Pyranocoumarins from Root Extracts of Peucedanum praeruptorum Dunn with Multidrug Resistance Reversal and Anti-Inflammatory Activities
Abstract
:1. Introduction
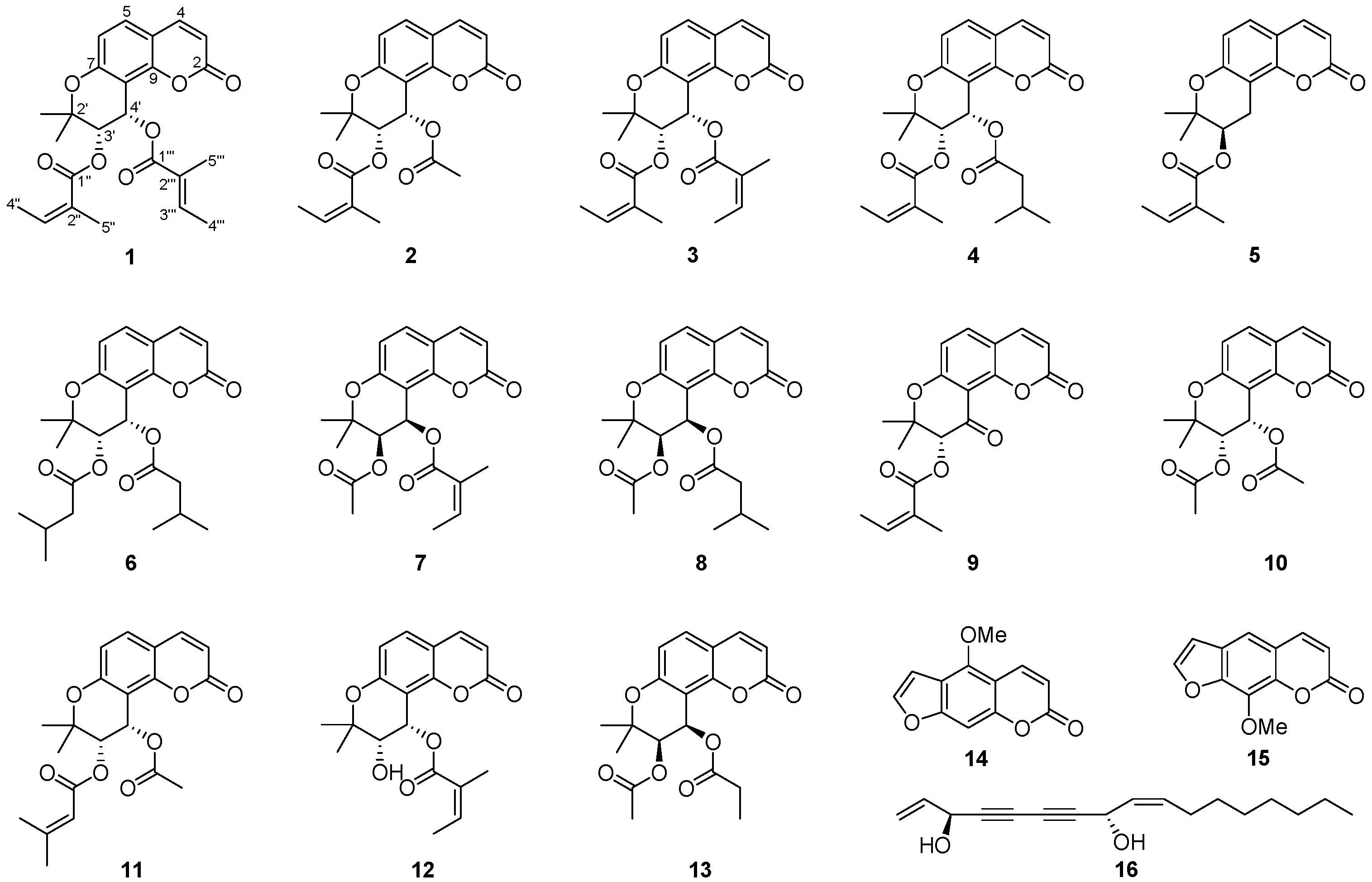
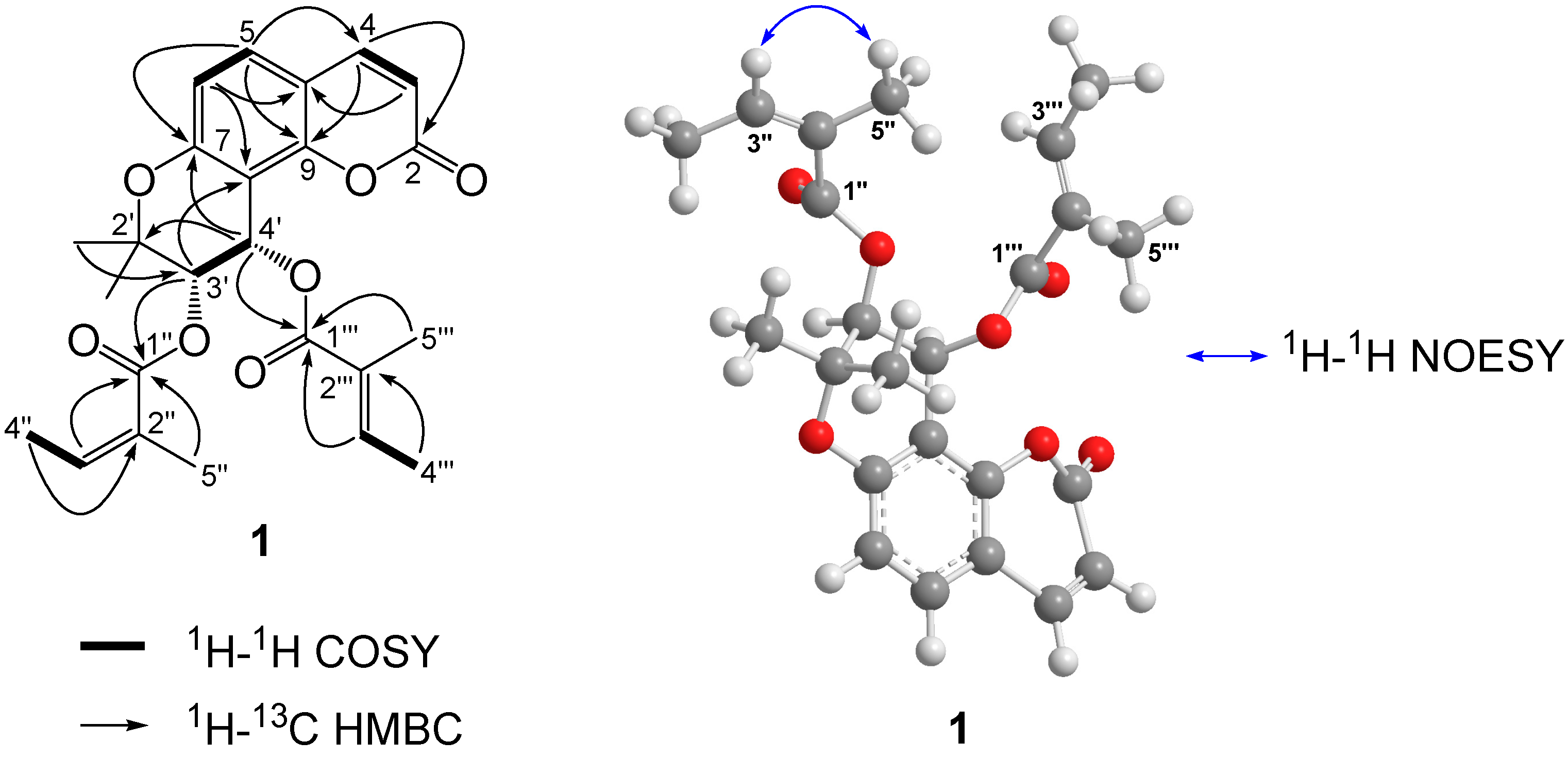
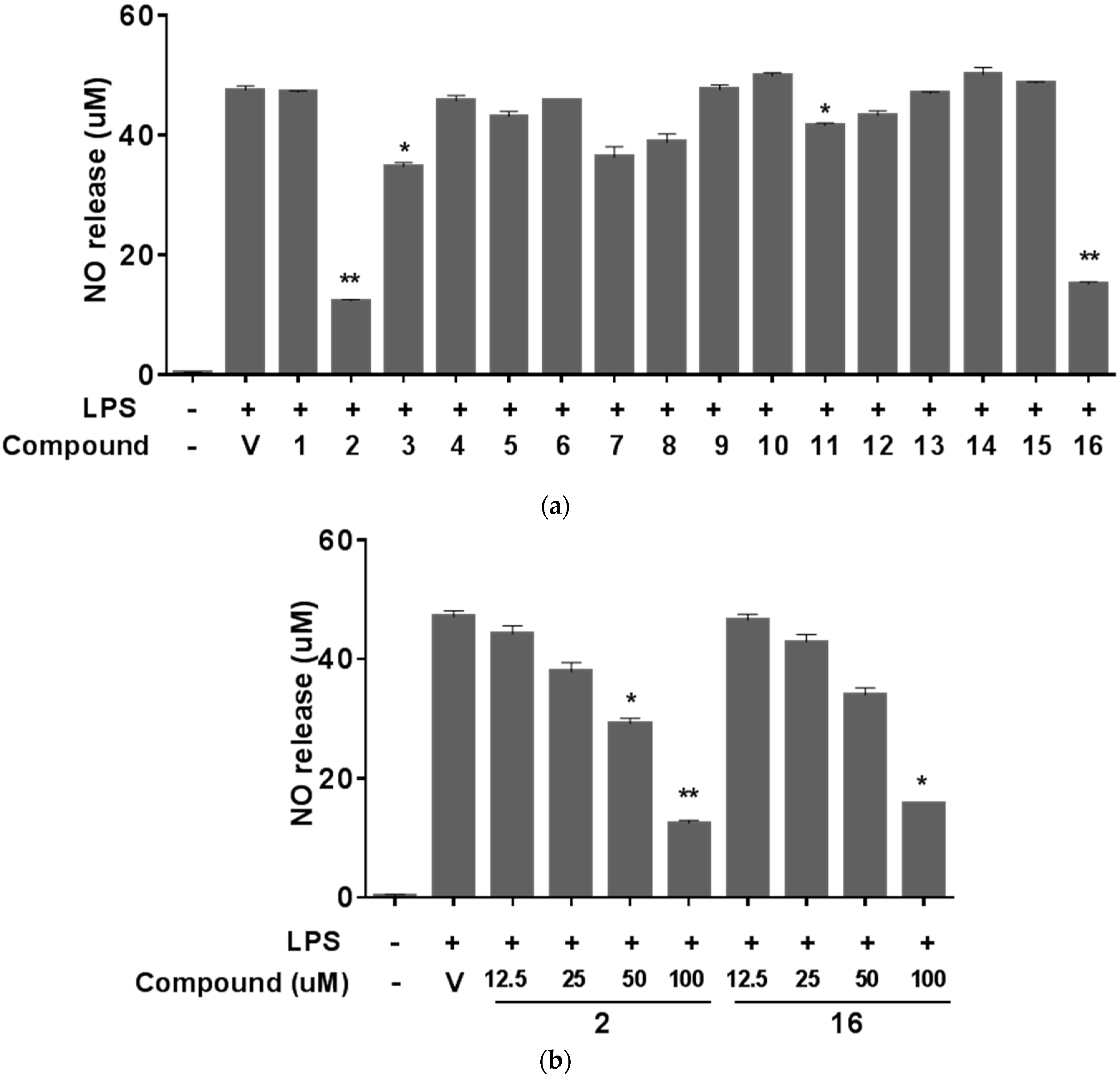
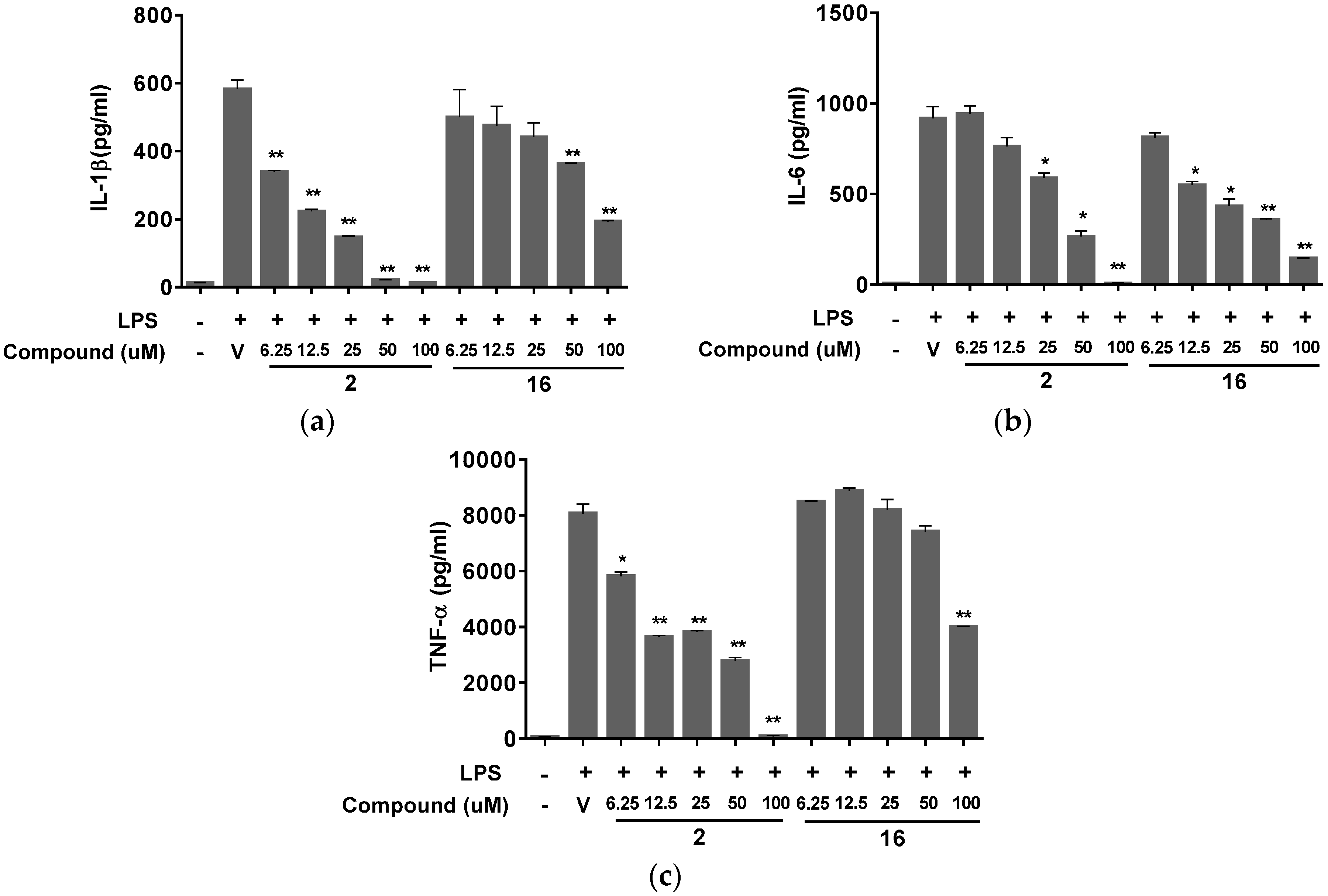
2. Results and Discussion

3. Experimental Section
3.1. Materials and Major Equipment
3.2. Plant Material
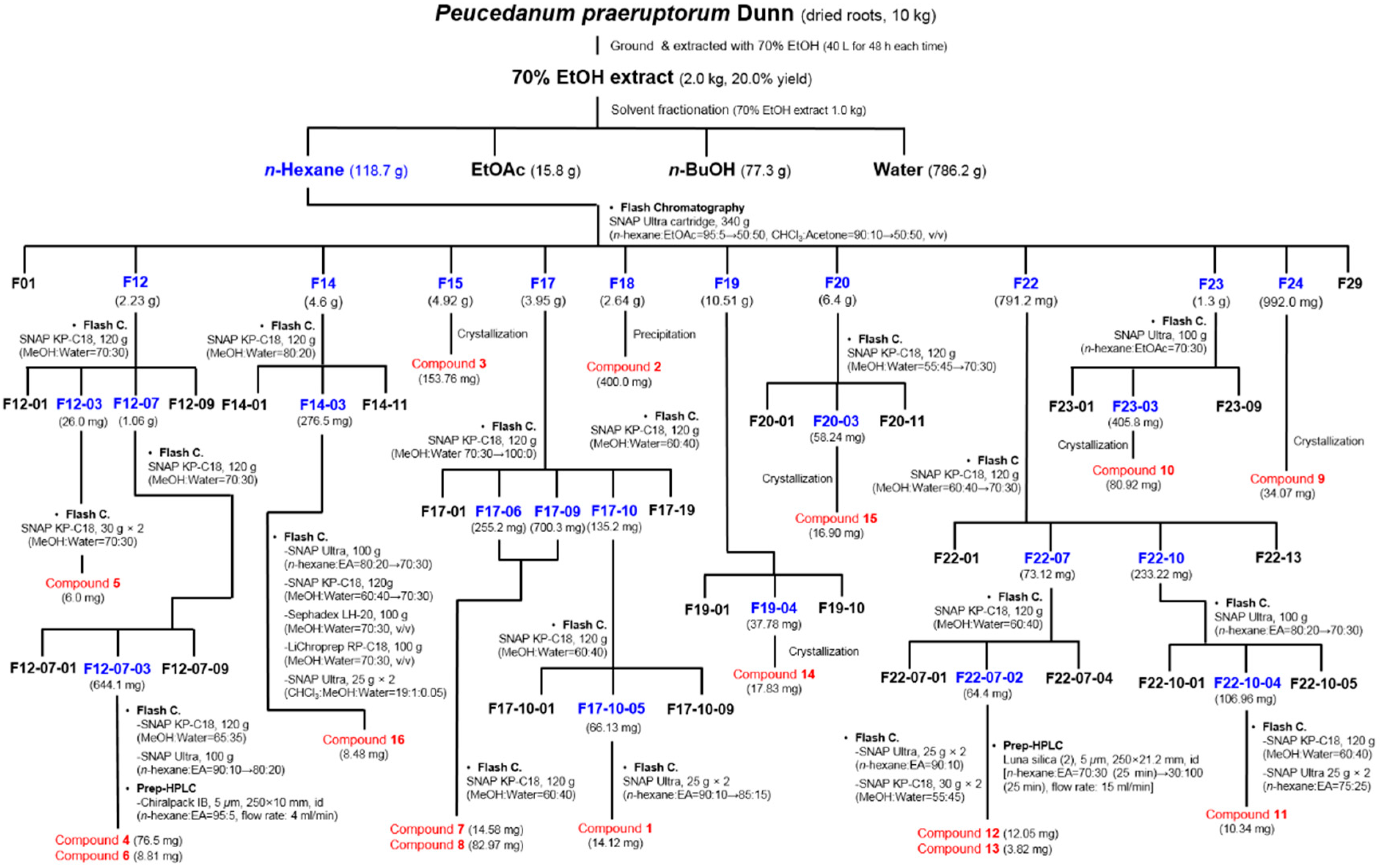
3.3. Extraction and Isolation of Compounds
3.4. Characterization Data of (+)-cis-(3′S,4'S)-3'-Angeloyl-4′-tigloylkhellactone (1)
3.5. Cell Culture and Cell Viability
3.6. NO Assay
3.7. Measurement of IL-1β, IL-6, and TNF-α
3.8. MDR Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Song, Y.; Jing, W.; Yan, R.; Wang, Y. Research progress of the studies on the roots of Peucedanum praeruptorum Dunn (peucedani radix). Pak. J. Pharm. Sci. 2015, 28, 71–81. [Google Scholar] [PubMed]
- Xiong, Y.; Wang, J.; Wu, F.; Li, J.; Zhou, L.; Kong, L. Effects of (±)-praeruptorin a on airway inflammation, airway hyperresponsiveness and NF-κB signaling pathway in a mouse model of allergic airway disease. Eur. J. Pharm. Sci. 2012, 683, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.T.; Okada, Y.; Ma, T.J.; Okuyama, T.; Tu, P.F. Two new coumarin glycosides from Peucedanum praeruptorum. J. Asian Nat. Prod. Res. 2008, 10, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Okada, Y.; Okuyama, T.; Tu, P. 1H- and 13C-NMR assignments for two new angular furanocoumarin glycosides from Peucedanum praeruptorum. Magn. Reson. Chem. 2007, 45, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.Y.; Pei, Y.H.; Li, X.; Zhu, T.R.; Okuyama, T. Isolation and structure elucidation of qianhucoumarin A. Yao Xue Xue Bao 1993, 28, 432–436. [Google Scholar] [PubMed]
- Takata, M.; Shibata, S.; Okuyama, T. Structures of angular pyranocoumarins of Bai-Hua Qian-Hu, the root of Peucedanum praeruptorum. Planta Med. 1990, 56, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.X.; Huang, B.S.; She, Q.L.; Zeng, G.F. The chemical constituents of Bai-Hua-Qian-Hu, the root of Peucedanum praeruptorum Dunn. (Umbelliferae)—Four new coumarins (author’s transl). Yao Xue Xue Bao 1979, 14, 486–496. [Google Scholar] [PubMed]
- Ye, J.S.; Zhang, H.Q.; Yuan, C.Q. Isolation and identification of coumarin praeruptorin E from the root of the Chinese drug Peucedanum praeruptorum Dunn (Umbelliferae). Yao Xue Xue Bao 1982, 17, 431–434. [Google Scholar] [PubMed]
- Okuyama, T.; Takata, M.; Shibata, S. Structures of linear furano- and simple-coumarin glycosides of Bai-Hua Qian-Hu. Planta Med. 1989, 55, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.J.; Jin, H.; Zhang, J.Y.; Wang, G.F.; Li, J.R.; Zhu, Z.G.; Tian, Y.X.; Wu, S.Y.; Xu, W.; Zhang, J.J.; et al. Pyranocoumarins isolated from Peucedanum praeruptorum Dunn suppress lipopolysaccharide-induced inflammatory response in murine macrophages through inhibition of NF-κB and stat3 activation. Inflammation 2012, 35, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wang, J.; Wu, F.; Li, J.; Kong, L. The effects of (±)-praeruptorin A on airway inflammation, remodeling and transforming growth factor-β1/Smad signaling pathway in a murine model of allergic asthma. Int. Immunopharmacol. 2012, 14, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.J.; Ci, W.; Wang, G.F.; Zhang, J.Y.; Wu, S.Y.; Xu, W.; Jin, H.; Zhu, Z.G.; Zhang, J.J.; Pang, J.X.; et al. Praeruptorin a inhibits lipopolysaccharide-induced inflammatory response in murine macrophages through inhibition of NF-κB pathway activation. Phytother. Res. 2011, 25, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.Y.; Wu, F.H.; Wang, J.S.; Li, J.; Kong, L.Y. Attenuation of airway hyperreactivity and T helper cell type 2 responses by coumarins from Peucedanum praeruptorum Dunn in a murine model of allergic airway inflammation. J. Ethnopharmacol. 2012, 141, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Yue, W.; Li, Q. Chemopreventive effects of Peucedanum praeruptorum Dunn and its major constituents on SGC7901 gastric cancer cells. Molecules 2010, 15, 8060–8071. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.C.; Jin, W.B.; Zhang, X.H.; Guan, F.L.; Sun, Y.B.; Adachi, H.; Okuyama, T. Relaxant effects of pyranocoumarin compounds isolated from a Chinese medical plant, Bai-Hua Qian-Hu, on isolated rabbit tracheas and pulmonary arteries. Biol. Pharm. Bull. 1999, 22, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, X.B.; Yang, Q.; Zhang, K.; Zhang, N.; Guo, Y.Y.; Feng, B.; Zhao, M.G.; Wu, Y.M. The neuroprotective effect of praeruptorin c against NMDA-induced apoptosis through down-regulating of GluN2B-containing NMDA receptors. Toxocol. In Vitro 2013, 27, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Yeon, J.T.; Kim, K.J.; Choi, S.W.; Moon, S.H.; Park, Y.S.; Ryu, B.J.; Oh, J.; Kim, M.S.; Erkhembaatar, M.; Son, Y.J.; et al. Anti-osteoclastogenic activity of praeruptorin a via inhibition of p38/Akt-c-Fos-NFATc1 signaling and plcgamma-independent Ca2+ oscillation. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.S.; Chang, C.T.; Sheen, W.S.; Teng, C.M.; Tsai, I.L.; Duh, C.Y.; Ko, F.N. Coumarins and antiplatelet aggregation constituents from formosan Peucedanum japonicum. Phytochemistry 1996, 41, 525–530. [Google Scholar] [CrossRef]
- Song, Y.L.; Zhang, Q.W.; Li, Y.P.; Yan, R.; Wang, Y.T. Enantioseparation and absolute configuration determination of angular-type pyranocoumarins from peucedani radix using enzymatic hydrolysis and chiral HPLC-MS/MS analysis. Molecules 2012, 17, 4236–4251. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Feng, L.; Sun, A.; Kong, L. Preparative isolation and purification of coumarins from Peucedanum praeruptorum Dunn by high-speed counter-current chromatography. J. Chromatogr. A 2004, 1057, 89–94. [Google Scholar] [CrossRef]
- Hou, Z.; Xu, D.; Yao, S.; Luo, J.; Kong, L. An application of high-speed counter-current chromatography coupled with electrospray ionization mass spectrometry for separation and online identification of coumarins from Peucedanum praeruptorum Dunn. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 2571–2578. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Chen, P.Y.; Wu, C.C.; Tsai, I.L.; Chen, I.S. Chemical constituents and anti-platelet aggregation activity from the root of Peucedanum formosanum. J. Food Drug Anal. 2008, 16, 15–25. [Google Scholar]
- Gupta, B.D.; Banerjee, S.K.; Handa, K.L. Coumarins of Ligusticum elatum. Phytochemistry 1975, 14, 598. [Google Scholar] [CrossRef]
- Jong, T.T.; Hwang, H.C.; Jean, M.Y.; Wu, T.S.; Teng, C.M. An antiplatelet aggregation principle and X-ray structural analysis of cis-khellactone diester from Peucedanum japonicum. J. Nat. Prod. 1992, 55, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Swager, T.M.; Cardellina, J.H., II. Coumarins from Musineon divaricatum. Phytochemistry 1985, 24, 805–813. [Google Scholar] [CrossRef]
- Hata, K.; Kozawa, M.; Baba, K.; Yen, K.; Yang, L. Coumarins from the roots of Angelica morii hayata. Chem. Pharm. Bull. 1974, 22, 957–961. [Google Scholar] [CrossRef]
- Okuyama, T.; Shibata, S. Studies on coumarins of a Chinese drug “Qian-Hu”. Planta Med. 1989, 42, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.L.; Wang, W.J.; Rao, G.X.; Sun, H.D. The chemical constituents of Ligusticum calophlebicum. Acta Bot. Yunnanica 2004, 26, 234–236. [Google Scholar]
- Lee, J.W.; Lee, C.; Jin, Q.; Yeon, E.T.; Lee, D.; Kim, S.Y.; Han, S.B.; Hong, J.T.; Lee, M.K.; Hwang, B.Y. Pyranocoumarins from Glehnia littoralis inhibit the LPS-induced NO production in macrophage RAW 264.7 cells. Bioorg. Med. Chem. Lett. 2014, 24, 2717–2719. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, O.; Khan, S.; Ha, I.J.; Park, Y.; Tosun, A.; Kim, Y.S. Application of stepwise gradients in counter-current chromatography: A rapid and economical strategy for the one-step separation of eight coumarins from Seseli resinosum. J. Chromatogr. A 2013, 1310, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, N.; Kouno, I.; Kawano, N. On the isolation of (+)-samidin from the roots of Peucedanum japonicum thunb. Yakugaku Zasshi 1982, 102, 392–394. [Google Scholar]
- Tosun, A.; Ozkal, N.; Baba, M.; Okuyama, T. Pyranocoumarins from Seseli gummiferum subsp. Corymbosum growing in Turkey. Turk. J. Chem. 2005, 29, 327–334. [Google Scholar]
- Valencia-Islas, N.; Abbas, H.; Bye, R.; Toscano, R.; Mata, R. Phytotoxic compounds from Prionosciadium watsoni. J. Nat. Prod. 2002, 65, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Chunyan, C.; Bo, S.; Ping, L.; Jingmei, L.; Ito, Y. Isolation and purification of psoralen and bergapten from Ficus carica L. leaves by high-speed countercurrent chromatography. J. Liq. Chromatogr. Relat. Technol. 2009, 32, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Intekhab, J.; Aslam, M. Coumarins from the roots of Clausena pentaphylla. FABAD J. Pharm. Sci. 2008, 33, 67–70. [Google Scholar]
- He, F.; Wang, M.; Gao, M.; Zhao, M.; Bai, Y.; Zhao, C. Chemical composition and biological activities of Gerbera anandria. Molecules 2014, 19, 4046–4057. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.R.; Cardellina, J.H., II. Native American medicinal plants. Falcarindiol and 3-O-methylfalcarindiol from Osmorhiza occidentalis. J. Nat. Prod. 1982, 45, 774–776. [Google Scholar] [CrossRef]
- Villegas, M.; Vargas, D.; Msonthi, J.D.; Marston, A.; Hostettmann, K. Isolation of the antifungal compounds falcarindiol and sarisan from Heteromorpha trifoliata. Planta Med. 1988, 54, 36–37. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Du, G.J.; Qi, L.W.; Williams, S.; Wang, C.Z.; Yuan, C.S. Hydrophobic constituents and their potential anticancer activities from devil’s club (Oplopanax horridus Miq.). J. Ethnopharmacol. 2010, 132, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, T.; Furumi, K.; Fujii, H.; Okabe, H.; Mihashi, K.; Nakano, Y.; Matsunaga, H.; Katano, M.; Mori, M. Antiproliferative constituents from Umbelliferae plants. V. A new furanocoumarin and falcarindiol furanocoumarin ethers from the root of Angelica japonica. Chem. Pharm. Bull. 1999, 47, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Krishna, R.; Mayer, L.D. Multidrug resistance (MDR) in cancer. Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 2000, 11, 265–283. [Google Scholar] [CrossRef]
- Wu, J.Y.; Fong, W.F.; Zhang, J.X.; Leung, C.H.; Kwong, H.L.; Yang, M.S.; Li, D.; Cheung, H.Y. Reversal of multidrug resistance in cancer cells by pyranocoumarins isolated from radix peucedani. Eur. J. Pharm. Sci. 2003, 473, 9–17. [Google Scholar] [CrossRef]
- Lala, P.K.; Chakraborty, C. Role of nitric oxide in carcinogenesis and tumour progression. Lancet Oncol. 2001, 2, 149–156. [Google Scholar] [CrossRef]
- Gallo, O.; Masini, E.; Morbidelli, L.; Franchi, A.; Fini-Storchi, I.; Vergari, W.; Ziche, M. Role of nitric oxide in angiogenesis and tumor progression in head and neck cancer. J. Natl. Cancer Inst. 1998, 90, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Jadeski, L.C.; Hum, K.O.; Chakraborty, C.; Lala, P.K. Nitric oxide promotes murine mammary tumour growth and metastasis by stimulating tumour cell migration, invasiveness and angiogenesis. Int. J. Cancer 2000, 86, 30–39. [Google Scholar] [CrossRef]
- Cardnell, R.J.; Mikkelsen, R.B. Nitric oxide synthase inhibition enhances the antitumor effect of radiation in the treatment of squamous carcinoma xenografts. PLoS ONE 2011, 6, e20147. [Google Scholar] [CrossRef] [PubMed]
- Marks-Konczalik, J.; Chu, S.C.; Moss, J. Cytokine-mediated transcriptional induction of the human inducible nitric oxide synthase gene requires both activator protein 1 and nuclear factor κB-binding sites. J. Biol. Chem. 1998, 273, 22201–22208. [Google Scholar] [CrossRef] [PubMed]
- Erwig, L.P.; Rees, A.J. Macrophage activation and programming and its role for macrophage function in glomerular inflammation. Kidney Blood Press. Res. 1999, 22, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, N.S.; Kim, H.; Yi, J.M.; Oh, S.M.; Bang, O.S.; Lee, J. Cytotoxic and anti-inflammatory constituents from the seeds of Descurainia sophia. Arch. Pharm. Res. 2013, 36, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Lee, Y.J.; Kim, J.; Bang, O.-S. Pyranocoumarins from Root Extracts of Peucedanum praeruptorum Dunn with Multidrug Resistance Reversal and Anti-Inflammatory Activities. Molecules 2015, 20, 20967-20978. https://doi.org/10.3390/molecules201219738
Lee J, Lee YJ, Kim J, Bang O-S. Pyranocoumarins from Root Extracts of Peucedanum praeruptorum Dunn with Multidrug Resistance Reversal and Anti-Inflammatory Activities. Molecules. 2015; 20(12):20967-20978. https://doi.org/10.3390/molecules201219738
Chicago/Turabian StyleLee, Jun, You Jin Lee, Jinhee Kim, and Ok-Sun Bang. 2015. "Pyranocoumarins from Root Extracts of Peucedanum praeruptorum Dunn with Multidrug Resistance Reversal and Anti-Inflammatory Activities" Molecules 20, no. 12: 20967-20978. https://doi.org/10.3390/molecules201219738
APA StyleLee, J., Lee, Y. J., Kim, J., & Bang, O.-S. (2015). Pyranocoumarins from Root Extracts of Peucedanum praeruptorum Dunn with Multidrug Resistance Reversal and Anti-Inflammatory Activities. Molecules, 20(12), 20967-20978. https://doi.org/10.3390/molecules201219738





