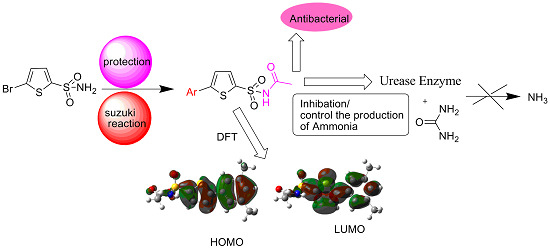
Synthesis, Density Functional Theory (DFT), Urease Inhibition and Antimicrobial Activities of 5-Aryl Thiophenes Bearing Sulphonylacetamide Moieties
Abstract
:1. Introduction
2. Results and Discussion
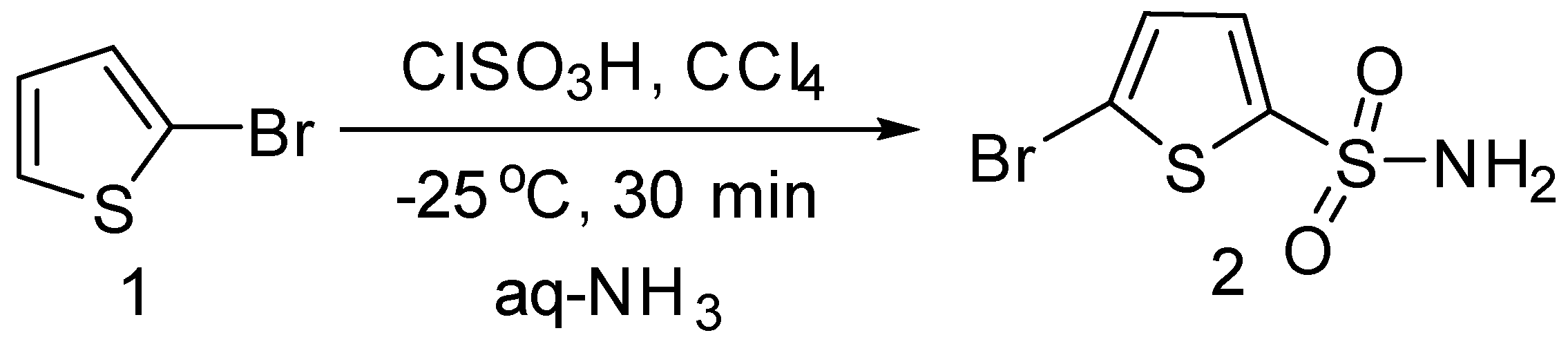
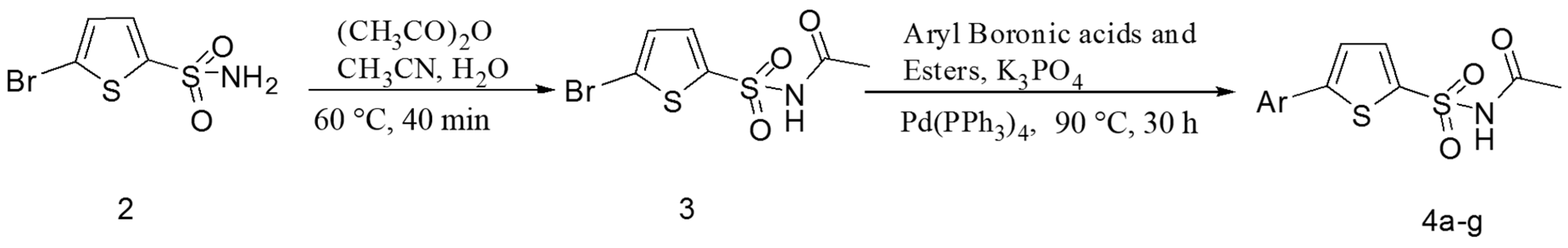
2.1. Chemistry
| Entry | Reagent | Product | Solvent/H2O (4:1) | Yields% a |
|---|---|---|---|---|
| 1 |  | 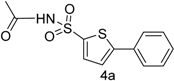 | 1,4-Dioxane | 77 |
| 2 |  |  | Toluene | 68 |
| 3 | 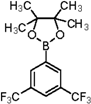 | 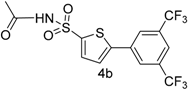 | 1,4-Dioxane | 66 |
| 4 | 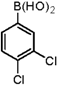 | 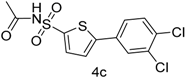 | 1,4-Dioxane | 68 |
| 5 |  |  | 1,4-Dioxane | 72 |
| 6 | 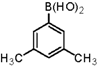 | 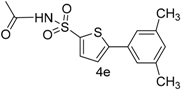 | 1,4-Dioxane | 74 |
| 7 | 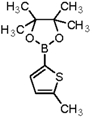 | 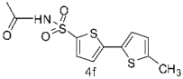 | 1,4-Dioxane | 70 |
| 8 | 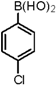 |  | 1,4-Dioxane | 65 |
2.2. DFT Studies
2.2.1. Geometry Optimization
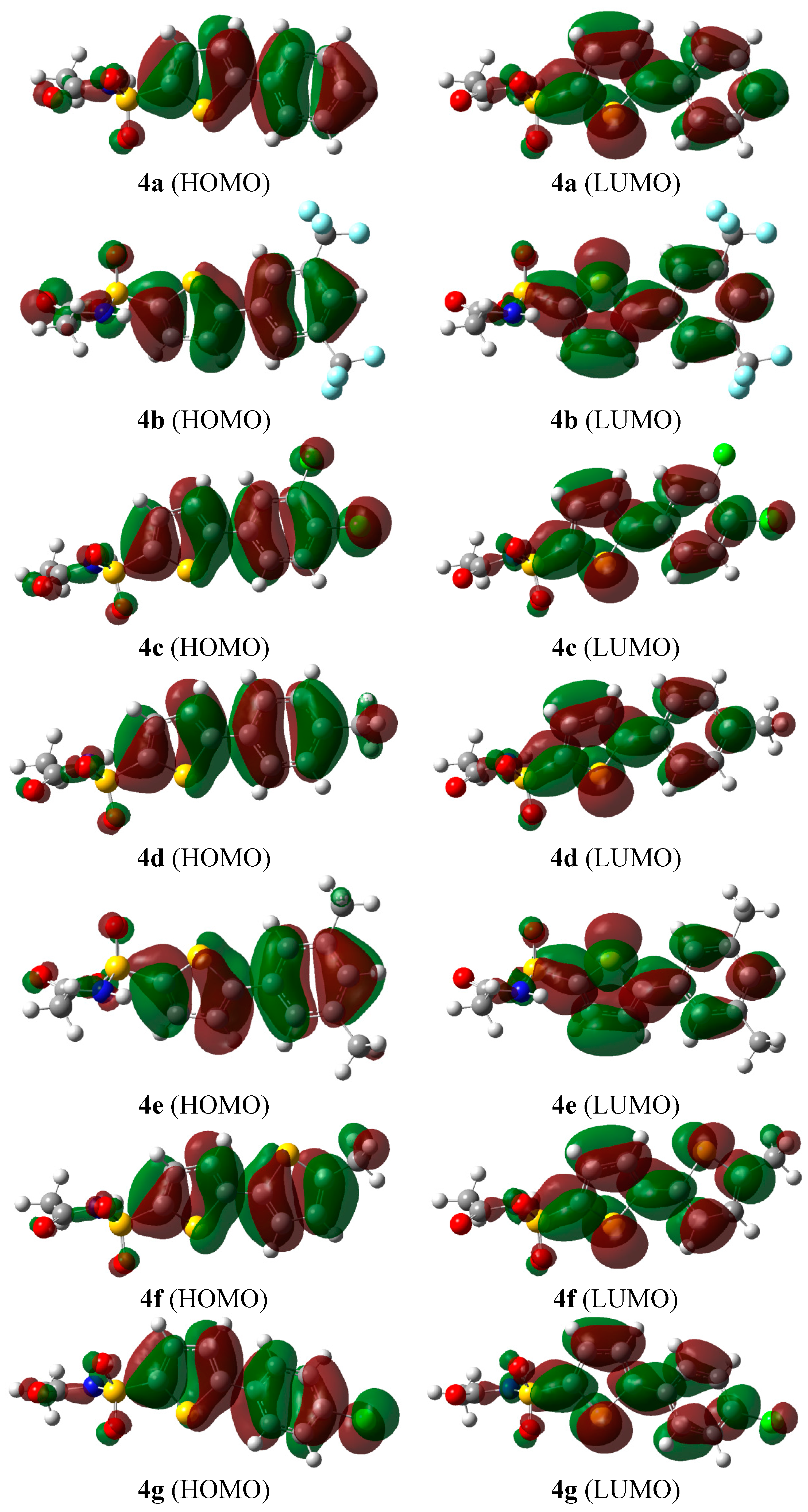
2.2.2. Frontier Molecular Orbital (FMO) Analysis
| Entry | HOMO (a.u.) | LUMO (a.u.) | HOMO-LUMO (ΔE/eV) |
|---|---|---|---|
| 4a | −0.24078 | −0.07273 | 4.57 |
| 4b | −0.26085 | −0.09138 | 4.60 |
| 4c | −0.24906 | −0.08507 | 4.46 |
| 4d | −0.23451 | −0.07014 | 4.47 |
| 4e | −0.23540 | −0.06935 | 4.51 |
| 4f | −0.22386 | −0.07710 | 3.99 |
| 4g | −0.24329 | −0.07937 | 4.45 |
2.2.3. Molecular Electrostatic Potential (MEP)
| Entry | −ve Potential (a.u.) | +ve Potential (a.u.) |
|---|---|---|
| 4a | −0.07342 | 0.07342 |
| 4b | −0.07182 | 0.07182 |
| 4c | −0.06974 | 0.06974 |
| 4d | −0.07444 | 0.07014 |
| 4e | −0.07459 | 0.07459 |
| 4f | −0.07400 | 0.07400 |
| 4g | −0.07096 | 0.07096 |

2.2.4. Hyperpolarizability and Non-Linear Optical (NLO) Properties
| Entry | 4a | 4b | 4c | 4d | 4e | 4f | 4g |
|---|---|---|---|---|---|---|---|
| βxxx | −546.18 | 118.57 | 1043.62 | −1159.69 | −804.94 | 1627.21 | 1301.99 |
| βxxy | −27.13 | 66.68 | −16.19 | −94.92 | 56.93 | −184.46 | −122.23 |
| βxyy | 26.92 | 53.51 | 10.45 | 45.99 | 22.73 | −31.44 | 11.45 |
| βyyy | 32.77 | −49.74 | −60.06 | 26.81 | −67.96 | 44.77 | 23.43 |
| βxxz | −37.90 | 59.80 | −83.06 | −18.56 | −47.42 | −66.36 | −2.37 |
| Βxyz | 6.16 | 5.61 | −5.87 | 0.29 | 13.17 | 2.75 | 4.35 |
| βyyz | 1.38 | −9.85 | 1.38 | 1.27 | −10.85 | −25.01 | 4.56 |
| βxzz | 95.61 | 33.85 | −19.52 | 109.00 | 58.63 | −112.32 | 80.98 |
| βyzz | 18.20 | −1.45 | 8.08 | 25.42 | 3.92 | 19.57 | 2.39 |
| βzzz | 33.17 | 10.04 | 19.56 | 41.49 | 56.31 | 4.35 | 51.02 |
| βtot × 10−33 (esu) | 3.67 | 1.857 | 8.973 | 8.690 | 6.251 | 12.879 | 11.998 |
2.3. Biological Activity
2.3.1. Urease Inhibition Activity
| Entry | Percentage Activity at 50 µg/mL | Percentage Activity at 250 µg/mL | IC50 µg/mL |
|---|---|---|---|
| 4a | 67.56 ± 0.007 | 89 ± 0.01 | 38.4 ± 0.32 |
| 4b | 29.98 ± 0.034 | 56 ± 0.006 | 82.01 ± 0.79 |
| 4c | 54.4 ± 0.002 | 78 ± 0.003 | 42.5 ± 0.41 |
| 4d | 13.34 ± 0.007 | 54 ± 0.004 | 218 ± 1.98 |
| Standard | 60 ± 0.032 | 95 ± 0.09 | 43 ± 0.38 |
| Entry | Percentage Activity at 15 µg/mL | Percentage Activity at 40 µg/mL | Percentage Activity at 80 µg/mL | IC50 µg/mL |
|---|---|---|---|---|
| 4e | 44.59 ± 0.14 | 91.21 ± 0.81 | 92.88 ± 0.14 | 17.9 ± 0.13 |
| 4f | 42.44 ± 0.11 | 92.12 ± 0.21 | 94.66 ± 0.11 | 17.1 ± 0.15 |
| 4g | 46.23 ± 0.11 | 90.97 ± 0.18 | 68 ± 0.02 | 23.3 ± 0.21 |
| Standard | 47.1 ± 0.31 | 65 ± 0.01 |
2.3.2. Antibacterial Activity
| % Activity at 100 µg | ||||||
|---|---|---|---|---|---|---|
| Entry | Gram Positive Bacteria | Gram Negative Bacteria | Gram Positive Bacteria | Gram Negative Bacteria | Gram Negative Bacteria | Gram Negative Bacteria |
| Bacillus subtiles | Escherichia coli | Staphylococcus aureus | Shigella dysenteriae | Salmonella typhae | Pseudomonas aeruginosa | |
| 4a | 17 ± 0.0007 | 13 ± 0.007 | 19.03 ± 0.0 | 55.7 ± 0.016 | 39.31 ± 0.008 | 39.32 ± 0.004 |
| 4b | 17 ± 0.005 | 20 ± 0.01 | 17.7 ± 0.007 | 38.59 ± 0.0007 | 36.57 ± 0.005 | 32.0 ± 0.006 |
| 4c | 14.1 ± 0.002 | 16 ± 0.00 | 13.89 ± 0.002 | 15.59 ± 0.004 | 34.95 ± 0.012 | 27.2 ± 0.004 |
| 4d | 20 ± 0.007 | 20 ± 0.007 | 18.35 ± 0.0007 | 19.93 ± 0.038 | 33.26 ± 0.002 | 24.1 ± 0.004 |
| 4e | 15 ± 0.035 | 20 ± 0.035 | 17.96 ± 0.0007 | 26.93 ± 0.038 | 33.70 ± 0.009 | 25.05 ± 0.005 |
| 4f | 19 ± 0.007 | 12 ± 0.001 | 19.86 ± 0.001 | 38.33 ± 0.0007 | 48.55 ± 0.014 | 35.05 ± 0.004 |
| 4g | 22 ± 0.019 | 12. ± 0.01 | 20.17 ± 0.02 | 31.1 ± 0.009 | 32.51 ± 0.006 | 32.06 ± 0.004 |
| Ampicillin | 60.2 ± 0.32 | 82 ± 0.2 | 65 ± 0.22 | 60 ± 0.18 | 86 ± 0.5 | 55 ± 0.12 |
| % Activity at 300 µg | ||||||
|---|---|---|---|---|---|---|
| Entry | Gram Positive Bacteria | Gram Negative Bacteria | Gram Positive Bacteria | Gram Negative Bacteria | Gram Negative Bacteria | Gram Negative Bacteria |
| Bacillus subtiles | Escherichia coli | Staphylococcus aureus | Shigella dysenteriae | Salmonella typhae | Pseudomonas aeruginosa | |
| 4a | 34 ± 0.06 | 29 ± 0.06 | 32.22 ± 0.002 | 51.78 ± 0.001 | 29.06 ± 0.016 | 38.53 ± 0.010 |
| 4b | 28.2 ± 0.01 | 30 ± 0.02 | 32.16 ± 0.0007 | 51.7 ± 0.000 | 36.12 ± 0.006 | 44.81 ± 0.009 |
| 4c | 26.3 ± 0.001 | 27 ± 0.006 | 22.50 ± 0.003 | 50.17 ± 0.001 | 31.2 ± 0.0091 | 40.64 ± 0.03 |
| 4d | 40.0 ± 0.000 | 34 ± 0.05 | 27.35 ± 0.0003 | 52.36 ± 0.002 | 34.22 ± 0.0007 | 40.05 ± 0.038 |
| 4e | 40.01 ± 0.001 | 40 ± 0.01 | 27.80 ± 0.0004 | 45.96 ± 0.017 | 28.8 ± 0.021 | 40.74 ± 0.06 |
| 4f | 24.5 ± 0.00 | 25 ± 0.00 | 28.74 ± 0.0007 | 49.30 ± 0.023 | 33.58 ± 0.007 | 41.86 ± 0.043 |
| 4g | 37 ± 0.144 | 35 ± 0.144 | 29.57 ± 0.0007 | 52.94 ± 0.001 | 40.2 ± 0.016 | 38.4 ± 0.003 |
| Ampicillin | 85 ± 0.51 | 88 ± 0.6 | 78 ± 0.45 | 76.2 ± 0.29 | 89 ± 0.18 | 72 ± 0.61 |
| % Activity at 1000 µg | ||||||
|---|---|---|---|---|---|---|
| Entry | Gram Positive Bacteria | Gram Negative Bacteria | Gram Positive Bacteria | Gram Negative Bacteria | Gram Negative Bacteria | Gram Negative Bacteria |
| Bacillus subtiles | Escherichia coli | Staphylococcus aureus | Shigella dysenteriae | Salmonella typhae | Pseudomonas aeruginosa | |
| 4a | 60 ± 0.004 | 64 ± 0.004 | 64 ± 0.004 | 80 ± 0.00 | 55 ± 0.009 | 46 ± 0.12 |
| 4b | 32 ± 0.004 | 62 ± 0.004 | 59 ± 0.0021 | 73 ± 0.0034 | 56 ± 0.007 | 45 ± 0.0021 |
| 4c | 31 ± 0.0003 | 44 ± 0.001 | 58 ± 0.005 | 74 ± 0.015 | 64 ± 0.0012 | 48 ± 0.045 |
| 4d | 54 ± 0.009 | 59 ± 0.01 | 59 ± 0.00 | 75 ± 0.023 | 62 ± 0.003 | 65 ± 0.03 |
| 4e | 75 ± 0.01 | 74 ± 0.01 | 55.5 ± 0.0012 | 65 ± 0.004 | 58 ± 0.0054 | 67 ± 0.04 |
| 4f | 34 ± 0.0021 | 54 ± 0.00 | 56 ± 0.004 | 67 ± 0.045 | 65 ± 0.001 | 64 ± 0.0012 |
| 4g | 78 ± 0.007 | 71 ± 0.007 | 61 ± 0.006 | 75 ± 0.02 | 68 ± 0.0004 | 43 ± 0.5 |
| Ampicillin | 92 ± 0.55 | 95.9 ± 0.21 | 92.3 ± 0.32 | 91.6 ± 0.61 | 98.9 ± 0.26 | 92 ± 0.44 |
3. Experimental Section
3.1. General Information
3.2. Synthesis of 5-Bromothiophene-2-sulphonamide (2)
3.3. Synthesis of 5-Bromothiophene-2-sulfonylacetamide (Sulfacetamide) (3)
3.4. General Procedure for the Synthesis of 5-Arylthiophene-2-sulfonylacetamides 4a–g
3.5. Computational Methods
3.6. Urease Inhibition Activity
3.7. Antibacterial Activity Assay
Determination of Minimum Inhibitory Concentrations
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martin, M.T.; Roschanger, F.; Eddy, J.F. Practical acid catalyzed acylation of sulfonamides with carboxylic acid anhydrides. Tetrahedron Lett. 2003, 44, 5461–5463. [Google Scholar] [CrossRef]
- Berredjem, M.; Fouzia, B.; Samira, A.K.; Maazouz, D.; Nour-Eddine, A. Synthesis and antibacterial activity of novel N-acylsulfonamides. Arabian J. Chem. 2013. [Google Scholar] [CrossRef]
- Ashton, W.T.; Chang, L.L.; Flanagan, K.L.; Hutchins, S.M.; Naylor, E.M.; Chakravarty, P.K.; Patchett, A.A.; Greenlee, W.J.; Chen, T.B. Triazolinone biphenylsulfonamide derivatives as orally active angiotensin II antagonists with potent AT1 receptor affinity and enhanced AT2 affinity. J. Med. Chem. 1994, 37, 2808–2824. [Google Scholar] [CrossRef] [PubMed]
- Winters, M.P.; Crysler, C.; Subasinghe, N.; Ryan, D.; Leong, L.; Zhao, S.; Donatelli, R.; Yurkow, E.; Mazzulla, M.; Boczon, L. Carboxylic acid bioisosteres acylsulfonamides, acylsulfamides, and sulfonylureas as novel antagonists of the CXCR2 receptor. Bioorg. Med. Chem. Lett. 2008, 18, 1926–1930. [Google Scholar] [CrossRef] [PubMed]
- Reitz, A.B.; Smith, G.R.; Parker, M.H. The role of sulfamide derivatives in medicinal chemistry: A patent review (2006–2008). Exprt Opin. Ther. Pat. 2009, 19, 1449–1453. [Google Scholar] [CrossRef] [PubMed]
- Ozbek, N.; Katircioglu, H.; Karacan, N.; Baykal, T. Synthesis, characterization and antimicrobial activity of new aliphatic sulfonamide. Bioorg. Med. Chem. 2007, 15, 5105–5109. [Google Scholar] [CrossRef] [PubMed]
- Hassan, J.; Sevignon, M.; Gozzi, C.; Schulz, E.; Lamaire, M.C. Aryl-aryl bond formation one century after the discovery of the Ullmann reaction. Chem. Rev. 2002, 102, 1359–1469. [Google Scholar] [CrossRef] [PubMed]
- Arshad, M.N.; Mahmood, T.; Khan, A.F.; Zia-Ur-Rehman, M.; Asiri, A.M.; Khan, I.; Nisa, R.U.; Ayub, K.; Mukhtar, A.; Saeed, M.T. Synthesis, crystal structure and spectroscopic properties of 1,2-benzothiazine derivatives: An experimental and DFT study. Chin. J. Struct. Chem. 2014, 34, 15–25. [Google Scholar]
- Arshad, M.N.; Asiri, A.M.; Alamry, K.A.; Mahmood, T.; Gilani, M.A.; Ayub, K.; Birinji, A.S. Synthesis, crystal structure, spectroscopic and density functional theory (DFT) study of N-[3-anthracen-9-yl-1-(4-bromo-phenyl)-allylidene]-N-benzenesulfonohydrazine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 142, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Singh, D.U.; Pankajkumar, R.S.; Shriniwas, D.S. Fe-exchanged montmorillonite K10—The first heterogenous catalyst for acylation of sulfonamides with carboxylic acid anhydrides. Tetrahedron Lett. 2004, 45, 4805–4807. [Google Scholar] [CrossRef]
- Rozentsveig, I.B.; Aizina, Y.A.; Chernyshev, K.A.; Klyna, L.V.; Zhanchipova, E.R.; Sukhomazova, E.N.; Krivdin, L.B.; Levkovskaya, G.G. 2,5-Dihalothiophenes in the reaction with chlorosulfonic acid. Russ. J. Gen. Chem. 2007, 77, 926–931. [Google Scholar] [CrossRef]
- Tung, D.T.; Tuan, D.T.; Rasool, N.; Villinger, A.; Reinke, H.; Fischer, C.; Langer, P. Regioselective palladium (0)-catalyzed cross-coupling reactions and metal-halide exchange reactions of tetrabromothiophene: Optimization, scope and limitations. Adv. Synth. Catal. 2009, 351, 1595–1609. [Google Scholar] [CrossRef]
- Reinhard, B. Advance Organic Chemistry; Harcourt/Academic Press: San Diego, CA, USA, 2001; pp. 530–531. [Google Scholar]
- Arshad, M.N.; Bibi, A.; Mahmood, T.; Asiri, A.M.; Ayub, K. Synthesis, crystal structures and spectroscopic properties of triazine based hydrazone derivatives; a comparative experimental-theoretical study. Molecules 2015, 20, 5851–5874. [Google Scholar] [CrossRef] [PubMed]
- Szabo, L.; Chis, V.; Pirnau, A.; Leopold, N.; Cozar, O.; Orosz, S. Spectroscopic and theoretical study of amlodipine besylate. J. Mol. Struct. 2009, 924, 385–392. [Google Scholar] [CrossRef]
- Scrocco, E.; Tomasi, J. Electronic molecular structure, reactivity and intermolecular forces: An euristic interpretation by means of electrostatic molecular potentials. Adv. Quantum Chem. 1978, 11, 115–121. [Google Scholar]
- Sagadevan, S. Investigation on the optical properties of nonlinear optical (NLO) single crystal: l-valine zinc hydrochloride. Am. J. Optics Photogr. 2014, 2, 24–27. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, X.; Jiang, Y.; Wang, G.; Qu, C.; Zhao, B. Computational study on the second-order nonlinear optical properties of coumarin derivatives with N-p-vinylphenyl carbazole chromophores. Asian J. Chem. 2014, 26, 7404–7408. [Google Scholar]
- Hameed, A.; Anwar, A.; Khan, K.M.; Malik, R.; Shahab, F.; Siddiq, S.; Basha, F.M.; Choudhary, M.I. Urease inhibition and anticancer activity of novel polyfunctional 5,6-dihydropyridine derivatives and their structure-activity relationship. Eur. J. Chem. 2013, 4, 49–52. [Google Scholar] [CrossRef]
- Sokmen, B.B.; Hulya, C.O.; Ayse, Y.; Refiye, Y. Anti-elastic, antiurease and antioxidant activities of (3–13)-monohydroxyeicosanoic acid isomers. J. Serbian Chem. Soc. 2012, 77, 1353–1361. [Google Scholar] [CrossRef]
- Rauf, A.; Ahmad, F.; Qureshi, A.M.; Rehman, A.; Khan, A.; Qadir, M.I.; Choudhary, M.I.; Chohan, Z.H.; Youssoufi, M.H.; Hadda, T.B. Synthesis and urease inhibition studies of barbituric and thiobarbituric acid derived sulphonamides. J. Chin. Chem. Soc. 2011, 58, 528–537. [Google Scholar] [CrossRef]
- Gull, Y.; Rasool, N.; Noreen, M.; Nasim, F.U.H.; Yaqoob, A.; Kousar, S.; Rasheed, U.; Bukhari, I.H.; Zubair, M.; Islam, M.S. Efficient synthesis of 2-amino-6-arylbenzothiazoles via Pd (0) Suzuki cross coupling reactions: Potent urease enzyme inhibition and nitric oxide scavenging activities of the products. Molecules 2013, 18, 8845–8857. [Google Scholar] [CrossRef] [PubMed]
- Kulsoom, S.; Hassan, F.; Naqvi, S.B.S. Antibacterial studies of sulfacetamide ophthalmic solutions and suspensions. Pakistan J. Pharmacol. 2005, 22, 25–34. [Google Scholar]
- Paramashivappa, R.; Kumar, P.P.; Vithayathil, P.J.; Rao, A.S. Novel method for isolation of major phenolic constituents from cashew (Anacardium occidentale L.) nut shell liquid. J. Agric. Food Chem. 2001, 49, 2548–2551. [Google Scholar] [CrossRef] [PubMed]
- Andrighetti-Fröhner, C.R.; de Oliveira, K.N.; Gaspar-Silva, D.; Pacheco, L.K.; Joussef, A.C.; Steindel, M.; Simoes, C.M.O.; de Souza, A.M.T.; Magalhaes, U.O.; Afonso, I.F. Synthesis, biological evaluation and SAR of sulfonamide 4-methoxychalcone derivatives with potential antileishmanial activity. Eur. J. Med. Chem. 2009, 44, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Roy, D.; Todd, K.; John, M. Gauss View; Version 5; Semichem, Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Pervez, H.; Muhammad, R.; Muhammad, Y.; Faizul-Hassan, N.; Khalid, M.K. Synthesis and biological evaluation of some new N4-aryl substituted 5-chloroisatin-3-thiosemicarbazones. Med. Chem. 2012, 8, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 4a–g are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noreen, M.; Rasool, N.; Gull, Y.; Zubair, M.; Mahmood, T.; Ayub, K.; Nasim, F.-u.-H.; Yaqoob, A.; Zia-Ul-Haq, M.; De Feo, V. Synthesis, Density Functional Theory (DFT), Urease Inhibition and Antimicrobial Activities of 5-Aryl Thiophenes Bearing Sulphonylacetamide Moieties. Molecules 2015, 20, 19914-19928. https://doi.org/10.3390/molecules201119661
Noreen M, Rasool N, Gull Y, Zubair M, Mahmood T, Ayub K, Nasim F-u-H, Yaqoob A, Zia-Ul-Haq M, De Feo V. Synthesis, Density Functional Theory (DFT), Urease Inhibition and Antimicrobial Activities of 5-Aryl Thiophenes Bearing Sulphonylacetamide Moieties. Molecules. 2015; 20(11):19914-19928. https://doi.org/10.3390/molecules201119661
Chicago/Turabian StyleNoreen, Mnaza, Nasir Rasool, Yasmeen Gull, Muhammad Zubair, Tariq Mahmood, Khurshid Ayub, Faiz-ul-Hassan Nasim, Asma Yaqoob, Muhammad Zia-Ul-Haq, and Vincenzo De Feo. 2015. "Synthesis, Density Functional Theory (DFT), Urease Inhibition and Antimicrobial Activities of 5-Aryl Thiophenes Bearing Sulphonylacetamide Moieties" Molecules 20, no. 11: 19914-19928. https://doi.org/10.3390/molecules201119661
APA StyleNoreen, M., Rasool, N., Gull, Y., Zubair, M., Mahmood, T., Ayub, K., Nasim, F.-u.-H., Yaqoob, A., Zia-Ul-Haq, M., & De Feo, V. (2015). Synthesis, Density Functional Theory (DFT), Urease Inhibition and Antimicrobial Activities of 5-Aryl Thiophenes Bearing Sulphonylacetamide Moieties. Molecules, 20(11), 19914-19928. https://doi.org/10.3390/molecules201119661