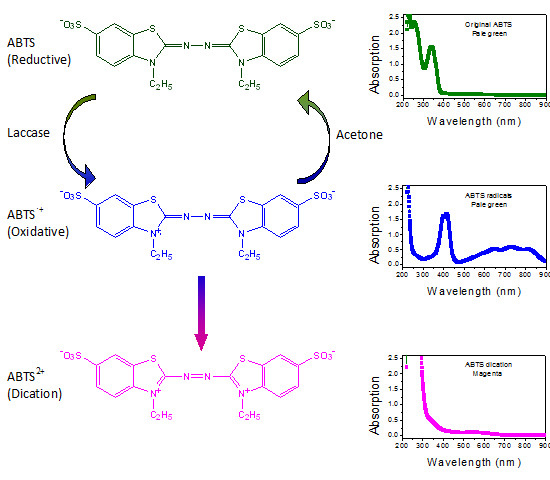
Radical Scavenging by Acetone: A New Perspective to Understand Laccase/ABTS Inactivation and to Recover Redox Mediator
Abstract
:1. Introduction
2. Results and Discussion
2.1. Inhibition of Laccase-Catalyzed ABTS Oxidation by Acetone
| Acetone Content (%, v/v) | Km (μM) | Vmax (μM·min−1·[E]−1 *) | Vmax/Km (L·mg−1·min−1) |
|---|---|---|---|
| 0 | 7.67 ± 0.44 | 42.86 ± 0.49 | 5.588 |
| 12.5 | 25.07 ± 0.65 | 28.76 ± 0.45 | 1.147 |
| 25 | 58.66 ± 2.24 | 8.13 ± 0.24 | 0.139 |
| 37.5 | 91.99 ± 0.99 | 4.03 ± 0.08 | 0.044 |
| 50 | 370.08 ± 20.55 | 1.94 ± 0.04 | 0.005 |
2.2. Scavenging ABTS Radicals by Acetone
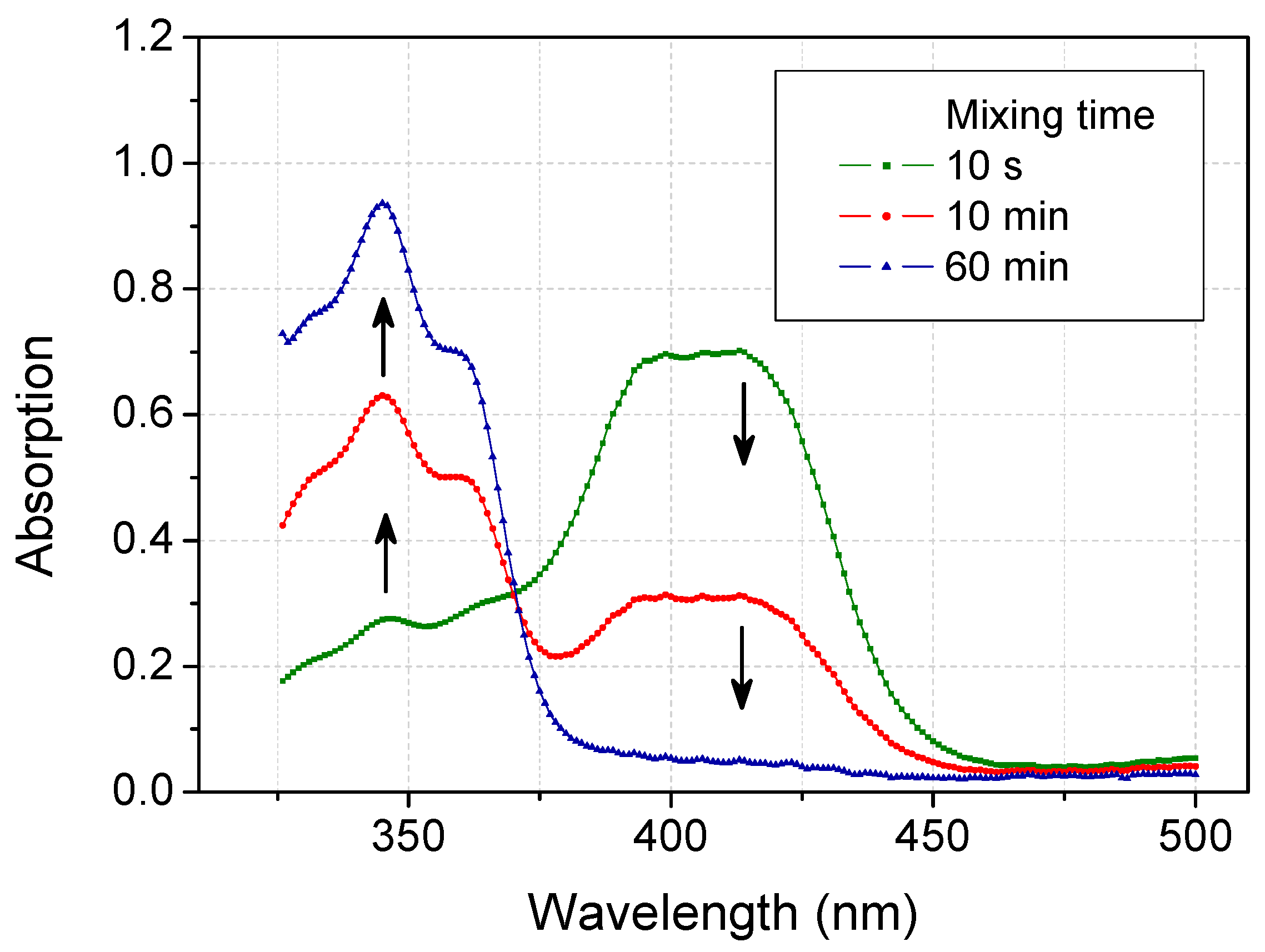
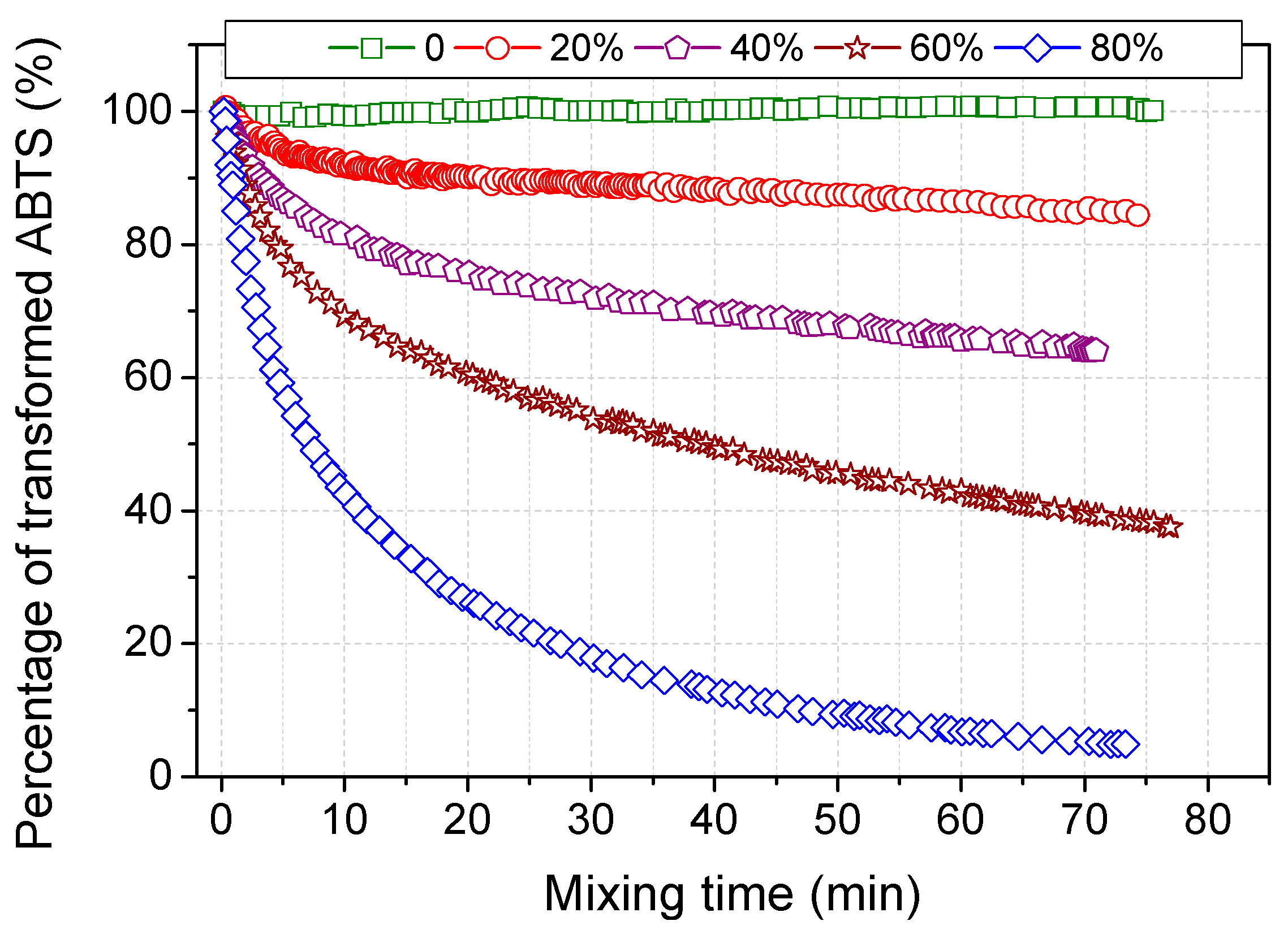
| Solvents | Reduction Percentage at 45 min (%) | Time for 50% Reduction (min) | Time for 90% Reduction (min) |
|---|---|---|---|
| Acetic acid | 9.6 ± 0.6 | ND * | ND |
| Methanol | 39.3 ± 1.6 | 83 ± 2.9 | 346 ± 4.5 |
| Ethanol | 50.5 ± 0.4 | 41 ± 1.8 | 209 ± 3.5 |
| Isopropanol | 84.5 ± 0.9 | 15 ± 0.4 | 51 ± 1.3 |
| Acetone | 88.7 ± 1.1 | 7 ± 0.1 | 47 ± 0.4 |
| 1,4-Dioxane | 94.1 ± 0.9 | 2 ± 0.1 | 13 ± 0.7 |
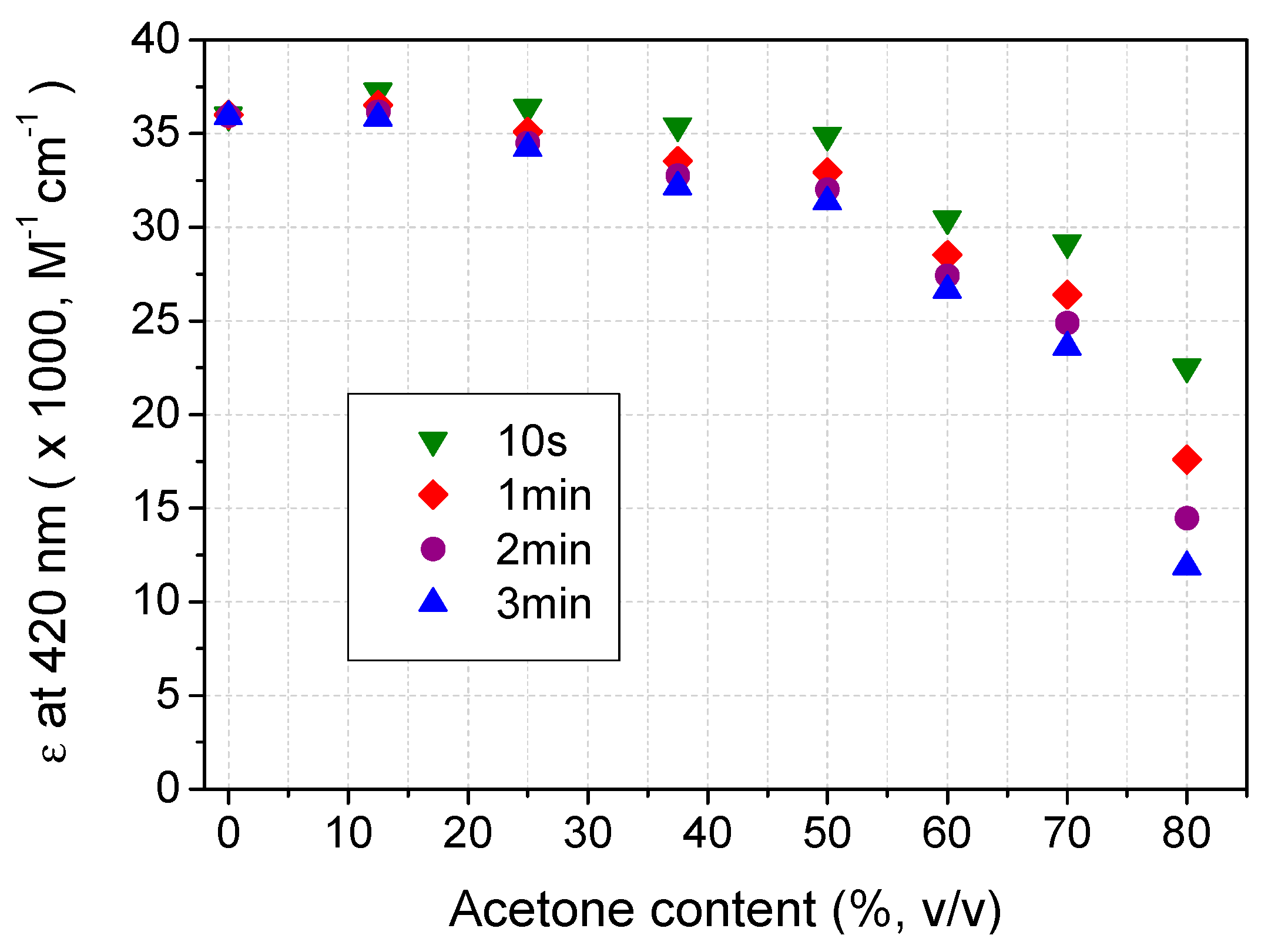
2.3. Recovery of ABTS in Aqueous Acetone Media
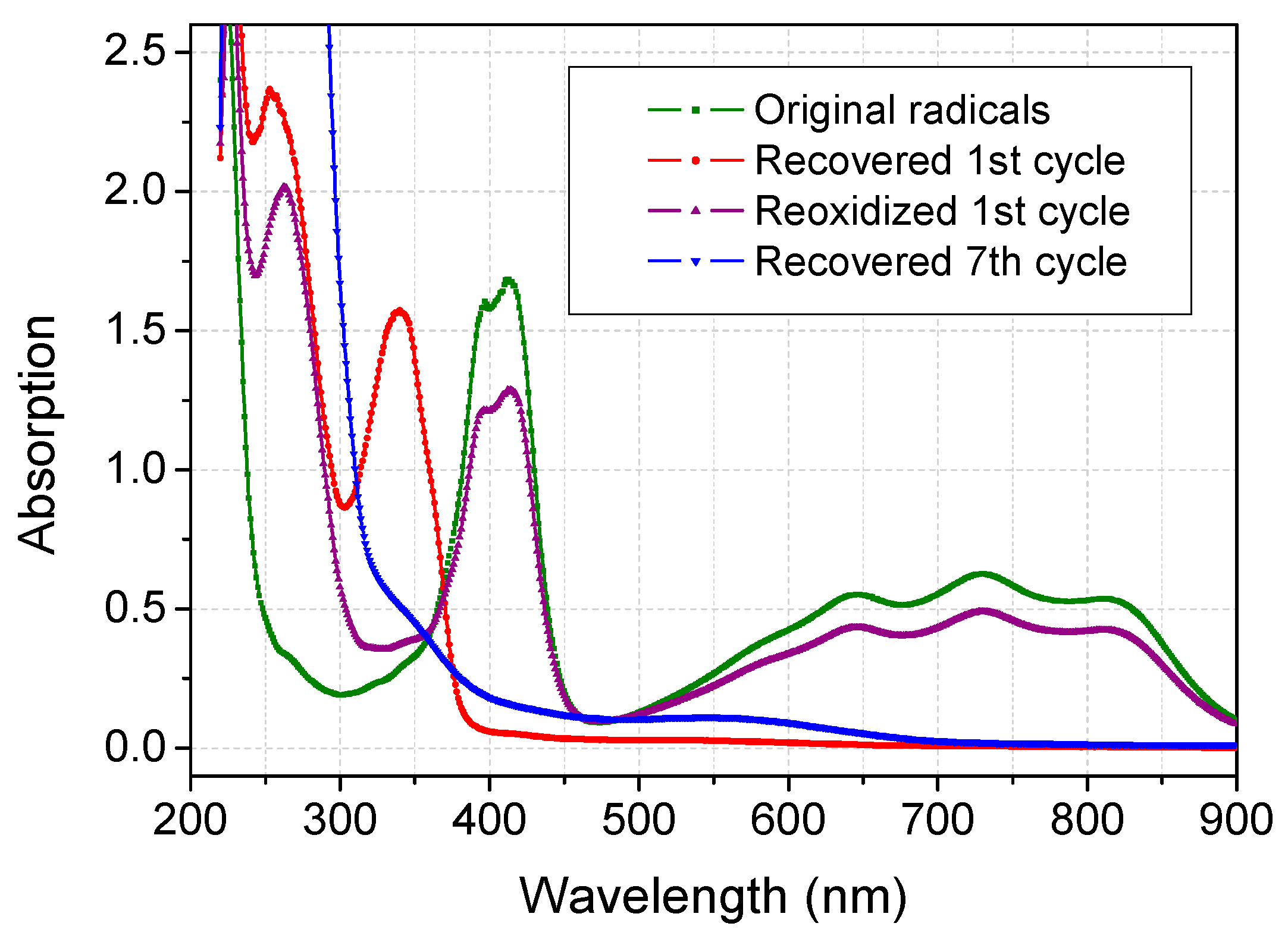
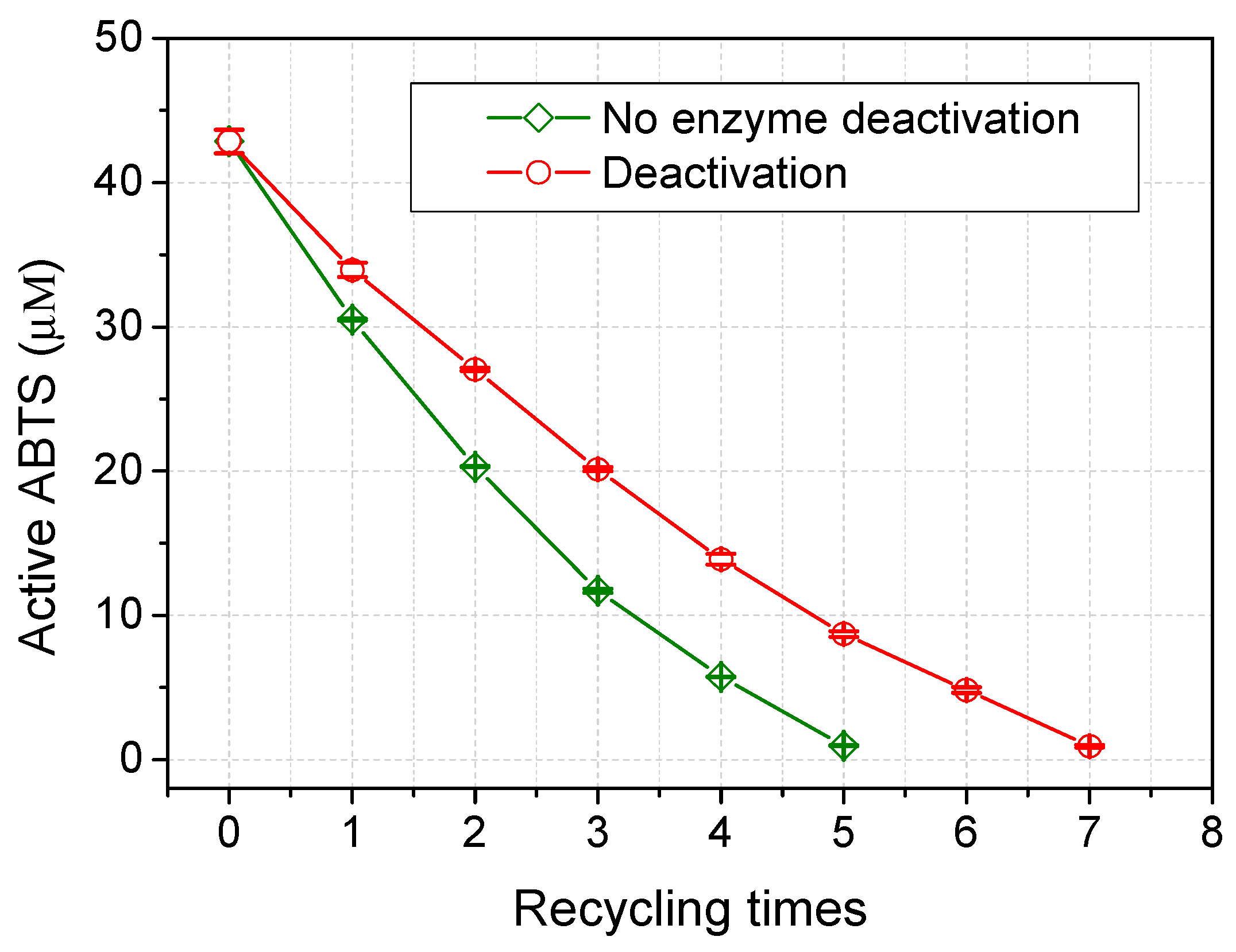
3. Experimental Section
3.1. Chemicals and Reagents
3.2. Laccase/ABTS Reaction
3.3. ABTS Recovery Procedure
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, X.; Bai, R.; Zhang, Y.; Wang, Q.; Fan, X.; Yuan, J.; Cui, L.; Wang, P. Laccase-catalyzed oxidative polymerization of phenolic compounds. Appl. Biochem. Biotechnol. 2013, 171, 1673–1680. [Google Scholar] [CrossRef] [PubMed]
- Mogharabi, M.; Faramarzi, M.A. Laccase and laccase-mediated systems in the synthesis of organic compounds. Adv. Synth. Catal. 2014, 356, 897–927. [Google Scholar] [CrossRef]
- Mai, C.; Schormann, W.; Hüttermann, A.; Kappl, R.; Hüttermann, J. The influence of laccase on the chemo-enzymatic synthesis of lignin graft-copolymers. Enzyme Microb. Technol. 2002, 30, 66–72. [Google Scholar] [CrossRef]
- Bourbonnais, R.; Paice, M.G. Oxidation of non-phenolic substrates: An expanded role for laccase in lignin biodegradation. FEBS Lett. 1990, 267, 99–102. [Google Scholar] [CrossRef]
- Kunamneni, A.; Camarero, S.; García-Burgos, C.; Plou, F.J.; Ballesteros, A.; Alcalde, M. Engineering and applications of fungal laccases for organic synthesis. Microb. Cell Fact. 2008, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, R.; Uyama, H.; Kobayashi, S. Novel synthetic pathway to a poly (phenylene oxide). Laccase-catalyzed oxidative polymerization of syringic acid. Macromolecules 1996, 29, 3053–3054. [Google Scholar] [CrossRef]
- Rodakiewicz-Nowak, J.; Kasture, S.M.; Dudek, B. Effect of various water-miscible solvents on enzymatic activity of fungal laccases. J. Mol. Catal. B Enzym. 2000, 11, 1–11. [Google Scholar] [CrossRef]
- Ruiz, A.I.; Malavé, A.J.; Felby, C.; Griebenow, K. Improved activity and stability of an immobilized recombinant laccase in organic solvents. Biotechnol. Lett. 2000, 22, 229–233. [Google Scholar] [CrossRef]
- Chhabra, M.; Mishra, S.; Sreekrishnan, T.R. Laccase/mediator assisted degradation of triarylmethane dyes in a continuous membrane reactor. J. Biotechnol. 2009, 143, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Rodakiewicz-Nowak, J.; Jarosz-Wilkołazka, A. Catalytic activity of Cerrena unicolor laccase in aqueous solutions of water-miscible organic solvents—Experimental and numerical description. J. Mol. Catal. B Enzym. 2007, 44, 53–59. [Google Scholar] [CrossRef]
- Van Erp, S.H.; Kamenskaya, E.O.; Khmelnitsky, Y.L. The effect of water content and nature of organic solvent on enzyme activity in low-water media: A quantitative description. Eur. J. Biochem. 1991, 202, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Bell, G.; Janssen, A.E.M.; Halling, P.J. Water activity fails to predict critical hydration level for enzyme activity in polar organic solvents: Interconversion of water concentrations and activities. Enzyme Microb. Technol. 1997, 20, 471–477. [Google Scholar] [CrossRef]
- Yang, L.; Dordick, J.S.; Garde, S. Hydration of enzyme in nonaqueous media is consistent with solvent dependence of its activity. Biophys. J. 2004, 87, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, R.; Muniglia, L.; Rovel, B.; Girardin, M. Phenolic colorants obtained by enzymatic synthesis using a fungal laccase in a hydro-organic biphasic system. Food Res. Int. 2005, 38, 995–1000. [Google Scholar] [CrossRef]
- Díaz-González, M.; Vidal, T.; Tzanov, T. Phenolic compounds as enhancers in enzymatic and electrochemical oxidation of veratryl alcohol and lignins. Appl. Microbiol. Biotechnol. 2011, 89, 1693–1700. [Google Scholar] [CrossRef] [PubMed]
- El-Agamey, A.; McGarvey, D.J. Acyl/aroylperoxyl radicals: A comparative study of the reactivity of peroxyl radicals resulting from the α-cleavage of ketones. Phys. Chem. Chem. Phys. 2002, 4, 1611–1617. [Google Scholar] [CrossRef]
- Collins, P.J.; Dobson, A.D.W.; Field, J.A. Reduction of the 2, 2′-azinobis (3-ethylbenzthiazoline-6-sulfonate) cation radical by physiological organic acids in the absence and presence of manganese. Appl. Environ. Microbiol. 1998, 64, 2026–2031. [Google Scholar] [PubMed]
- Osman, A.M.; Wong, K.K.Y.; Hill, S.J.; Fernyhough, A. Isolation and the characterization of the degradation products of the mediator ABTS-derived radicals formed upon reaction with polyphenols. Biochem. Biophys. Res. Commun. 2006, 340, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Brezova, V.; Dvoranova, D.; Staško, A. Characterization of titanium dioxide photoactivity following the formation of radicals by EPR spectroscopy. Res. Chem. Intermediat. 2007, 33, 251–268. [Google Scholar] [CrossRef]
- Solís-Oba, M.; Ugalde-Saldívar, V.M.; González, I.; Viniegra-González, G. An electrochemical-spectrophotometrical study of the oxidized forms of the mediator 2, 2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid) produced by immobilized laccase. J. Electroanal. Chem. 2005, 579, 59–66. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the ABTS radicals and ABTS dication are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhou, P.; Wu, X.; Sun, J.; Chen, S. Radical Scavenging by Acetone: A New Perspective to Understand Laccase/ABTS Inactivation and to Recover Redox Mediator. Molecules 2015, 20, 19907-19913. https://doi.org/10.3390/molecules201119672
Liu H, Zhou P, Wu X, Sun J, Chen S. Radical Scavenging by Acetone: A New Perspective to Understand Laccase/ABTS Inactivation and to Recover Redox Mediator. Molecules. 2015; 20(11):19907-19913. https://doi.org/10.3390/molecules201119672
Chicago/Turabian StyleLiu, Hao, Pandeng Zhou, Xing Wu, Jianliang Sun, and Shicheng Chen. 2015. "Radical Scavenging by Acetone: A New Perspective to Understand Laccase/ABTS Inactivation and to Recover Redox Mediator" Molecules 20, no. 11: 19907-19913. https://doi.org/10.3390/molecules201119672
APA StyleLiu, H., Zhou, P., Wu, X., Sun, J., & Chen, S. (2015). Radical Scavenging by Acetone: A New Perspective to Understand Laccase/ABTS Inactivation and to Recover Redox Mediator. Molecules, 20(11), 19907-19913. https://doi.org/10.3390/molecules201119672