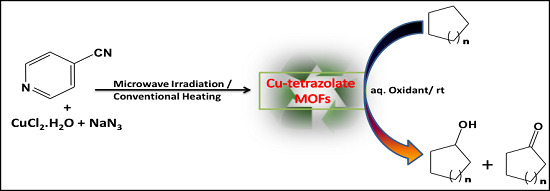
Greener Selective Cycloalkane Oxidations with Hydrogen Peroxide Catalyzed by Copper-5-(4-pyridyl)tetrazolate Metal-Organic Frameworks
Abstract
:1. Introduction
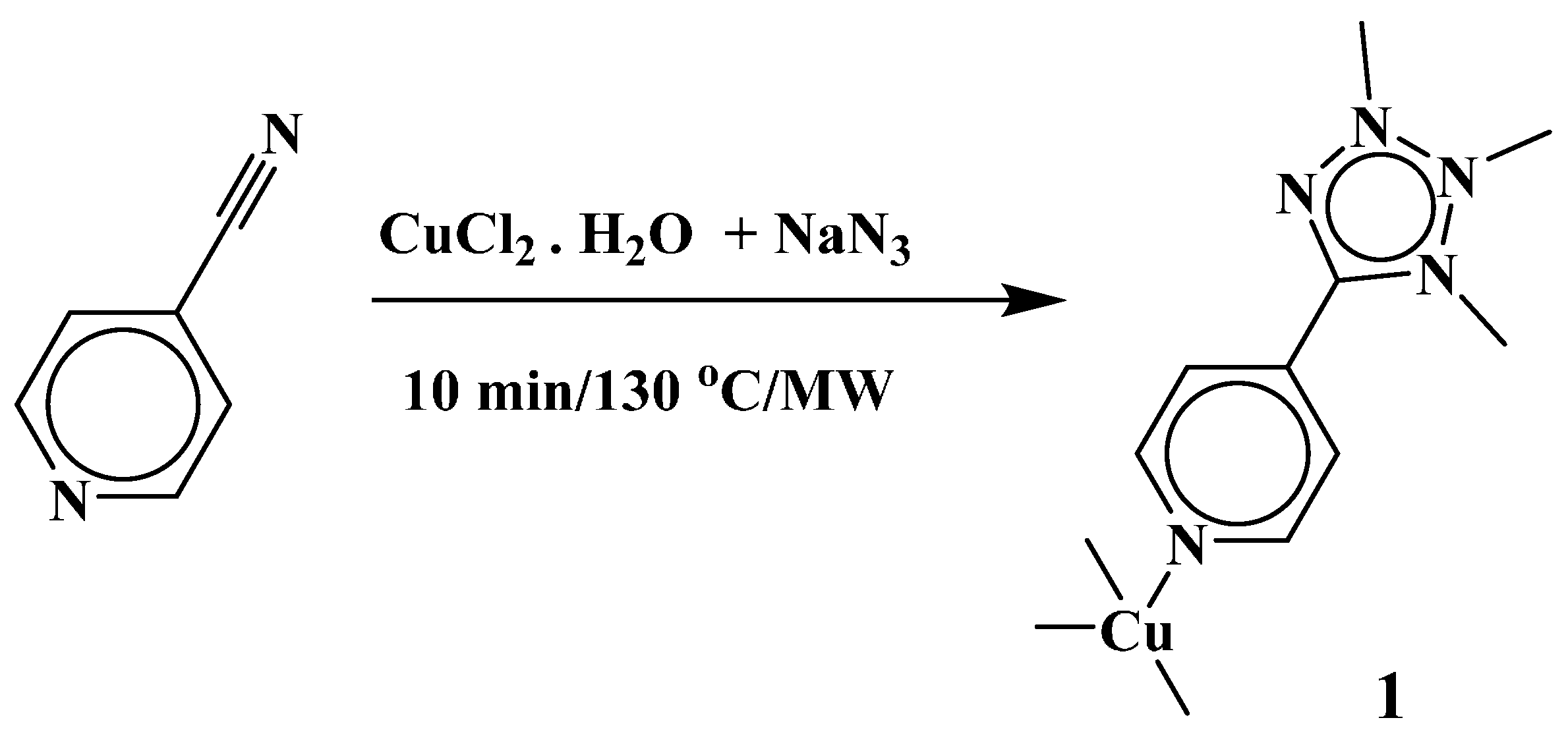
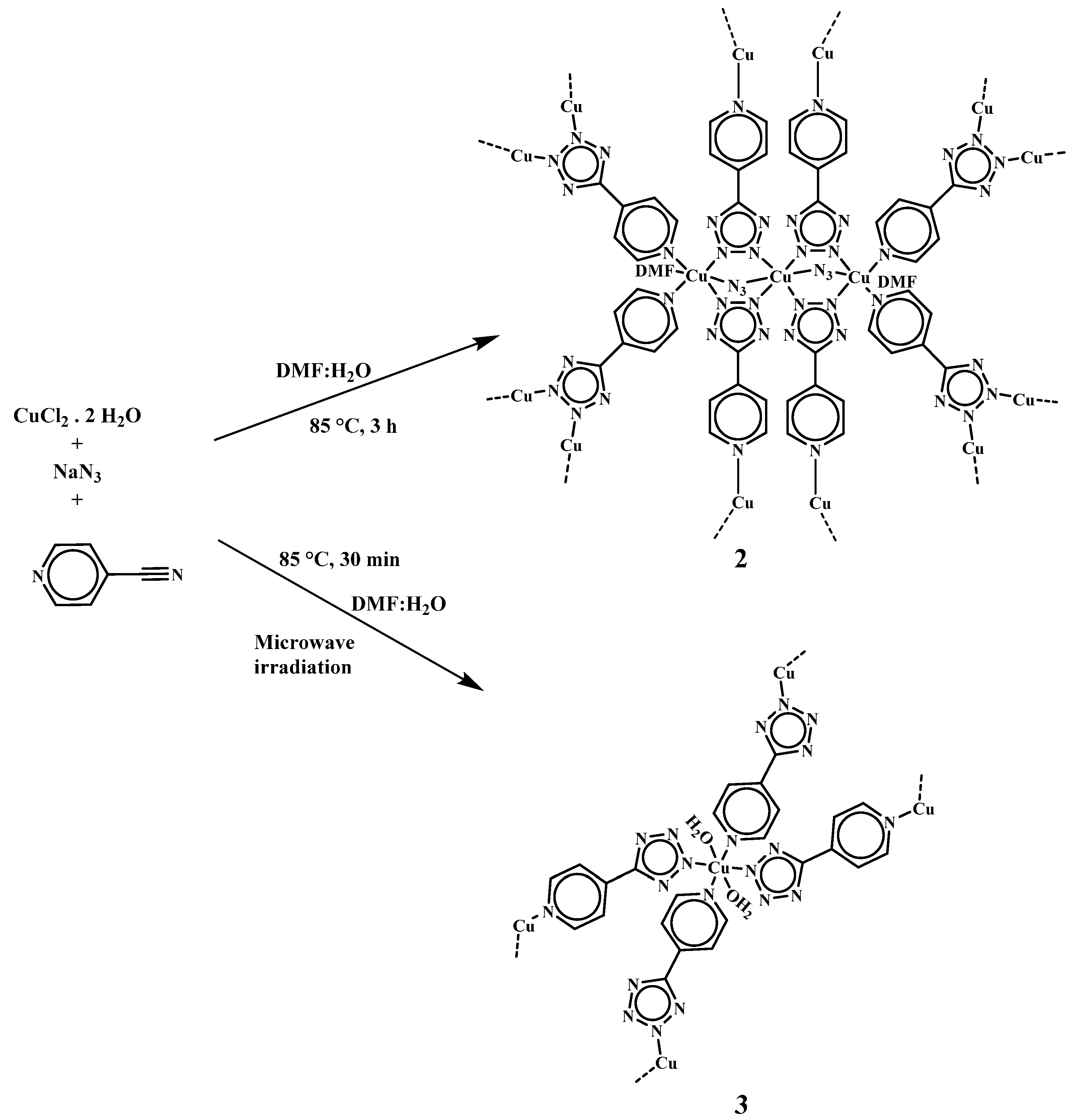
2. Results and Discussion
2.1. Synthesis and Spectroscopic Characterization of [Cu(µ4-4-ptz)]n (1)
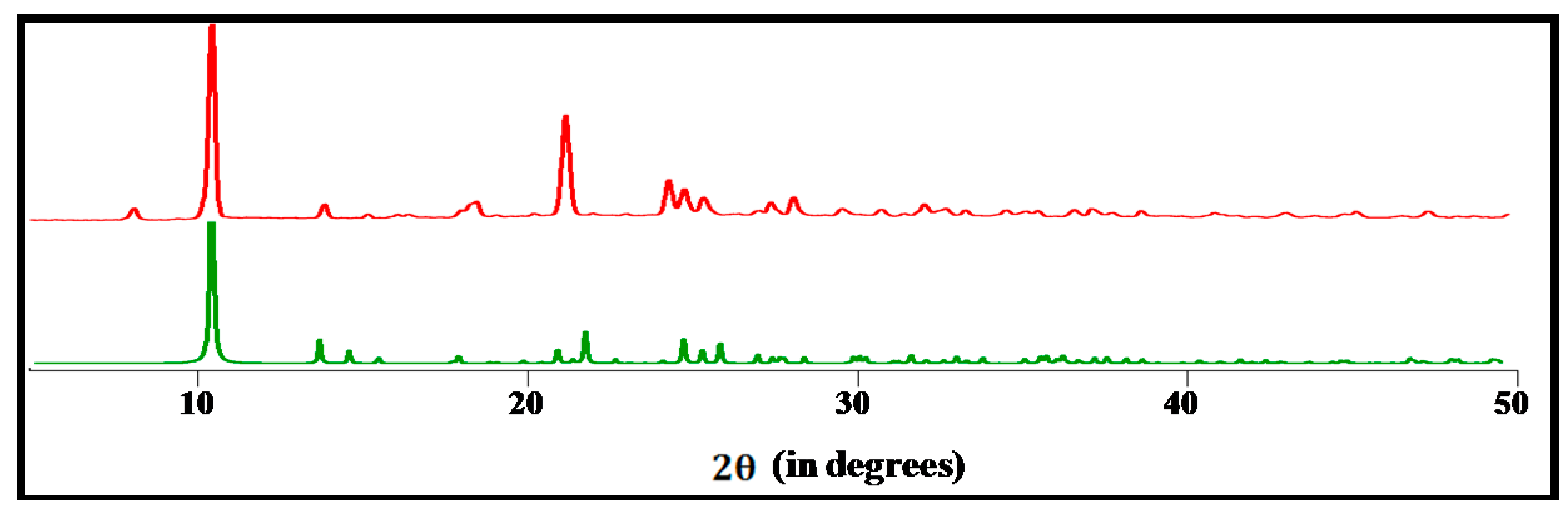
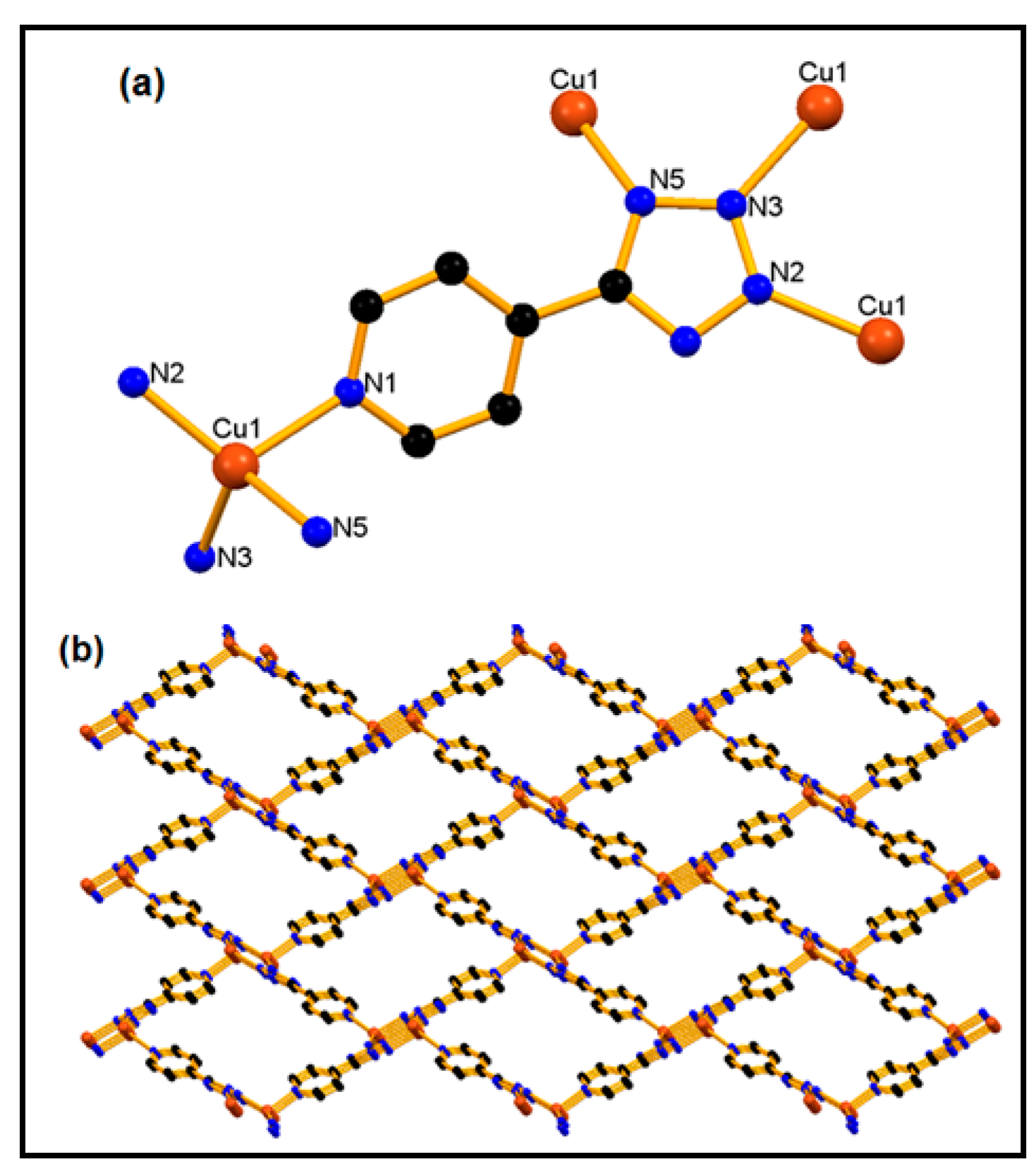
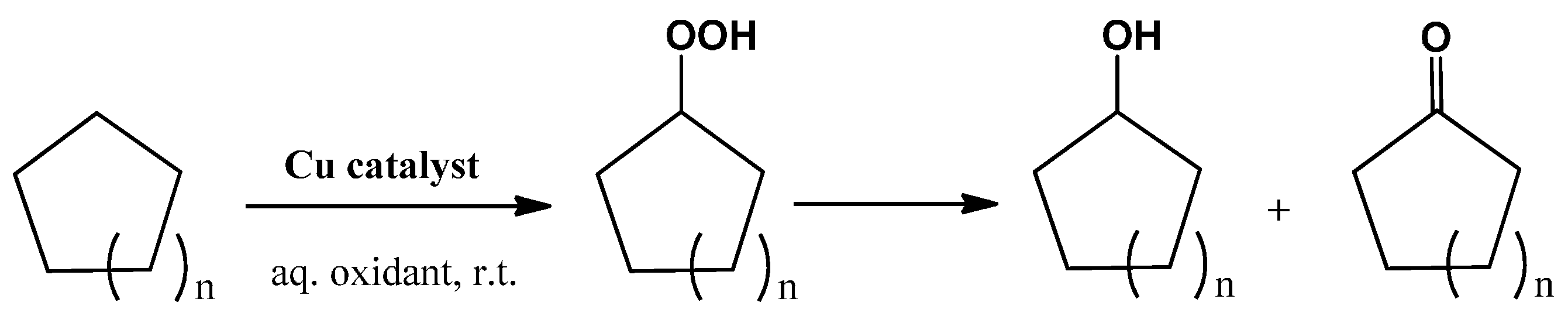
2.2. Catalytic Oxidation of Cycloalkanes
| Entry | Catalyst | Substrate | Oxidant | TON [TOF (h−1)] b | OL | Yield (%) c ONE | Total d | OL/ONE Ratio |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | cyclopentane | H2O2 | 260 (26) | 14.3 | 11.7 | 26.0 | 1.2 |
| 2 | TBHP | 440 (44) | 5.5 | 16.7 | 22.2 | 0.3 | ||
| 3 | cyclohexane | H2O2 | 369 (37) | 18.8 | 18.1 | 36.9 | 1.0 | |
| 4 | TBHP | 664 (66) | 11.3 | 21.9 | 33.2 | 0.5 | ||
| 5 | cyclooctane | H2O2 | 218 (22) | 11.7 | 10.1 | 21.8 | 1.2 | |
| 6 | TBHP | 434 (43) | 8.3 | 13.4 | 21.7 | 0.6 | ||
| 7 | 2 | cyclopentane | H2O2 | 276 (28) | 13.1 | 14.5 | 27.6 | 0.9 |
| 8 | TBHP | 416 (42) | 7.6 | 13.2 | 20.8 | 0.6 | ||
| 9 | cyclohexane | H2O2 | 396 (40) | 20.8 | 18.8 | 39.6 | 1.1 | |
| 10 | TBHP | 704 (70) | 12.9 | 22.3 | 35.2 | 0.6 | ||
| 11 | cyclooctane | H2O2 | 343 (34) | 21.4 | 12.9 | 34.3 | 1.7 | |
| 12 | TBHP | 620 (62) | 5.3 | 25.7 | 31.0 | 0.2 | ||
| 13 | 3 | cyclopentane | H2O2 | 215 (22) | 11.9 | 9.6 | 21.5 | 1.2 |
| 14 | TBHP | 218 (22) | 4.0 | 6.9 | 10.9 | 0.6 | ||
| 15 | cyclohexane | H2O2 | 366 (37) | 16.5 | 20.1 | 36.6 | 0.8 | |
| 16 | TBHP | 710 (71) | 13.9 | 21.6 | 35.5 | 0.6 | ||
| 17 | cyclooctane | H2O2 | 285 (29) | 17.8 | 10.7 | 28.5 | 1.7 | |
| 18 | TBHP | 468 (47) | 8.7 | 14.7 | 23.4 | 0.6 | ||
| 19 | none | cyclopentane | H2O2 | - | 2.1 | 1.1 | 3.2 | 1.9 |
| 20 | TBHP | - | 1.1 | 1.8 | 2.9 | 0.6 | ||
| 21 | cyclohexane | H2O2 | - | 2.4 | 1.4 | 3.8 | 1.7 | |
| 22 | TBHP | - | 1.3 | 2.5 | 3.8 | 0.5 | ||
| 23 | cyclooctane | H2O2 | - | 2.2 | 1.5 | 3.7 | 1.5 | |
| 24 | TBHP | - | 0.7 | 1.3 | 2.0 | 0.5 |
| Entry | Catalyst | n(cat.)/n(CyH) × 103 | n(H2O2)/n(cat.) × 10−3 | Reaction Time (h) | Yield (%) b | TON [TOF (h−1)] c | ||
|---|---|---|---|---|---|---|---|---|
| OL | ONE | Total d | ||||||
| 1 | 1 | 4 | 0.5 | 10 | 11.1 | 6.7 | 17.8 | 45 (4.5) |
| 2 | 2 | 1 | 10 | 16.6 | 11.5 | 28.1 | 141 (14) | |
| 3 | 1.3 | 1.5 | 10 | 16.2 | 17.8 | 34.0 | 262 (26) | |
| 4 | 0.8 | 2.5 | 10 | 12.4 | 21.9 | 34.3 | 429 (43) | |
| 5 | 0.4 | 5 | 10 | 2.1 | 18.0 | 19.9 | 498 (50) | |
| 6 | 1 | 2 | 0.25 | 5.5 | 2.6 | 8.1 | 81 (8.1) | |
| 7 | 1 | 2 | 0.5 | 8.4 | 2.6 | 11.0 | 110 (11) | |
| 8 | 1 | 2 | 1 | 9.1 | 5.8 | 14.9 | 149 (15) | |
| 9 | 1 | 2 | 2.5 | 10.5 | 11.3 | 21.8 | 218 (22) | |
| 10 | 1 | 2 | 5 | 17.1 | 13.7 | 30.8 | 308 (31) | |
| 11 | 1 | 2 | 12.5 | 18.7 | 16.0 | 34.7 | 347 (35) | |
| 12 | 1 | 2 | 24 | 6.0 | 19.3 | 25.3 | 253 (25) | |
| 13 e | 1 | 2 | 10 | 1.3 | 1.9 | 3.2 | 32 (3.2) | |
| 14 f | 1 | 2 | 10 | 2.8 | 2.3 | 5.1 | 51 (5.1) | |
| 15 g | 1 | 2 | 10 | 1.1 | 0.2 | 1.3 | 13 (1.3) | |
| 16 h | 1 | 2 | 10 | 1.6 | 1.1 | 2.7 | 27 (2.7) | |
| 17 i | 1 | 2 | 10 | 9.3 | 25.0 | 34.3 | 343 (34) | |
| 18 | 2 | 4 | 0.5 | 10 | 10.2 | 5.4 | 15.6 | 39 (4) |
| 19 | 2 | 1 | 10 | 17.6 | 10.8 | 28.4 | 142 (14) | |
| 20 | 1.3 | 1.5 | 10 | 16.7 | 20.3 | 37.0 | 285 (29) | |
| 21 | 0.8 | 2.5 | 10 | 16.9 | 19.5 | 36.4 | 455 (46) | |
| 22 | 0.4 | 5 | 10 | 5.8 | 10.7 | 16.5 | 413 (41) | |
| 23 | 1 | 2 | 0.25 | 7.5 | 2.4 | 9.9 | 99 (9.9) | |
| 24 | 1 | 2 | 0.5 | 10.6 | 3.3 | 13.9 | 139 (14) | |
| 25 | 1 | 2 | 1 | 14.4 | 4.9 | 19.3 | 193 (19) | |
| 26 | 1 | 2 | 2.5 | 19.7 | 8.8 | 28.5 | 285 (29) | |
| 27 | 1 | 2 | 5 | 17.8 | 18.1 | 35.9 | 359 (36) | |
| 28 | 1 | 2 | 12.5 | 18.0 | 19.6 | 37.6 | 376 (38) | |
| 29 | 1 | 2 | 24 | 5.5 | 21.7 | 27.2 | 272 (27) | |
| 30 e | 1 | 2 | 10 | 0.6 | 0.7 | 1.3 | 13 (1.3) | |
| 31 f | 1 | 2 | 10 | 1.1 | 3.5 | 4.5 | 45 (4.5) | |
| 32 g | 1 | 2 | 10 | 1.7 | 0.8 | 2.5 | 25 (2.5) | |
| 33 h | 1 | 2 | 10 | 0.9 | 1.2 | 2.1 | 21 (2.1) | |
| 34 i | 1 | 2 | 10 | 11.9 | 26.0 | 37.9 | 379 (38) | |
| 35 | 3 | 4 | 0.5 | 10 | 10.2 | 8.7 | 18.9 | 47 (4.7) |
| 36 | 2 | 1 | 10 | 17.1 | 12.9 | 30.0 | 150 (15) | |
| 37 | 1.3 | 1.5 | 10 | 16.3 | 18.8 | 35.1 | 270 (27) | |
| 38 | 0.8 | 2.5 | 10 | 15.2 | 20.1 | 35.3 | 441 (44) | |
| 39 | 0.4 | 5 | 10 | 8.4 | 15.3 | 23.7 | 593 (59) | |
| 40 | 1 | 2 | 0.25 | 7.1 | 4.4 | 11.5 | 115 (12) | |
| 41 | 1 | 2 | 0.5 | 8.3 | 5.7 | 14.0 | 140 (14) | |
| 42 | 1 | 2 | 1 | 9.1 | 8.5 | 17.6 | 176 (18) | |
| 43 | 1 | 2 | 2.5 | 15.6 | 11.9 | 27.5 | 275 (28) | |
| 44 | 1 | 2 | 5 | 19.5 | 14.1 | 33.6 | 336 (34) | |
| 45 | 1 | 2 | 12.5 | 22.9 | 20.2 | 35.1 | 351 (35) | |
| 46 | 2 | 1 | 24 | 14.9 | 40.1 | 23.5 | 235 (24) | |
| 47 e | 1 | 2 | 10 | 0.8 | 0.6 | 1.4 | 14 (1.4) | |
| 48 f | 1 | 2 | 10 | 1.2 | 1.1 | 2.3 | 23 (2.3) | |
| 49 g | 1 | 2 | 10 | 1.4 | 0.7 | 2.1 | 21 (2.1) | |
| 50 h | 1 | 2 | 10 | 0.7 | 1.2 | 1.9 | 19 (1.9) | |
| 51 i | 1 | 2 | 10 | 8.4 | 26.9 | 35.3 | 353 (35) | |
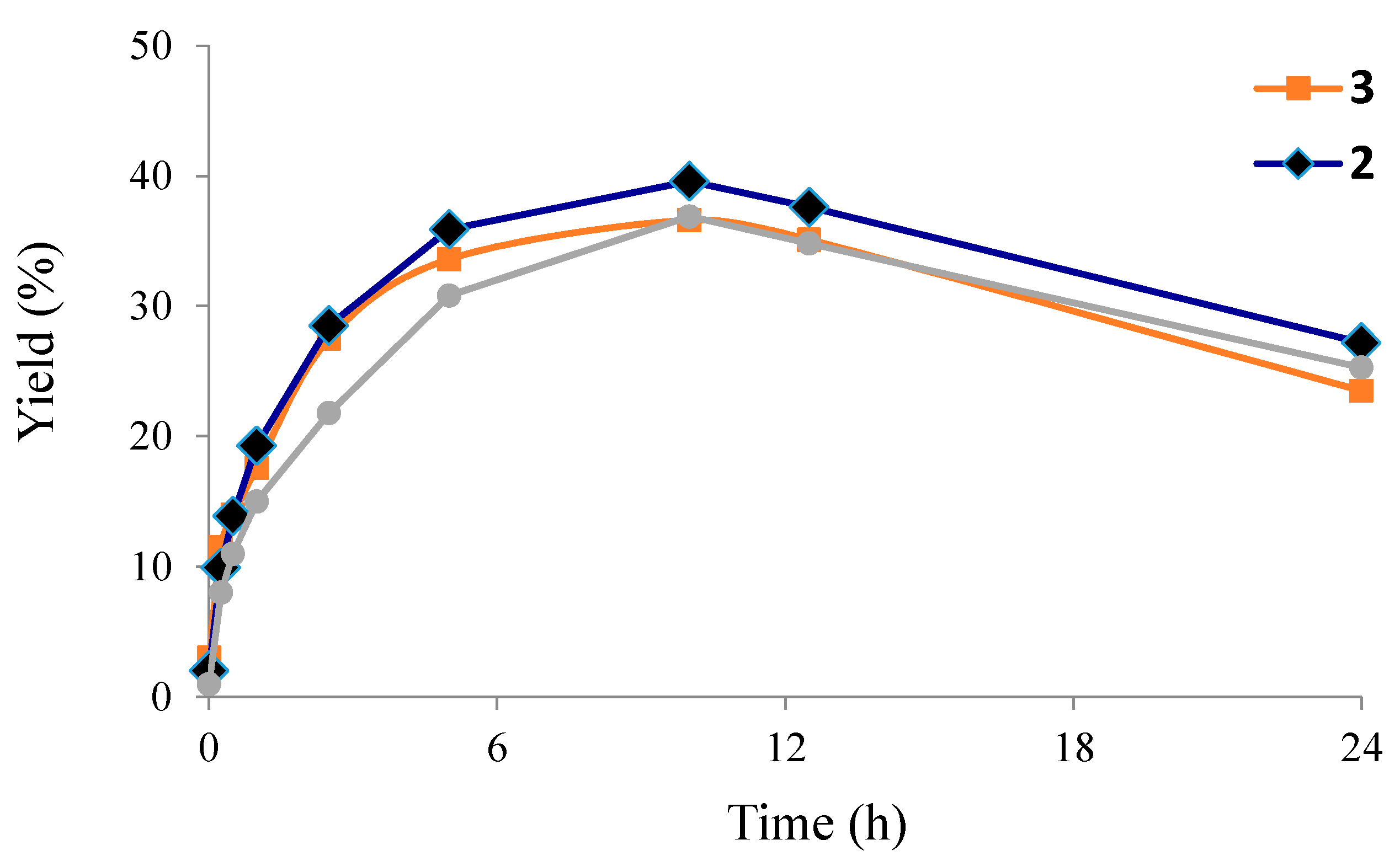
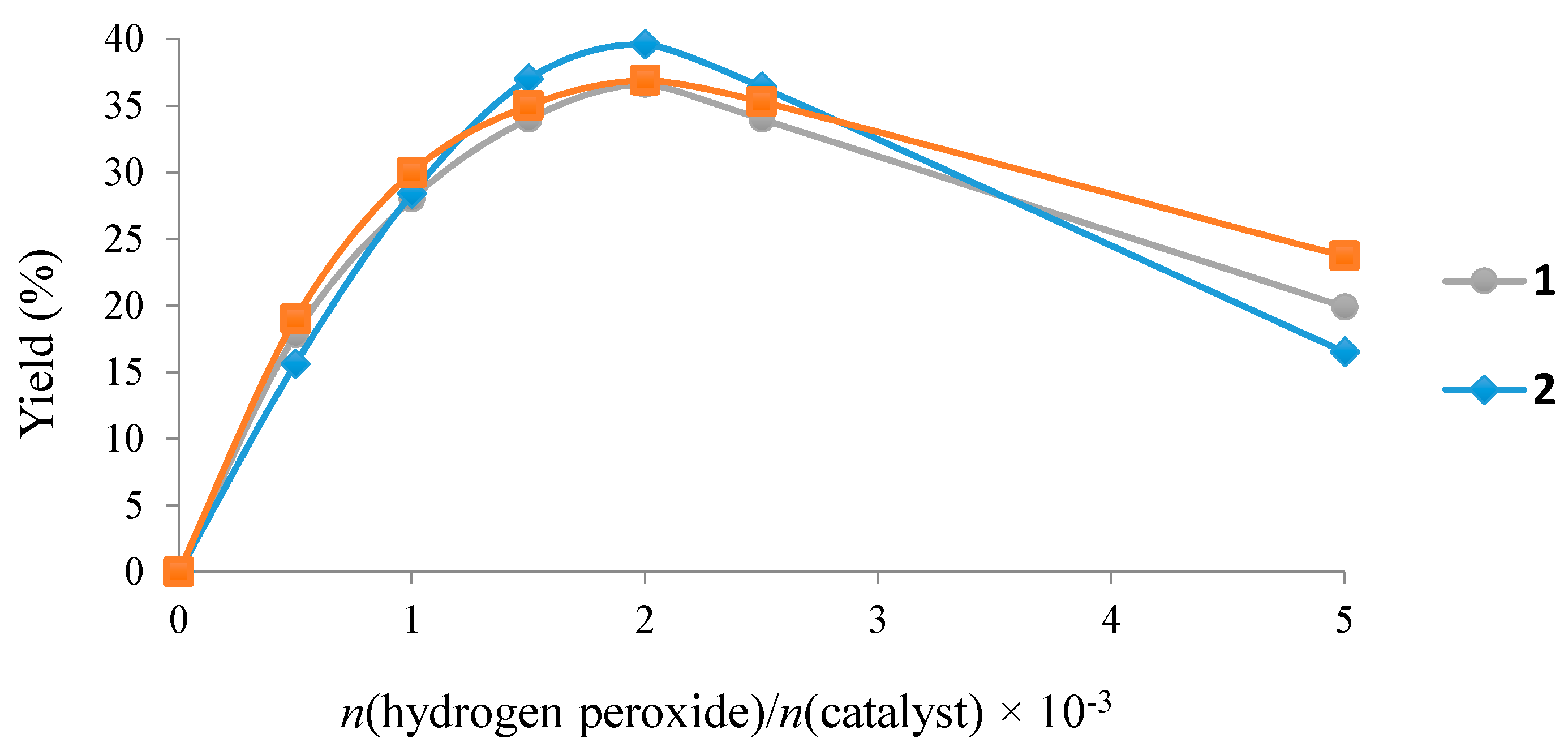
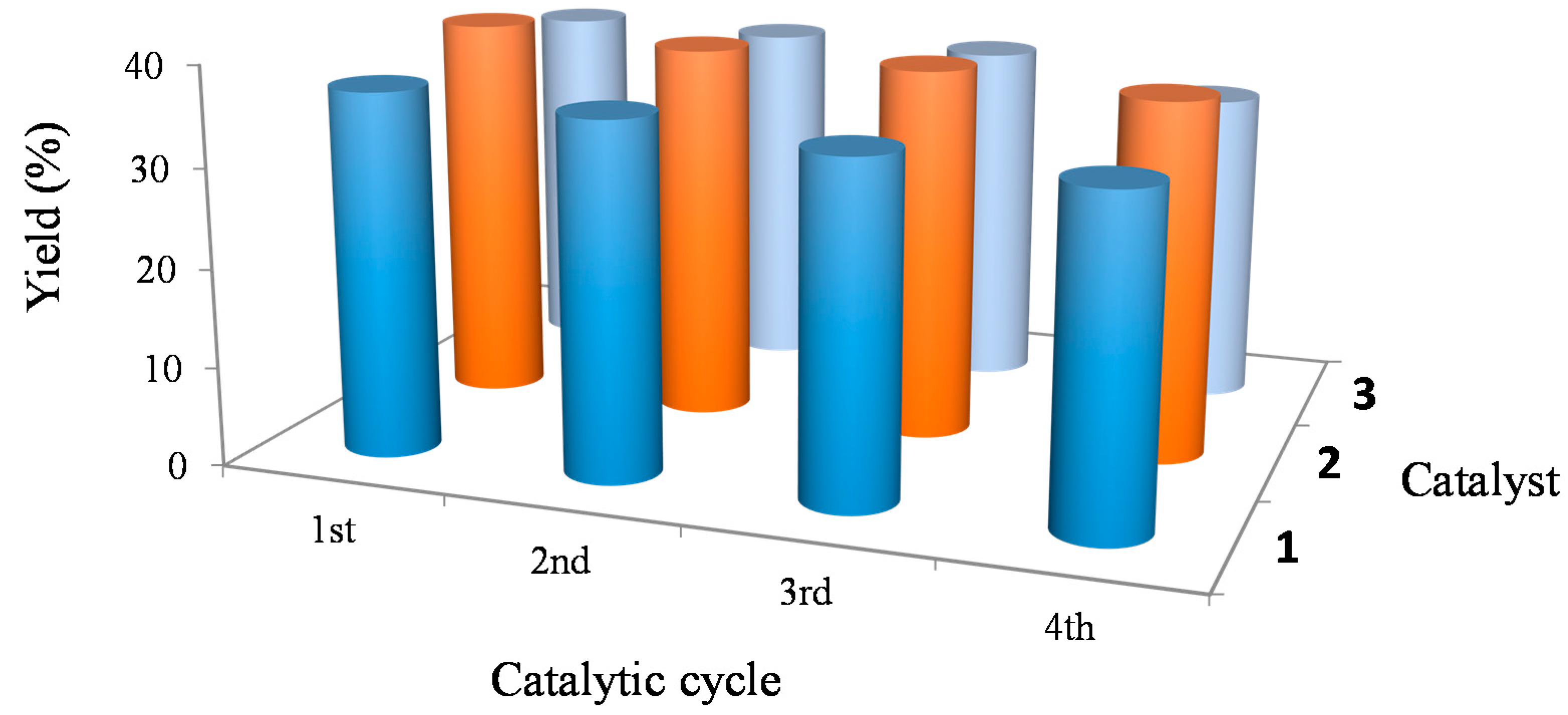
3. Experimental Section
3.1. General Information
| 1 | |
|---|---|
| Empirical formula | C6H4CuN5 |
| Mr (g·mol−1) | 209.68 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| a (Å) | 5.8169(2) |
| b (Å) | 16.8804(6) |
| c (Å) | 9.0264(5) |
| α (°) | 90 |
| β (°) | 94.070 |
| γ (°) | 90 |
| V (Å3) | 884.08(7) |
| Z | 4 |
| Dcalcd (mgm−3) | 1.575 |
| F(000) | 416 |
| GOF | 1.259 |
| Reflections collected/unique | 5386/1546 |
| Final R indices | R1 = 0.0355, wR2 = 0.1032 |
| R indices (all data) | R1 = 0.0367, wR2 = 0.1036 |
3.2. Synthesis and Characterization of Complex [Cu(µ4-4-ptz)]n (1)
3.3. Typical Procedures for the Catalytic Oxidation of Cycloalkanes and Product Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, X.-L.; Li, N.; Tian, A.-X.; Ying, J.; Zhao, D.; Liu, X.-J. A hexa-nuclear {Cu6(ptz)6} cluster containing five [Cu2N4] units with cycle connecting cycle mode induced by tetrazole-based ligand to modify Keggin anions. Inorg. Chem. Commun. 2012, 25, 60–64. [Google Scholar] [CrossRef]
- Sha, J.-Q.; Sun, J.-W.; Li, M.-T.; Wang, C.; Li, G.-M.; Yan, P.-F.; Sun, L.-J. Secondary spacer modulated assembly of polyoxometalate based metal–organic frameworks. Dalton Trans. 2013, 42, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Darling, K.; Ouellette, W.; Zubieta, J. Solid state coordination chemistry of copper with pyridyltetrazoles: Structural consequences of incorporation of coordinating anions. Inorg. Chim. Acta 2012, 392, 52–60. [Google Scholar] [CrossRef]
- Myznikov, L.V.; Roh, J.; Artamonova, T.V.; Hrabalek, A.; Koldobskii, G.I. Tetrazoles: LI. Synthesis of 5-Substituted Tetrazoles under Microwave Activation. J. Org. Chem. 2007, 43, 765–767. [Google Scholar] [CrossRef]
- Himo, F.; Demko, Z.P.; Noodleman, L.; Sharpless, K.B. Why Is Tetrazole Formation by Addition of Azide to Organic Nitriles Catalyzed by Zinc(II) Salts? J. Am. Chem. Soc. 2003, 125, 9983–9987. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yao, C.; Ni, R.; Yang, G. Amine Salt–Catalyzed Synthesis of 5-Substituted 1H-Tetrazoles from Nitriles. Synth. Commun. 2010, 40, 2624–2632. [Google Scholar] [CrossRef]
- Aureggi, V.; Sedelme, G. 1,3-Dipolar Cycloaddition: Click Chemistry for the Synthesis of 5-Substituted Tetrazoles from Organoaluminum Azides and Nitriles. Angew. Chem. Int. Ed. 2007, 46, 8440–8444. [Google Scholar] [CrossRef] [PubMed]
- Nasani, R.; Saha, M.; Mobin, S.M.; Martins, L.M.D.R.S.; Pombeiro, A.J.L.; Kirillov, A.M.; Mukhopadhyay, S. Copper-organic frameworks assembled from in-situ generated 5-(4-pyridyl)tetrazole building blocks: Synthesis, structural features, topological analysis and catalytic oxidation of alcohols. Dalton Trans. 2014, 43, 9944–9954. [Google Scholar] [CrossRef] [PubMed]
- Pombeiro, A.J.L. Advances in Organometallic Chemistry and Catalysis, The Silver/Gold Jubilee ICOMC Celebratory Book; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Kirillov, A.M.; Kirilova, M.V.; Pombeiro, A.J.L. Multicopper complexes and coordination polymers for mild oxidative functionalization of alkanes. Coord. Chem. Rev. 2012, 256, 2741–2759. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Tris(pyrazol-1yl)methane metal complexes for catalytic mild oxidative functionalizations of alkanes, alkenes and ketones. Coord. Chem. Rev. 2014, 265, 74–88. [Google Scholar] [CrossRef]
- Timokhin, I.; Pettinari, C.; Marchetti, F.; Pettinari, R.; Condello, F.; Galli, S.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Novel Coordination Polymers with (Pyrazolato)-based Tectons: Catalytic Activity in the Peroxidative Oxidation of Alcohols and Cyclohexane. Cryst. Growth Des. 2015, 15, 2303–2317. [Google Scholar] [CrossRef]
- Di Nicola, C.; Garau, F.; Karabach, Y.Y.; Martins, L.M.D.R.S.; Monari, M.; Pandolfo, L.; Pettinari, C.; Pombeiro, A.J.L. Trinuclear Triangular Copper(II) Clusters. Synthesis, Electrochemical Studies and Catalytic Peroxidative Oxidation of Cycloalkanes. Eur. J. Inorg. Chem. 2009, 666–676. [Google Scholar] [CrossRef]
- Sutradhar, M.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Liu, C.-M.; Pombeiro, A.J.L. Trinuclear Cu(II) structural isomers: Coordination, magnetism, electrochemistry and catalytic activity toward oxidation of alkanes. Eur. J. Inorg. Chem. 2015, 2015, 3959–3969. [Google Scholar] [CrossRef]
- Conde, A.; Vilella, L.; Balcells, D.; Diaz-Requejo, M.; Lledos, A.; Perez, P. Introducing Copper as Catalyst for Oxidative Alkane Dehydrogenation. J. Am. Chem. Soc. 2013, 135, 3887–3896. [Google Scholar] [CrossRef] [PubMed]
- Perraud, O.; Sorokin, A.B.; Dutasta, J.-P.; Martinez, A. Oxidation of cycloalkanes by H2O2 using a copper–hemicryptophane complex as a catalyst. Chem. Commun. 2013, 49, 1288–1290. [Google Scholar] [CrossRef] [PubMed]
- Kopylovich, M.N.; Nunes, A.C.C.; Mahmudov, K.T.; Haukka, M.; Mac Leod, T.C.O.; Martins, L.M.D.R.S.; Kuznetsov, M.L.; Pombeiro, A.J.L. Complexes of copper(II) with 3-(ortho-substituted phenylhydrazo)pentane-2,4-diones: Syntheses, properties and catalytic activity for cyclohexane oxidation. Dalton Trans. 2011, 40, 2822–2836. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.K.; Férey, G.; Lee, U.-H.; Chang, J.-S. Liquid Phase Oxidation of Organic Compounds by Metal-Organic Frameworks. In Liquid Phase Oxidation via Heterogeneous Catalysis, Organic Synthesis and Industrial Applications; Clerici, M.G., Kholdeeva, O., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; Chapter 8. [Google Scholar]
- Weissermel, K.; Arpe, H.J. Industrial Organic Chemistry, 2nd ed.; VCH Press: Weinheim, Germany, 1993. [Google Scholar]
- Clark, J.H.; Macquarrie, D.J. Handbook of Green Chemistry and Technology; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar]
- Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Wiley-VCH: Weinheim, Germany, 2002.
- Crabtree, R.H. Organometallic alkane CH activation. J. Organomet. Chem. 2004, 689, 4083–4091. [Google Scholar] [CrossRef]
- Shilov, A.E.; Shul’pin, G.B. Activation and Catalytic Reactions of Saturated Hydrocarbons in the Presence of Metal Complexes; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000. [Google Scholar]
- Shul’pin, G.B. Transition Metals for Organic Synthesis, 2nd ed.; Beller, M., Bolm, C., Eds.; Wiley-VCR: New York, NY, USA, 2004; Volume 2, p. 215. [Google Scholar]
- Derouane, E.G.; Parmon, V.; Lemos, F.; Râmoa Ribeiro, F. Sustainable Strategies for the Upgrading of Natural Gas: Fundamentals, Challenges, and Opportunities; Springer: Dordrecht, The Netherlands, 2005; Volume 191. [Google Scholar]
- Sheldon, R.A.; Arends, I.; Hanefeld, U. Green Chemistry and Catalysis; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Schuchardt, U.; Cardoso, D.; Sercheli, R.; Pereira, R.; Da Cruz, R.S.; Guerreiro, M.C.; Mandelli, D.; Spinacé, E.V.; Pires, E.L. Cyclohexane oxidation continues to be a challenge. Appl. Catal. A Gen. 2001, 211, 1–17. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Nizova, G.V. Formation of alkyl peroxides in oxidation of alkanes by H2O2 catalyzed by transition metal complexes. React. Kinet. Catal. Lett. 1992, 48, 333–338. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Attanasio, D.; Suber, L. Efficient H2O2 Oxidation of Alkanes and Arenes to Alkyl Peroxides and Phenols Catalyzed by the System Vanadate-Pyrazine-2-Carboxylic Acid. J. Catal. 1993, 142, 147–152. [Google Scholar] [CrossRef]
- Schuchardt, U.; Mandelli, D.; Shul’pin, G.B. Methyltrioxorhenium catalyzed oxidation of saturated and aromatic hydrocarbons by H2O2 in air. Tetrahedron Lett. 1996, 37, 6487–6490. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalyzed hydrocarbon oxygenations in solutions: The dramatic role of additives: A review. J. Mol. Catal. A Chem. 2002, 189, 39–66. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Nizova, G.V.; Kozlov, Y.N.; Cuervo, L.G.; Süss-Fink, G. Hydrogen Peroxide Oxygenation of Alkanes Including Methane and Ethane Catalyzed by Iron Complexes in Acetonitrile. Adv. Synth. Catal. 2004, 346, 317–332. [Google Scholar] [CrossRef]
- Anisia, K.S.; Kumar, A. Oxidation of cyclohexane with molecular oxygen using heterogeneous silica gel catalyst bonded with [1,2-bis(salicylidene amino)-phenylene] zirconium complex. Appl. Catal. A Gen. 2004, 273, 193–200. [Google Scholar] [CrossRef]
- Strukul, G.; Scarso, A. Environmentally Benign Oxidants. In Liquid Phase Oxidation via Heterogeneous Catalysis, Organic Synthesis and Industrial Applications; Clerici, M.G., Kholdeeva, O., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013; Chapter 1. [Google Scholar]
- Mukhopadhyay, S.; Lasri, J.; Charmier, M.A.J.; Silva, M.F.C.G.; Pombeiro, A.J.L. Microwave synthesis of mono- and bis-tetrazolato complexes via 1,3-dipolar cycloaddition of organonitriles with platinum(II)-bound azides. Dalton Trans. 2007, 5297–5304. [Google Scholar] [CrossRef]
- Smolenski, P.; Mukhopadhyay, S.; Guedes Silva, M.F.C.; Charmier, M.A.J.; Pombeiro, A.J.L. New water-soluble azido- and derived tetrazolato-platinum(II) complexes with PTA. Easy metal-mediated synthesis and isolation of 5-substituted tetrazoles. Dalton Trans. 2008, 6546–6555. [Google Scholar] [CrossRef] [PubMed]
- Shul’pin, G.B. Metal-catalysed hydrocarbon oxidations. C. R. Chim. 2003, 6, 163–178. [Google Scholar] [CrossRef]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Kudinov, A.R.; Mandelli, D. Extremely Efficient Alkane Oxidation by a New Catalytic Reagent H2O2/Os3(CO)12/Pyridine. Inorg. Chem. 2009, 48, 10480–10482. [Google Scholar]
- Shul’pin, G.B.; Kozlov, Y.N.; Shul’pina, L.S.; Petrovskiy, P.V. Oxidation of alkanes and alcohols with hydrogen peroxide catalyzed by complex Os3(CO)10(µ-H)2. Appl. Organomet. Chem. 2010, 24, 464–472. [Google Scholar] [CrossRef]
- Shul’pin, G.B. C–H functionalization: thoroughly tuning ligands at a metal ion, a chemist can greatly enhance catalyst’s activity and selectivity. Dalton Trans. 2013, 42, 12794–12818. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.M.D.R.S.; Pombeiro, A.J.L. Carbon-scorpionate Complexes in Oxidation Catalysis. In Advances in Organometallic Chemistry and Catalysis, the Silver/Gold Jubilee ICOMC Celebratory Book; Pombeiro, A.J.L., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014; Chapter 22; pp. 285–294. [Google Scholar]
- Milunovic, M.N.M.; Martins, L.M.D.R.S.; Alegria, E.C.B.A.; Pombeiro, A.J.L.; Krachler, R.; Trettenhahn, G.; Turta, C.; Shova, S.; Arion, V.B. Hexanuclear and Undecanuclear Iron(III) Carboxylates as Catalyst Precursors for Cyclohexane Oxidation. Dalton Trans. 2013, 42, 14388–14401. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sutradhar, M.; Kirilova, M.V.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. A Hexanuclear Oxovanadium(IV,V) Complex Bearing N,O-Ligand as a Highly Efficient Alkane Oxidation Catalyst. Inorg. Chem. 2012, 51, 11229–11231. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.F.S.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.; Kuznetsov, M.L.; Fernandes, A.R.; Silva, A.; Santos, S.; Pan, C.-J.; Lee, J.-F.; Hwang, B.-J.; et al. Cobalt complexes with pyrazole ligands as catalysts for the peroxidative oxidation of cyclohexane. XAS studies and biological applications. Chem. Asian J. 2014, 9, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.F.S.; Alegria, E.C.B.A.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Scorpionate vanadium, iron and copper complexes as selective catalysts for the peroxidative oxidation of cyclohexane under mild conditions. Adv. Synth. Catal. 2008, 350, 706–716. [Google Scholar] [CrossRef]
- Contaldi, S.; di Nicola, C.; Garau, F.; Karabach, Y.Y.; Martins, L.M.D.R.S.; Monari, M.; Pandolfo, L.; Pettinari, C.; Pombeiro, A.J.L. New coordination polymers based on triangular [Cu3(m3-OH)(m-pz)3]2+ units and unsaturated carboxylates. Molecular structures, electrochemical behaviour and catalytic peroxidative oxidation of cycloalkanes. Dalton Trans. 2009, 4928–4941. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.F.S.; Mishra, G.S.; Silva, M.F.G.; Wanke, R.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. CuII complexes bearing the 2,2,2-tris(1-pyrazolyl)ethanol or 2,2,2-tris(1-pyrazolyl)ethyl methanesulfonate scorpionates. X-ray structural characterization and application in the mild catalytic peroxidative oxidation of cyclohexane. Dalton Trans. 2009, 9207–9215. [Google Scholar] [CrossRef] [PubMed]
- Nesterov, D.S.; Chygorin, E.N.; Kokozay, V.N.; Bon, V.V.; Boča, R.; Kozlov, Y.N.; Shul’pina, L.S.; Jezierska, J.; Ozarowski, A.; Pombeiro, A.J.L.; et al. Heterometallic CoIII4FeIII2 Schiff Base Complex: Structure, Electron Paramagnetic Resonance, and Alkane Oxidation Catalytic Activity. Inorg. Chem. 2012, 51, 9110–9122. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.F.S.; Luzyanin, K.V.; Kirilova, M.V.; Guedes da Silva, M.F.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Novel scorpionate and pyrazole dioxovanadium complexes, catalysts for carboxylation and peroxidative oxidation of alkanes. Adv. Synth. Catal. 2010, 352, 171–187. [Google Scholar] [CrossRef]
- Silva, T.F.S.; Guedes da Silva, M.F.; Mishra, G.S.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Synthesis and structural characterization of iron complexes with 2,2,2-tris(1-pyrazolyl)ethanol ligands: application in the peroxidative oxidation of cyclohexane under mild conditions. J. Organomet. Chem. 2011, 696, 1310–1318. [Google Scholar] [CrossRef]
- Mishra, G.S.; Silva, T.F.S.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Scorpionate V(III-V) Complexes as Catalyst Precursors for Solvent-free Cyclohexane Oxidation with Dioxygen. Pure Appl. Chem. 2009, 81, 1217–1227. [Google Scholar] [CrossRef]
- Silva, T.F.S.; MacLeod, T.C.O.; Martins, L.M.D.R.S.; Guedes da Silva, M.F.C.; Schiavon, M.A.; Pombeiro, A.J.L. Pyrazole or tris(pyrazolyl)ethanol oxo-vanadium(IV) complexes as homogeneous or supported catalysts for oxidation of cyclohexane under mild conditions. J. Mol. Catal. A Chem. 2013, 367, 52–60. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Peixoto de Almeida, M.; Carabineiro, S.A.C.; Figueiredo, J.L.; Pombeiro, A.J.L. Heterogenisation of a C-scorpionate Fe(II) complex in carbon materials for cyclohexane oxidation with hydrogen peroxide. ChemCatChem 2013, 5, 3847–3856. [Google Scholar] [CrossRef]
- Martins, L.M.D.R.S.; Martins, A.; Alegria, E.C.B.A.; Carvalho, A.P.; Pombeiro, A.J.L. Gold Nanoparticles Supported on Carbon Materials for Cyclohexane Oxidation with Hydrogen Peroxide. Appl. Cat. A Gen. 2013, 464–465, 43–50. [Google Scholar] [CrossRef]
- Mishra, G.S.; Alegria, E.C.B.; Martins, L.M.D.R.S.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Cyclohexane oxidation with dioxygen catalyzed by supported pyrazole rhenium complexes. J. Mol. Catal. A Chem. 2008, 285, 92–100. [Google Scholar] [CrossRef]
- Peixoto de Almeida, M.; Martins, L.M.D.R.S.; Carabineiro, S.A.C.; Lauterbach, T.; Rominger, F.; Hashmi, A.S.K.; Pombeiro, A.J.L.; Figueiredo, J.L. Homogeneous and Heterogenised New Gold C-Scorpionate Complexes as Catalysts for Cyclohexane Oxidation. Catal. Sci. Technol. 2013, 3, 3056–3069. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Shul’pin, G.B. Pyrazinecarboxylic acid and analogs: Highly efficient co-catalysts in the metal-complex-catalyzed oxidation of organic compounds. Coord. Chem. Rev. 2013, 257, 732–754. [Google Scholar] [CrossRef]
- Kuznetsov, M.L.; Pombeiro, A.J.L. Radical Formation in the [MeReO3]-Catalyzed Aqueous Peroxidative Oxidation of Alkanes: A Theoretical Mechanistic Study. Inorg. Chem. 2009, 48, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Kirilova, M.V.; Kuznetsov, M.L.; Romakh, V.B.; Shul’pina, L.S.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L.; Shul’pin, G.B. Mechanism of H2O2 Oxidations Catalyzed by Vanadate Anion or Oxovanadium(V) triethanolaminate (Vanadatrane) in Combination with Pyrazine-2-carboxylic acid (PCA): Kinetic and DFT studies. J. Catal. 2009, 267, 140–157. [Google Scholar] [CrossRef]
- Kirilova, M.V.; Kuznetsov, M.L.; Kozlov, Y.N.; Shul’pina, L.S.; Kitaygorodskiy, A.; Pombeiro, A.J.L.; Shul’pin, G.B. Participation of Oligovanadates in Alkane Oxidation with H2O2 Catalyzed by Vanadate Anion in Acidified Acetonitrile: Kinetic and DFT Studies. ACS Catal. 2011, 1, 1511–1520. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Program for Crystal Structure Solution and Refinement; University of Goettingen: Goettingen, Germany, 1997.
- Sample Availability: Samples of the compounds 1–3 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, L.; Nasani, R.; Saha, M.; Mobin, S.; Mukhopadhyay, S.; Pombeiro, A. Greener Selective Cycloalkane Oxidations with Hydrogen Peroxide Catalyzed by Copper-5-(4-pyridyl)tetrazolate Metal-Organic Frameworks. Molecules 2015, 20, 19203-19220. https://doi.org/10.3390/molecules201019203
Martins L, Nasani R, Saha M, Mobin S, Mukhopadhyay S, Pombeiro A. Greener Selective Cycloalkane Oxidations with Hydrogen Peroxide Catalyzed by Copper-5-(4-pyridyl)tetrazolate Metal-Organic Frameworks. Molecules. 2015; 20(10):19203-19220. https://doi.org/10.3390/molecules201019203
Chicago/Turabian StyleMartins, Luísa, Rajendar Nasani, Manideepa Saha, Shaikh Mobin, Suman Mukhopadhyay, and Armando Pombeiro. 2015. "Greener Selective Cycloalkane Oxidations with Hydrogen Peroxide Catalyzed by Copper-5-(4-pyridyl)tetrazolate Metal-Organic Frameworks" Molecules 20, no. 10: 19203-19220. https://doi.org/10.3390/molecules201019203
APA StyleMartins, L., Nasani, R., Saha, M., Mobin, S., Mukhopadhyay, S., & Pombeiro, A. (2015). Greener Selective Cycloalkane Oxidations with Hydrogen Peroxide Catalyzed by Copper-5-(4-pyridyl)tetrazolate Metal-Organic Frameworks. Molecules, 20(10), 19203-19220. https://doi.org/10.3390/molecules201019203