses of essential oils of Lippia origanoides H.B.K grown in Colombia. Salud UIS 2011, 43, 141–148. [Google Scholar]
- Pinto, C.P.; Rodrigues, V.D.; Pinto, F.P.; Pinto, R.P.; Uetanabaro, A.P.T.; Pinheiro, C.S.R.; Gadea, S.F.M.; Silva, T.R.S.; Lucchese, A.M. Antimicrobial activity of Lippia species from the Brazilian semiarid region traditionally used as antiseptic and anti-infective agents. Evid. Based Complement. Altern. Med. 2013. [Google Scholar] [CrossRef]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E.E. Repellent activity of essential oils from seven aromatic plants grown in Colombia against Sitophilus zeamais Motschulsky (Coleoptera). J. Stored Prod. Res. 2009, 45, 212–214. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Olivero-Verbel, J.; Stashenko, E.E. Repellency and toxicity of essential oils from Cymbopogon martinii, Cymbopogon flexuosus and Lippia origanoides cultivated in Colombia against Tribolium castaneum. J. Stored Prod. Res. 2012, 50, 62–65. [Google Scholar] [CrossRef]
- Meneses, R.; Ocazionez, R.E.; Martínez, J.R.; Stashenko, E.E. Inhibitory effect of essential oils obtained from plants grown in Colombia on yellow fever virus replication in vitro. Ann. Clin. Microbiol. Antimicrob. 2009, 8, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Escobar, P.; Leal, M.S.; Herrera, L.V.; Martinez, J.R.; Stashenko, E.E. Chemical composition and antiprotozoal activities of Colombian Lippia spp essential oils and their major components. Mem. Inst. Oswaldo Cruz 2010, 105, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.R.; Aires, J.R.A.; Higino, T.M.M.; Medeiros, M.G.F.; Citó, A.M.G.L.; Lopes, J.A.D.; Figueiredo, R.C.B.Q. Trypanocidal and cytotoxic activities of essential oils from medicinal plants of Northeast of Brazil. Exp. Parasitol. 2012, 132, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Vicuña, G.C.; Stashenko, E.E.; Fuentes, J.L. Chemical composition of the Lippia origanoides essential oils and their antigenotoxicity against bleomycin-induced DNA damage. Fitoterapia 2010, 81, 342–349. [Google Scholar] [CrossRef]
- Teles, S.; Pereira, J.A.; de Oliveira, L.M.; Malheiro, R.; Lucchese, A.M.; Silva, F. Lippia origanoides H.B.K. essential oil production, composition, and antioxidant activity under organic and mineral fertilization: Effect of harvest moment. Ind. Crops Prod. 2014, 60, 217–225. [Google Scholar] [CrossRef]
- Castelo, A.V.M.; Del Menezzi, C.H.S.; Resck, I.S. Seasonal variation in the yield and the chemical composition of essential oils from two Brazilian native arbustive species. J. Appl. Sci. 2012, 12, 753–760. [Google Scholar] [CrossRef]
- NIST (National Institute of Standards and Technology). Mass Spectral Library (NIST/EPA/NIH, v. 2.0d); The NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publ. Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Chorianopoulos, N.; Evergetis, E.; Mallouchos, A.; Kalpoutzakis, E.; Nychas, G.J.; Haroutounian, S.A. Characterization of the essential oil volatiles of Satureja thymbra and Satureja parnassica: Influence of harvesting time and antimicrobial activity. J. Agric. Food Chem. 2006, 54, 3139–3145. [Google Scholar] [CrossRef] [PubMed]
- Jerković, I.; Mastelić, J.; Miloš, M. The impact of both the season of collection and drying on the volatile constituents of Origanum vulgare L. ssp. hirtum grown wild in Croatia. Int. J. Food Sci. Technol. 2001, 36, 649–654. [Google Scholar] [CrossRef]
- Sangwan, N.S.; Farooqi, A.H.A.; Shabih, F.; Sangwan, R.S. Regulation of essential oil production in plants. Plant Growth Regul. 2001, 34, 3–21. [Google Scholar] [CrossRef]
- Terblanché, F.C.; Kornelius, G. Essential oil constituents of the genus Lippia (Verbenaceae)—A literature review. J. Essent. Oil Res. 1996, 8, 471–485. [Google Scholar] [CrossRef]
- Matos, F.J.A.; Machado, M.I.L.; Craveiro, A.A.; Alencar, J.W. Essential oil composition of two chemotypes of Lippia alba grown in Northeast Brazil. J. Essent. Oil Res. 1996, 8, 695–698. [Google Scholar] [CrossRef]
- Zoghbi, M.G.B.; Andrade, E.H.A.; Santos, A.S.; Silva, M.H.L.; Maia, J.G.S. Essential oils of Lippia alba (Mill.) N.E. Br. growing wild in the Brazilian Amazon. Flavour Fragr. J. 1998, 13, 47–48. [Google Scholar] [CrossRef]
- Zoghbi, M.G.B.; Andrade, E.H.A.; Silva, M.H.L.; Maia, J.G.S. Volatile constituents of Lippia lupulina Cham. Flavour Fragr. J. 2001, 17, 29–31. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Taveira, F.S.N.; Andrade, E.H.A.; Silva, M.H.L.; Zoghbi, M.G.B. Essential oils of Lippia grandis Schau. Flavour Fragr. J. 2003, 18, 417–420. [Google Scholar] [CrossRef]
- Sarrazin, S.L.F.; Oliveira, R.B.; Barata, L.E.S.; Mourão, R.H.V. Chemical composition and antimicrobial activity of the essential oil of Lippia grandis Schauer (Verbenaceae) from the western Amazon. Food Chem. 2012, 134, 1474–1478. [Google Scholar] [CrossRef] [PubMed]
- Maia, J.G.S.; Silva, M.H.L.; Andrade, E.H.A.; Carreira, L.M.M. Essential oil variation in Lippia glandulosa Schauer. J. Essent. Oil Res. 2005, 17, 676–680. [Google Scholar] [CrossRef]
- Celiktas, O.Y.; Kocabas, E.E.H.; Bedir, E.; Sukan, F.V.; Ozek, T.; Baser, K.H.C. Antimicrobial activities of methanol extracts and essential oils of Rosmarinus officinalis, depending on location and seasonal variations. Food Chem. 2007, 100, 553–559. [Google Scholar] [CrossRef]
- Nostro, A.; Blanco, A.R.; Cannatelli, M.A.; Enea, V.; Flamini, G.; Morelli, I.; Roccaro, A.S.; Alonzo, V. Susceptibility of methicillin-resistant staphylococci to oregano essential oil, carvacrol and thymol. FEMS Microbiol. Lett. 2004, 230, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Ben Arja, A.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial activity of carvacrol related to its chemical structure. Lett. Appl. Microbiol. 2006, 43, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Van den Dool, H.; Kratz, P.D.J.A. Generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- National Committee for Clinical Laboratory Standards (NCCLS). Performance Standards for Antimicrobial Disk Susceptibility Tests, Approved Standard Eighth Edition; NCCLS: Wayne, PA, USA, 2003. [Google Scholar]
- Sample Availability: Sample of Lippia origanoides essential oil is available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarrazin, S.L.F.; Da Silva, L.A.; De Assunção, A.P.F.; Oliveira, R.B.; Calao, V.Y.P.; Da Silva, R.; Stashenko, E.E.; Maia, J.G.S.; Mourão, R.H.V. Antimicrobial and Seasonal Evaluation of the Carvacrol-Chemotype Oil from Lippia origanoides Kunth. Molecules 2015, 20, 1860-1871. https://doi.org/10.3390/molecules20021860
Sarrazin SLF, Da Silva LA, De Assunção APF, Oliveira RB, Calao VYP, Da Silva R, Stashenko EE, Maia JGS, Mourão RHV. Antimicrobial and Seasonal Evaluation of the Carvacrol-Chemotype Oil from Lippia origanoides Kunth. Molecules. 2015; 20(2):1860-1871. https://doi.org/10.3390/molecules20021860
Chicago/Turabian StyleSarrazin, Sandra Layse F., Leomara Andrade Da Silva, Ana Paula F. De Assunção, Ricardo B. Oliveira, Victor Y. P. Calao, Rodrigo Da Silva, Elena E. Stashenko, José Guilherme S. Maia, and Rosa Helena V. Mourão. 2015. "Antimicrobial and Seasonal Evaluation of the Carvacrol-Chemotype Oil from Lippia origanoides Kunth." Molecules 20, no. 2: 1860-1871. https://doi.org/10.3390/molecules20021860
APA StyleSarrazin, S. L. F., Da Silva, L. A., De Assunção, A. P. F., Oliveira, R. B., Calao, V. Y. P., Da Silva, R., Stashenko, E. E., Maia, J. G. S., & Mourão, R. H. V. (2015). Antimicrobial and Seasonal Evaluation of the Carvacrol-Chemotype Oil from Lippia origanoides Kunth. Molecules, 20(2), 1860-1871. https://doi.org/10.3390/molecules20021860






