Abstract
A highly porous metal-organic framework (Cu-TDPAT), constructed from a paddle-wheel type dinuclear copper cluster and 2,4,6-tris(3,5-dicarboxylphenylamino)-1,3,5-triazine (H6TDPAT), has been tested in Ullmann and Goldberg type C–N coupling reactions of a wide range of primary and secondary amines with halobenzenes, affording the corresponding N-arylation compounds in moderate to excellent yields. The Cu-TDPAT catalyst could be easily separated from the reaction mixtures by simple filtration, and could be reused at least five times without any significant degradation in catalytic activity.
1. Introduction
The N-aryl heterocycles are very important structural motifs in biological, pharmaceutical and material science products [1,2,3]. Formation of C–N bonds by Ullmann and Goldberg coupling reactions represents a powerful tool for the preparation of these nitrogen-containing compounds on both laboratory and industrial scales [4,5,6,7]. However the classic Ullmann and Goldberg coupling reactions suffer from drawbacks such as harsh reaction conditions, including high reaction temperatures, extended reaction times, and the use of large amounts of copper. Since two separate research groups achieved an important breakthrough with the discovery of efficient copper/ligand systems that enable these cross coupling reactions to occur under much milder conditions at the beginning of the 21st century [8,9], the formation of C–N bonds by copper-mediated cross-coupling approaches has undergone a renaissance in the last decade [10,11,12]. Many classes of ligands, including diamines [4,13], amino acids [14,15,16], amino alcohols [17,18], diols [19], diethylamine [20], diphosphines [21], phenanthrolines [22,23], pyridine N-oxide [24], β-diketones [25,26,27] and metformin [28,29], have been used to promote the copper-catalyzed cross-coupling C–N bond-forming reactions. Moreover, a few metal- and ligand-free [30,31,32], and photoinduced [33,34,35] C–N coupling reactions have also been reported. Meanwhile, a few mechanistic [36,37] and computational studies [38] have been conducted on these copper-mediated cross-coupling reactions in order to understand these intriguing catalytic processes in detail.
Because of the increasing concern regarding environmental impact, the heterogeneous catalysis has received much attention recently due to its advantages of ease of product separation, catalyst recovery and recyclability. Several categories of copper-based heterogeneous catalysts for the Ullmann and Goldberg coupling reactions, including copper compound nanoparticles [39,40,41], inorganic materials (e.g., mesoporous nitrogen-doped carbon [42], SiO2 [43,44], fluoroapatite [45] and Fe3O4 [46,47])—supported catalytic systems, and organic polymer (e.g., polystyrene [48,49,50], chitosan [51] and polytriallylamine [52])-supported catalytic systems, have been reported. In the past decade, metal-organic frameworks (MOFs) have received much attention as catalytic materials in addition to their applications in gas storage and separation due to their unique features, including their crystallinity, porous structure, huge specific surface area and the high density of open metal sites in the framework [53,54,55]. Several studies on the catalytic activity of MOFs with active open metal sites have shown their potential applications in reactions like hydrogenation [56,57], isomerization [58], cyanosilylation [59,60], oxidation [61,62,63,64,65], photocatalysis [66,67], Friedel-Crafts reaction [68,69,70], and condensation reactions [71]. However, the Ullmann and Goldberg type C–O, C–N and C–S coupling reactions over MOFs have barely been explored [72,73,74,75]. Cu-TDPAT [76] based on [Cu2(COO)4] square paddle-wheel secondary building units (SBUs) with rht-topology is highly porous, with a large surface area and pore volume, and should be a good potential copper-based heterogeneous catalyst. Herein, we report the applications of Cu-TDPAT as an efficient and reusable heterogeneous catalyst for Ullmann and Goldberg type C–N coupling reactions.
2. Results and Discussion
Cu-TDPAT catalyst was prepared by a solvothermal method according to the reported procedure [76]. The SEM image of Cu-TDPAT sample clearly showed that most of the MOF particles presented polyhedral shapes (Figure S1). The crystal size of the Cu-TDPAT sample was in the range of 10–40 µm. The powder XRD pattern of the as-synthesized Cu-TDPAT (Figure 1) matched well with the published results [76], confirming the formation of the intended crystalline framework. Nitrogen physisorption measurements demonstrated the porosity and stability of Cu-TDPAT after removing the included and coordinated solvents (Figure S2, Table S1). Its BET surface area was 1855 m2/g. Thermogravimetric data showed that Cu-TDPAT was stable up to a temperature of 250 °C (Figure S4).
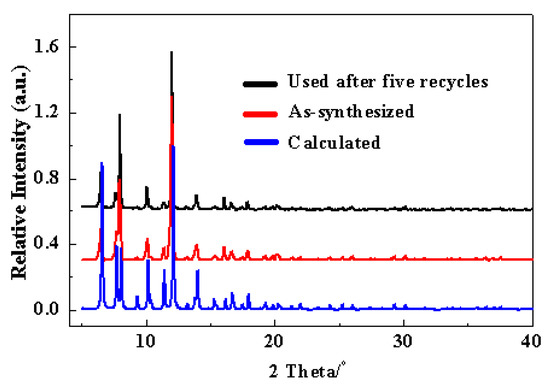
Figure 1.
X-ray diffraction patterns for Cu-TDPAT samples, as-synthesized (red); and used after five catalysis cycles (black).
2.1. Cu-TDPAT as a Solid Catalyst for the N-Arylation of 5-Methyl-2-(1H)-Pyridone
To test the use of Cu-TDPAT as a catalytic copper complex, iodobenzene and 5-methyl-2-(1H)-pyridone were selected as coupling partners in the initial screening of optimal reaction conditions (Scheme 1) as the coupling product of these two substrates, 5-methyl-1-phenyl-2-(1H)-pyridone, also known as pirfenidone (1), has been a widely used drug for the treatment of idiopathic pulmonary fibrosis (IPF) [77,78].
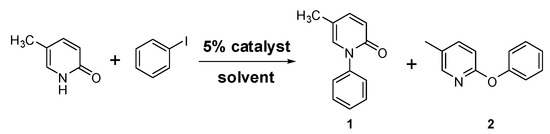
Scheme 1.
Coupling reaction of iodobenzene and 5-methyl-2-(1H)-pyridone.
The experimental results under various conditions are presented in Table 1. It was found that no product was observed after 2 h at 80 °C (Table 1, entry 2), indicating that the coupling reaction proceeded with difficulty at or below this temperature. With increasing temperature, the yields of pirfenidone (1) increased gradually (Table 1, compare entries 3, 4, 5). The highest yield of 1 was obtained at 140 °C. A further temperature increase to 160 °C only gave 1 in 14% yield after 2 h due to the decomposition of the MOF (Figure S5), revealing that the combined effects of temperature and reaction media, such as base and solvent molecules, reduced the stability of the MOF. Considering the stability of Cu-TDPAT, all the subsequent experiments were conducted at 120 °C. In the absence of Cu-TDPAT, no product was obtained (Table 1, entry 1), illustrating that Cu-TDPAT promoted the N-arylation reaction.

Table 1.
Goldberg-type C–N coupling reaction of iodobenzene and 5-methyl-2-(1H)-pyridone over copper MOFs and copper salts a.
| Entry | Catalyst | Solvent | Base | T (°C) | 1 (%) | 2 (%) |
|---|---|---|---|---|---|---|
| 1 | \ | DMSO | K2CO3 | 120 | n.r | n.r |
| 2 | Cu-TDPAT | DMSO | K2CO3 | 80 | n.r | n.r |
| 3 | Cu-TDPAT | DMSO | K2CO3 | 100 | 9 | 1 |
| 4 | Cu-TDPAT | DMSO | K2CO3 | 120 | 63 (90 b) | 3 (4 b) |
| 5 | Cu-TDPAT | DMSO | K2CO3 | 140 | 70 | 3 |
| 6 | Cu-TDPAT | DMSO | K2CO3 | 160 | 14 | 1 |
| 7 | Cu-TDPAT | Toluene | K2CO3 | 120 | n.r | n.r |
| 8 | Cu-TDPAT | 1,2-Dichlorobenzene | K2CO3 | 120 | n.r | n.r |
| 9 | Cu-TDPAT | DMF | K2CO3 | 120 | 34 | 2 |
| 10 | Cu-TDPAT | 1,4-Dioxane | K2CO3 | 120 | n.r | n.r |
| 11 | Cu-TDPAT | DMSO | KOH | 120 | 61 | 3 |
| 12 | Cu-TDPAT | DMSO | Cs2CO3 | 120 | 57 | 3 |
| 13 | Cu-TDPAT | DMSO | Et3N | 120 | n.r | n.r |
| 14 | Cu-TDPAT | DMSO | NaOMe | 120 | 49 | 4 |
| 15 | CuI | DMSO | K2CO3 | 120 | 80 | 4 |
| 16 | CuCl | DMSO | K2CO3 | 120 | 71 | 5 |
| 17 | Cu(OAc)2 | DMSO | K2CO3 | 120 | 38 | 4 |
| 18 | Cu(NO3)2 | DMSO | K2CO3 | 120 | 29 | 2 |
| 19 | CuBTC | DMSO | K2CO3 | 120 | 88 | 5 |
| 20 | Cu(NO3)2 + BTC | DMSO | K2CO3 | 120 | 43 | 2 |
| 21 | Cu(NO3)2 + TDPAT | DMSO | K2CO3 | 120 | 31 | 2 |
a Reaction conditions: PhI (1 mmol), pyridone (1 mmol), MOFs (0.05 mmol, based on copper), base (2 mmol), solvent (5 mL), 2 h; b Data of reaction for 8 h; The symbol n.r represents no reaction.
It has been found that the choice of solvent is crucial to the outcome of C–N coupling reactions [16,29]. In order to determine the best reaction medium, the coupling reaction was also conducted in other solvents (Table 1, entries 7–10). DMSO was found to be the best solvent for the N-arylation of 5-methyl-2-(1H)-pyridone (Table 1, entry 4). Reaction in DMF gave the coupled product in a low yield. On the other hand, weakly polar solvents, like 1,2-dichlorobenzene and 1,4-dioxane, and non-polar solvents, such as toluene, were not suitable for this process, revealing the dramatic effects of the solvent on the C–N coupling reactions. Recent studies showed that DMSO acts as an oxidant for metal species in the formation of C–S and C–Se bonds by cross coupling reactions [79,80], but in our experiments, no dimethyl sulfide, the reduction product of DMSO, was detected, implying that here DMSO is only acting as an effective polar solvent. The effects of bases on the C–N coupling reaction was also investigated. Among the bases tested potassium carbonate, cesium carbonate, potassium hydroxide and sodium methoxide were effective for the formation of the desired product and gave similar results (Table 1, compare entries 4, 11, 12 and 14). On the other hand, triethylamine (Table 1, entry 13) did not effectively improve the N-arylation reaction, contrary to previously reported results [79]. One possibility is that the interaction of substrate molecules with catalyst was impeded due to the coordination of triethylamine with the copper ion on the MOF.
According to the above results, we conducted the C–N coupling reactions in DMSO at 120 °C for 8 h in the presence of K2CO3 as the standard reaction conditions. The coupling reaction afforded 1 as the main product in 90% yield and the by-product 2 in 4% yield, respectively (Figure 2, Table 1, entry 4). The existence of small amount of 2 reflected the fact that 5-methyl-2-(1H)-pyridone has an accessible tautomer, 5-methyl-2-hydroxypyridine, in the reaction system [81].
2.2. Heterogeneity of the Reaction
To verify whether the catalysis of Cu-TAPAT is truly heterogeneous or, on the contrary, is due to some leached copper species present in the reaction solutions, we performed a hot-filtration experiment: the Cu-TAPAT solid catalyst was removed from a hot solution by filtration one hour after initiating the catalytic test. The filtrate was further reacted for another 7 h. No significant catalytic conversion was observed (Figure 2), indicating that the reaction was terminated upon removal of the catalyst. On the other hand, inductively coupled plasma atomic emission spectroscopy (ICP-AES) analysis of the filtrate showed that there was only about 1 ppm of copper in the solution. These results suggested that the reaction proceeded over the MOF surface in a heterogeneous fashion, with the open copper sites on the axis of paddle-wheel SBUs within the MOF being responsible for the promotion of the C–N coupling reaction [82].
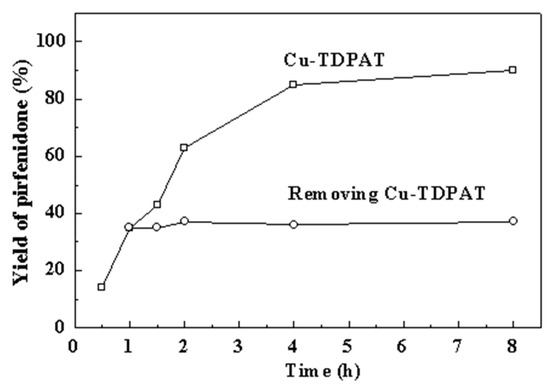
Figure 2.
Yield as a function of reaction time in the coupling reaction of 5-methyl-2-(1H)-pyridone and iodobenzene with Cu-TDPAT as catalyst at 120 °C. □: catalyst present throughout; ○: catalyst removed from the suspension after 1 h. Reaction conditions: PhI (1 mmol), pyridone (1 mmol), Cu-TDPAT (0.05 mmol, based on copper), K2CO3 (2 mmol), DMSO (5 mL).
2.3. Comparison with Other Homogeneous Copper Catalysts
For comparison, several copper salts, CuI, CuCl, Cu(OAc)2 and Cu(NO3)2, were used as homogeneous catalysts for the same reaction (Table 1, entries 15–18). The experimental results showed that both copper(I) salts—CuI and CuCl—produced pirfenidone in high yield, while the copper(II) salts resulted in relative low yields of coupled product. A widely used MOF, namely CuBTC [83] (Figures S6 and S7) that was constructed from paddle-wheel type copper clusters and 1,3,5-benzenetricarboxylate molecules, also exhibited high activity in the C–N coupling reaction between iodobenzene and 5-methyl-2-(1H)-pyridone (Table 1, entry 19), but the poor stability of CuBTC under the reaction conditions prevented its recycling. Similar phenomena were reported by Garcia [75] and Kantam [84]. In the case of CuBTC, a high yield (88%) of 1 was obtained, which was much higher than that of the homogeneous catalytic processes catalyzed by Cu(NO3)2 or both Cu(NO3)2 and BTC (Table 1, entries 18 and 20). Moreover, the same result was also observed in the comparison of Cu-TDPAT and Cu(NO3)2/TDPAT (Table 1, entries 4 and 21). Owing to the same amount of copper species being used in the heterogeneous and homogeneous reaction systems, the above results further reveal that the high density of open copper sites within the MOF were the active sites of the MOF catalyst and responsible for the enhanced C–N coupling reaction rates.
2.4. Reusability of the Cu-TDPAT Catalyst
A marked advantage of heterogeneous catalysis is the possibility of recovering and reusing the catalysts after the reaction. The recycle of the Cu-TDPAT catalyst was further examined in the coupling reaction of 5-methyl-2-(1H)-pyridone with iodobenzene (Figure 3). Pirfenidone was obtained in 85%, 83%, 85%, 81% and 81% yields in successive 4 h cycles. The results demonstrated that Cu-TDPAT exhibited good reusability in the Goldberg-type C–N coupling reaction. The image (Figure S1b) and XRD patterns (Figure 1) of the Cu-TDPAT catalyst after five reaction runs indicated that the particle shape, the crystallinity and the chemical structure of Cu-TDPAT were almost completely maintained. The presence of a little bit of powder can be attributed to the degradation of a few crystal particles induced by the stirring and the effect of the reaction medium. The nitrogen physisorption measurements further demonstrated the porosity and stability of Cu-TDPAT after use (Figure S3). The changes in surface area and pore volume of the used Cu-TDPAT (Table S1) are probably due to the adsorption of a few reactant or product molecules in the MOF cavities [85,86]. This hypothesis was supported by the changes of thermogravimetric data, in which more weight loss (about 2%) was observed for the used Cu-TDPAT sample (Figure S4).
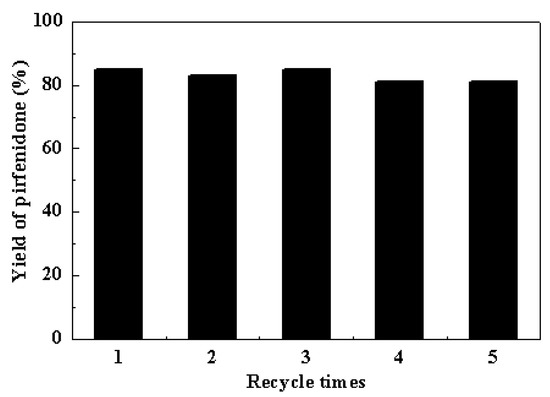
Figure 3.
Reuse of Cu-TDPAT in the Goldberg-type C–N coupling reaction of iodobenzene and 5-methyl-2-(1H)-pyridone. Reaction condition: PhI (1 mmol), pyridone (1 mmol), Cu-TDPAT (0.05 mmol, based on copper), K2CO3 (2 mmol), DMSO (5 mL), 4 h, temperature 120 °C.
2.5. Generality of the N-Arylation Reaction
The generality of the C–N coupling reaction was investigated under the standard reaction conditions. Initially various halobenzenes were subjected to the reaction conditions. It was found that bromobenzene provided the corresponding N-arylated pyridone (Table 2, entry 1) and N-arylated pyrrolidone (Table 2, entry 2) derivatives in moderate yield, while chlorobenzene was unreactive under these conditions (Table 2, entry 3). Then, the N-arylation reactions of other primary and secondary amines with iodobenzene were examined (Table 2, entries 4–12). The results showed that a wide range of amines, including aliphatic amines, aryl amines and N–H heterocycles, could undergo the C–N coupling reactions smoothly to afford the expected N-arylation products in moderate yields.

Table 2.
Ullmann and Goldberg type C–N coupling reactions of halobenzenes with various amines and amides over Cu-TDPAT *.
| Entry | (R1R2)NH | ArX | Yield (%) |
|---|---|---|---|
| 1 | 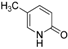 | 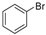 | 39 (2 a) |
| 2 | 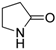 |  | 37 |
| 3 | 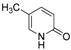 | 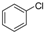 | n.r |
| 4 | 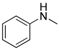 | 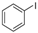 | 51 |
| 5 | 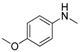 |  | 54 |
| 6 | 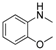 |  | 43 |
| 7 | 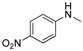 |  | 35 |
| 8 | 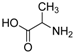 |  | 59 |
| 9 | 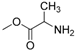 |  | 55 |
| 10 | 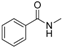 |  | 56 |
| 11 | 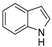 |  | 72 (93 b) |
| 12 | 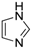 |  | 61 (91 b) |
* Reaction conditions: halobenzenes (1 mmol), amine derivatives (1 mmol), MOFs (0.05 mmol, based on copper), K2CO3 (2 mmol), DMSO (5 mL), 2 h, temperature 120 °C. a The yield of 2; b Data of reaction for 8 h. The symbol of n.r represents no reaction.
In the case of indole, the coupling product, 1-phenylindole, can be obtained in 93% yield after 8 h, which is comparable to the homogeneous system results [28,79], indicating the high activity of Cu-TDPAT. N-Methylaniline derivatives bearing electron-withdrawing and electron-donating groups on the benzene ring afforded obviously different results under the standard reaction conditions. When 4-methoxy-N-methylaniline was subjected to the reaction, a good yield (54%) of the desired product was obtained thanks to the electron-donating effect of the methoxy group. However the coupling reactions of 4-nitro-N-methylaniline proceeded with difficulty to give the N-arylation product in a low yield (Table 2, entry 7). These results demonstrated that the electronic properties of the substituent(s) on the aryl amine play an important role in determining its reactivity in the C–N coupling reactions. Compared to 4-methoxy-N-methylaniline, the reaction of 2-methoxy-N-methylaniline gave a relative low yield of the expected product due to the steric hindrance of the adjacent methoxy group (Table 2, entry 6). Secondary amides are a particularly challenging substrate class for cross couplings due to the large size of secondary amides and their relatively low nucleophilicity (compared to amines) [87,88]. The reaction of N-methylbenzamide (Table 2, entry 10) with iodobenzene afforded a 56% yield of the tertiary amide, N-methyl-N-phenylbenzamide, within 2 h in the N-arylation reaction, implying that Cu-TDPAT showed a higher activity than copper(I) thiophenecarboxylate (CuTC) [88] in the N-arylation reaction of secondary amides.
Table 3 provides a comparison of the results obtained for the Cu-TDPAT catalytic system with other reported heterogeneous catalytic systems in the cross-coupling reaction of imidazole with iodobenzene, further indicating the higher activity of this Cu(II) coordination polymer compared to the other reported systems. Considering the chemical structure and rht-topology of Cu-TDPAT, its high catalytic activity can be attributed to the high density of open copper sites within Cu-TDPAT framework and the high pore volume and large open windows that may reduce the diffusional limitation of the substrate and product [85].

Table 3.
Comparison of activity of different heterogeneous catalysts in the N-arylation reaction of imidazole and iodobenzene.
| Catalyst * | Reaction Conditions | Yield | Reference |
|---|---|---|---|
| CuI/Meso-N-C-1 | DMSO, KOH, 125 °C, 24 h | 88% | [42] |
| CuI/PSP | H2O, K3PO4, 120 °C, 8 h | 65% | [48] |
| Cu-MPTA-1 | H2O, KOH, 120 °C, 12 h | 91% | [52] |
| Cu/SiO2 | Toluene, Cs2CO3, 100 °C, 8 h | 23%–92% | [44] |
| Cu-TDPAT | DMSO, K2CO3, 120 °C, 8 h | 91% | This study |
* Meso-N-C-1, PSP, MPTA-1 and SiO2 represent mesoporous nitrogen doped carbon, polystyrene-supported pyrrole-2-carbohydrazide, mesoporous polytriallylamine, and amine or imine-modified silica, respectively.
2.6. Mechanistic Considerations
Although the mechanism of Ullmann and Goldberg type C–N coupling reactions in the presence of copper compounds has been widely studied, and four mechanistic pathways involving oxidative addition/reductive elimination, single electron transfer (SET), σ-bond metathesis and π-complexation, respectively, have been proposed [89,90], copper-mediated C–N coupling reactions are still in some sense unpredictable. In order to investigate the function of Cu-TDPAT in the coupling reaction, X-band (9.06 GHz) electron paramagnetic resonance (EPR) spectra of the reaction system were recorded at 90 °C. The EPR spectra of the reaction mixture were dominated by a broad signal at g = 2.107 (Figure S8), which could be attributed to Cu2+–Cu2+ dimers or mononuclear Cu2+ ions [91,92], indicating that the copper atoms on the MOF catalyst exist mainly as Cu(II) species in the reaction mixtures. Compared to the spectra of pure Cu-TDPAT (Figure S9), the decrease of the intensity of EPR signal of the reaction mixture with time and the change of g-value indicated the occurrence of interaction between reaction substrates (iodobenzene and 5-methyl-2-(1H)-pyridone) and copper sites. No any organic radical species was detected, revealing that the SET mechanism could be discounted. This was further supported by the experiment in which no effect on the turnover frequency of the coupling reaction was observed when the free radical scavenger 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) was added to the reaction system. Moreover, the σ-bond metathesis pathway and oxidative addition/reductive elimination pathway could also be eliminated due to the difficulty in forming the corresponding intermediates, because the halobenzene molecules and amine species bonding to the same copper ion from axis might dissociate the MOF, rendering it non-recyclable. Therefore a possible mechanism for the N-arylation is that the halobenzene molecule interacts with copper ion first and forms a π-complex, then the amine species attacks the halobenzene activated by copper, giving the coupling product.
3. Experimental Section
3.1. General Information
All solvents and chemicals were obtained commercially and were used as received without further purification. Cu-TDPAT [76] and CuBTC [83] were synthesized according to the reported procedures, respectively. The catalysts samples were activated as follows: the as-synthesized MOF sample was soaked in dry methanol for 12 h, separated from the mixture, and then the process was repeated four times to remove the high boiling point solvates used in preparations.
Powder X-ray diffraction was performed on a D8 Advance instrument (Bruker, Karlsruhe, Germany) using Cu–Kα radiation (λ = 1.5406 Å) at room temperature with a scan speed of 0.5 s per step and a step size of 0.02°. 1H-NMR and 13C-NMR data were collected on Bruker ARX-400 or Bruker ARX-600 spectrometers (Bruker) at 400 MHz and 101 or 151 MHz, respectively, using CDCl3 or DMSO-d6 solutions with tetramethylsilane as an internal standard. Molecular weights were obtained on an Aglilent 6410 Triple Quad LC-MS mass spectrometer (Agilent Technologies, Palo Alto, CA, USA). Scanning electron microscope (SEM) images were obtained on Hitachi S 4300 (Hitachi, Tokyo, Japan) and JEOL-2010 (JEOL, Tokyo, Japan) instruments at room temperature. Inductively coupled plasma atomic emission spectroscopy (ICP-AES) analysis was performed on an IRIS Intrepid ER/S instrument (Thermo Elemental, Waltham, MA, USA). Nitrogen sorption measurement was performed at −196 °C on a ASAP 2020 (Micromeritics, Norcross, GA, USA). A sample of approximately 50 mg was outgassed at 150 °C for 12 h and then nitrogen isotherm at −196 °C was measured in liquid nitrogen bath using UHP-grade (99.999%) gas source. The Brunauer-Emmett-Teller (BET) surface area of Cu-TDPAT and CuBTC was calculated based on the nitrogen absorption isotherm. Thermogravimetric analysis (TGA) was performed on a TG 209F3 instrument (Netzsch, Selb, Germany) under nitrogen atmosphere (250 mL/min). The sample was heated at a constant rate of 10 °C/min from 40 °C to 400 °C. The continuous wave (CW) EPR spectra of the reaction mixture in toluene at X-Band were measured using a JES-FA200 spectrometer (JEOL), microwave frequency 9.06 GHz, power of the microwave 0.998 mW) at 90 °C. The data for pure Cu-TDPAT were obtained from powder samples at room temperature.
3.2. Catalytic Reactions
The coupling reaction was performed as follows: in a typical process, 5-methyl-2-(1H)-pyridone (1 mmol), aryl halide (1 mmol), base (2 mmol) and solvent (5 mL) were added to an oven-dried tube containing 5% (based on copper) MOF catalyst or copper salt. The mixture was stirred at desired temperature for 2 h. After being cooled to room temperature, the catalyst was filtrated and washed with ethyl acetate. The products were isolated by a series 1500 preparative high performance liquid chromatography system (SSI, Charlotte, NC, USA) equipped with a UV-VIS detector, using a Kromasil C18 column (50 × 250 mm) and gradient elution with a H2O (A)-acetonitrile (B) the mobile phase. The gradient program was 0 min, 10% B; 20 min, 35% B. The flow rate of mobile phase was 40 mL/min, and the detection wavelength was 220 nm. Fractions were collected and evaporated to afford the pure products.
5-Methyl-1-phenyl-2-(1H)-pyridone. 1H-NMR (400 MHz; CDCl3): δ 7.41 (t, J = 7.0 Hz, 2H), 7.36 (t, J = 7.7 Hz, 1H), 7.32 (d, J = 7.8 Hz, 2H), 7.21 (d, J = 9.2 Hz, 1H), 7.07 (s, 1H), 6.53 (d, J = 9.2 Hz, 1H), 2.04 (s, 3H); 13C-NMR (151 MHz; DMSO-d6): δ 160.95, 143.52, 141.47, 136.49, 129.46, 128.42, 127.17, 120.67, 114.60, 16.77; MS (ESI) m/z: 186.23 ([M + H]+; Calcd for C12H11NO + H 186.09, found: 186.23).
5-Methyl-2-phenoxypyridine. 1H-NMR (400 MHz, DMSO-d6): δ 7.98 (d, J = 1.7 Hz, 1H), 7.66 (dd, J = 8.3 Hz, 2.3 Hz, 1H), 7.39 (t, J = 7.9 Hz, 2H), 7.17 (t, J = 7.4 Hz, 1H), 7.07 (d, J = 7.8 Hz, 2H), 6.92 (d, J = 8.3 Hz, 1H), 2.23 (s, 3H). 13C-NMR (151 MHz; DMSO-d6): δ 161.63, 154.89, 147.41, 141.16, 130.08, 128.52, 124.57, 121.15, 111.62, 17.38. MS (ESI) m/z: 186.23 ([M + H]+; Calcd for C12H11NO + H 186.09, found: 186.23).
N-Methyldiphenylamine. 1H-NMR (400 MHz, CDCl3): δ 7.40 (t, J = 7.9Hz, 4H), 7.15 (d, J = 8.2 Hz, 4H), 7.08 (t, J = 7.3 Hz, 2H), 3.43 (s, 3H). 13C-NMR (151 MHz; DMSO-d6): δ 149.09, 129.62, 121.57, 120.57, 40.01. MS (ESI) m/z: 184.11 ([M + H]+; Calcd for C13H13N + H 184.11, found: 184.11).
N-Methyl-N-(4-methoxy)phenylaniline. 1H-NMR (400 MHz, CDCl3): δ 7.24–7.17 (m, 2H), 7.17–7.12 (m, 2H), 7.00 (m, 1H), 6.84–6.77 (m, 2H), 6.69–6.64 (m, 2H), 3.88 (s, 3H), 3.31 (s, 3H). 13C-NMR (151 MHz; DMSO-d6): δ 156.36, 149.92, 142.13, 129.30, 126.35, 118.55, 115.89, 115.27, 55.65, 40.63. MS (ESI) m/z: 214.09 ([M + H]+; Calcd for C14H15NO + H 214.12, found: 214.09).
N-Methyl-N-(2-methoxy)phenylaniline. 1H-NMR (400 MHz, DMSO-d6): δ 7.33–7.22 (m, 1H), 7.17 (dd, J = 12.3 Hz, 3 Hz, 1H), 7.13 (d, J = 6.1 Hz, 1H), 7.11 (t, J = 7.9 Hz, 2H), 6.99 (t, J = 7.5 Hz, 1H), 6.62 (t, J = 7.2 Hz, 1H), 6.51 (d, J = 8.2 Hz, 2H), 3.71 (s, 3H), 3.13 (s, 3H). 13C-NMR (151 MHz; DMSO-d6): δ 152.13, 148.33, 142.22, 129.12, 123.74, 122.84, 121.88, 119.22, 119.13, 113.32, 56.79, 37.87. MS (ESI) m/z: 214.09 ([M + H]+; Calcd for C14H15NO + H 214.12, found: 214.09).
N-Methyl-N-(4-nitro)phenylaniline. 1H-NMR (400 MHz, DMSO-d6): δ 8.05 (d, J = 6.2 Hz, 2H), 7.50 (t, J = 7.8 Hz, 2H), 7.35 (t, J = 7.5 Hz, 1H), 7.31 (d, J = 6.7 Hz, 2H), 6.74 (d, J = 6.3 Hz, 2H), 3.38 (s, 3H). 13C-NMR (151 MHz; DMSO-d6): δ 154.13, 146.44, 137.58, 130.67, 127.16, 126.96, 126.10, 113.02, 40.85. MS (ESI) m/z: 229.11 ([M + H]+; Calcd for C13H12N2O2 + H 229.10, found: 229.11).
2-(Phenylamino)propanoic acid. 1H-NMR (400 MHz, DMSO-d6): δ 7.06 (t, J = 7.8 Hz, 2H), 6.55 (d, J = 8 Hz, 2H), 6.55–6.54 (m, 1H), 3.92 (q, J = 7.0 Hz, 1H), 1.36 (d, J = 7.0 Hz, 3H). 13C-NMR (101 MHz; DMSO-d6): δ 177.62, 147.78, 128.83, 116.25, 112.46, 51.32, 18.21. MS (ESI) m/z: 166.09 ([M + H]+; Calcd for C9H11NO2 + H 166.09, found: 166.09).
Methyl 2-(phenylamino)propanoate. 1H-NMR (400 MHz, DMSO-d6): δ 7.07 (t, J = 7.8 Hz, 2H), 6.58–6.56 (m, 1H), 6.53 (d, J = 7.7 Hz, 2H), 4.05 (q, J = 7.0 Hz, 1H), 3.62 (s, 3H), 1.37 (d, J = 7.0 Hz, 3H). 13C-NMR (151 MHz, DMSO-d6): δ 174.96, 147.57, 128.90, 116.45, 112.30, 50.90, 50.70, 18.12 (s, 1H). MS (ESI) m/z: 180.11 ([M + H]+; Calcd for C10H13NO2 + H 180.10, found: 180.11).
N-Phenyl-2-pyrrolidone. 1H-NMR (400 MHz, DMSO-d6): δ 7.65 (d, J = 7.8 Hz, 2H), 7.37 (t, J = 8.0 Hz, 2H), 7.12 (t, J = 7.4 Hz, 1H), 3.83 (t, J = 7.0 Hz, 2H), 2.48 (t, J = 8.4 Hz, 2H), 2.06 (m, J = 7.5 Hz, 2H). 13C-NMR (151 MHz; DMSO-d6): δ 174.28, 140.07, 129.06, 124.26, 119.80, 48.50, 32.78, 17.86. MS (ESI) m/z: 162.09 ([M + H]+; Calcd for C10H11NO + H 162.09, found: 162.09).
N-Methyl-N-benzoylaniline. 1H-NMR (400 MHz, CDCl3): δ 7.30 (t, J = 7.2 Hz, 2H), 7.21 (t, J = 7.4 Hz, 2H), 7.18 (t, J = 4.5 Hz, 1H), 7.14 (d, J = 8.1 Hz, 2H), 7.11 (t, J = 6.4 Hz, 1H), 7.02 (d, J = 7.5 Hz, 2H), 3.48 (s, 3H). 13C-NMR (151 MHz; DMSO-d6): δ 170.01, 145.02, 136.77, 129.82, 129.53, 128.64, 128.19, 127.50, 126.88, 38.34. MS (ESI) m/z: 212.11 ([M + H]+; Calcd for C14H13NO + H 212.11, found: 212.11).
N-Phenylindole. 1H-NMR (400 MHz, DMSO-d6): δ 7.68 (d, J = 7.6 Hz, 1H), 7.64 (d, J = 3.3 Hz, 1H), 7.59–7.54 (m, 5H), 7.45–7.35 (m, 1H), 7.09–7.24 (m, 2H), 6.75 (d, 1H). 13C-NMR (151 MHz; DMSO-d6): δ 139.63, 135.62, 130.26, 129.65, 128.84, 126.82, 124.24, 122.80, 121.48, 120.76, 110.82, 104.06. MS (ESI) m/z: 194.11 ([M + H]+; Calcd for C14H11N + H 194.10, found: 194.11).
N-Phenylimidazole. 1H-NMR (400 MHz, DMSO-d6): δ 8.32 (br, 1H), 7.79 (br, 1H), 7.66 (d, J = 7.6 Hz, 2H), 7.52 (t, J = 7.8 Hz, 2H), 7.43–7.29 (m, 1H), 7.17 (br, 1H). 13C-NMR (101 MHz; DMSO-d6): δ 136.94, 135.60, 129.93, 129.84, 126.92, 120.40, 118.30. MS (ESI) m/z: 145.08 ([M + H]+; Calcd for C9H8N2 + H 145.08, found: 145.08).
3.3. Reuse of Cu-TDPAT
The aforementioned procedure was used with 5-methyl-2-(1H)-pyridone (1 mmol), iodobenzene (1 mmol), K2CO3 (2 mmol), DMSO (5 mL) and 5% Cu-TDPAT (based on copper). The reaction mixture was magnetically stirred at 120 °C for 4 h. The liquid solution was removed, and the solid mixture was washed with ethyl acetate (5 mL). The resulting solid phase was reused for further reactions without previous purification.
4. Conclusions
In summary, a detailed investigation of N-arylation reaction of 5-methyl-2-(1H)-pyridone with halobenzenes was carried out using Cu-TDPAT, a metal-organic framework with high density of open copper sites within its framework, as an efficient heterogeneous catalyst. It has been demonstrated that Cu-TDPAT can improve the N-arylation reaction of a wide variety of amines and amides with iodobenzene or bromobenzene efficiently. The experimental results prove that Cu-TDPAT is stable to the conditions of Ullmann and Goldberg type coupling reactions, and the open copper sites on the axis of paddle-wheel SBUs within MOF are responsible for the promotion of the C–N coupling reaction. Further work is in progress to broaden the scope of this catalytic system to other substrates and to better understand the reaction mechanism.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/20/12/19756/s1.
Acknowledgments
The work was supported by the national natural science foundation of China (Grant No. 21346005, 21307168 and 21277009).
Author Contributions
All authors contributed to the appearance of this article. W.Q. and H.H. conceived and designed the experiments; W.L. performed experiments and collated data. C.G. carried out the synthesis of the Cu-TDPAT. C.L., L.S., G.B. and G.Z. analyzed the data. W.Q. and W.L. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bikker, J.A.; Brooijmans, N.; Wissner, A.; Mansour, T.S. Kinase domain mutations in cancer: Implications for small molecule drug design strategies. J. Med. Chem. 2009, 52, 1493–1509. [Google Scholar] [CrossRef] [PubMed]
- Balle, T.; Perregaard, J.; Ramirez, M.T.; Larsen, A.K.; Soby, K.K.; Liljefors, T.; Andersen, K. Synthesis and structure-affinity relationship investigations of 5-heteroaryl-substituted analogues of the antipsychotic sertindole. A new class of highly selective α1 adrenoceptor antagonists. J. Med. Chem. 2003, 46, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Popowycz, F.; Routier, S.; Joseph, B.; Merour, J.Y. Synthesis and reactivity of 7-azaindole (1H-pyrrolo[2,3-b]pyridine). Tetrahedron 2007, 63, 1031–1064. [Google Scholar] [CrossRef]
- Surry, D.S.; Buchwald, S.L. Diamine ligands in copper-catalyzed reactions. Chem. Sci. 2010, 1, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Monnier, F.; Taillefer, M. Catalytic C–C, C–N, and C–O Ullmann-type coupling reactions. Angew. Chem. Int. Ed. 2009, 48, 6954–6971. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.W.; Cai, Q. Copper/amino acid catalyzed cross-couplings of aryl and vinyl halides with nucleophiles. Acc. Chem. Res. 2008, 41, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Casitas, A.; Ribas, X. The role of organometallic copper(III) complexes in homogeneous catalysis. Chem. Sci. 2013, 4, 2301–2318. [Google Scholar] [CrossRef]
- Klapars, A.; Antilla, J.C.; Huang, X.H.; Buchwald, S.L. A general and efficient copper catalyst for the amidation of aryl halides and the N-arylation of nitrogen heterocycles. J. Am. Chem. Soc. 2001, 123, 7727–7729. [Google Scholar] [CrossRef] [PubMed]
- Taillefer, M.; Cristau, H.J.; Cellier, P.; Spindler, J.F. Method for Forming a Carbon-Carbon or Carbon-Heteroatom Linkage. WO Patent 053,885-A, 20 December 2002. [Google Scholar]
- Klapars, A.; Huang, X.; Buchwald, S.L. A general and efficient copper catalyst for the amidation of aryl halides. J. Am. Chem. Soc. 2002, 124, 7421–7428. [Google Scholar] [CrossRef] [PubMed]
- Audisio, D.; Messaoudi, S.; Peyrat, J.B.; Brion, J.D.; Alami, M. A general copper powder-catalyzed Ullmann-type reaction of 3-halo-4(1H)-quinolones with various nitrogen-containing nucleophiles. J. Org. Chem. 2011, 76, 4995–5005. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.T.; Guo, J.J.; Liu, J.G.; Ding, K.; Yu, S.Y.; Cai, Q. Copper-catalyzed desymmetric intramolecular Ullmann C–N coupling: An enantioselective preparation of indulines. J. Am. Chem. Soc. 2012, 134, 14326–14329. [Google Scholar] [CrossRef] [PubMed]
- Antila, J.C.; Klapars, A.; Buchwald, S.L. The copper-catalyzed N-arylation of indoles. J. Am. Chem. Soc. 2002, 124, 11684–11688. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, Y.; Yao, J.; Wu, S.; Tao, F. Accelerating effect induced by the structure of R-amino acid in the copper-catalyzed coupling reaction of aryl halides with R-amino acids. Synthesis of benzolactam-V8. J. Am. Chem. Soc. 1998, 120, 12459–12467. [Google Scholar] [CrossRef]
- Kim, J.; Chang, S. Ammonium salts as an inexpensive and convenient nitrogen source in the Cu-catalyzed amination of aryl halides at room temperature. Chem. Commun. 2008, 26, 3052–3054. [Google Scholar] [CrossRef] [PubMed]
- Hammoud, H.; Schmitt, M.; Bihel, F.; Antheaume, C.; Bourguignon, J.J. Direct guanidinylation of aryl and heteroarylhalides via copper-catalyzed cross-coupling reaction. J. Org. Chem. 2012, 77, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Twieg, R.J.; Huang, S.D. Copper-catalyzed amination of aromatic halides with 2-N,N-dimethylaminoethanol as solvent. Tetrahedron Lett. 2003, 44, 6289–6292. [Google Scholar] [CrossRef]
- Lu, Z.; Twieg, R.J. A mild and practical copper catalyzed amination of halothiophenes. Tetrahedron 2005, 61, 903–918. [Google Scholar] [CrossRef]
- Kwong, F.Y.; Klapars, A.; Buchwald, S.L. Copper-catalyzed coupling of alkylamines and aryl iodides: An efficient system even in an air atmosphere. Org. Lett. 2002, 4, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, B.; Ma, S.M. Studies on electrophilic cyclization of N-(buta-2,3-dienyl)amides with N-bromosuccinimide and its applications. Adv. Synth. Catal. 2014, 356, 485–492. [Google Scholar] [CrossRef]
- Tye, J.W.; Weng, Z.; Johns, A.M.; Incarvito, C.D.; Hartwig, J.F. Copper complexes of anionic nitrogen ligands in the amidation and imidation of aryl halides. J. Am. Chem. Soc. 2008, 130, 9971–9983. [Google Scholar] [CrossRef] [PubMed]
- Goodbrand, H.B.; Hu, N.X. Ligand-accelerated catalysis of the Ullmann condensation: Application to hole conducting triarylamines. J. Org. Chem. 1999, 64, 670–674. [Google Scholar] [CrossRef]
- Gujadhur, R.K.; Bates, C.G.; Venkataraman, D. Formation of aryl-nitrogen, aryl-oxygen, and aryl-carbon bonds using well-defined copper(I)-based catalysts. Org. Lett. 2001, 3, 4315–4317. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Qiu, Y.T.; Li, Z.; Wang, Z.Y.; Jiang, S. Ligands for copper-catalyzed C–N bond forming reactions with 1 mol % CuBr as Catalyst. J. Org. Chem. 2011, 76, 3151–3159. [Google Scholar] [CrossRef] [PubMed]
- Shafir, A.; Buchwald, S.L. Highly selective room-temperature copper-catalyzed C–N coupling reactions. J. Am. Chem. Soc. 2006, 128, 8742–8743. [Google Scholar] [CrossRef] [PubMed]
- Shafir, A.; Lichtor, P.A.; Buchwald, S.L. N-versus O-arylation of aminoalcohols: Orthogonal selectivity in copper-based catalysts. J. Am. Chem. Soc. 2007, 129, 3490–3491. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Taillefer, M. A very simple copper-catalyzed synthesis of anilines by employing aqueous ammonia. Angew. Chem. Int. Ed. 2009, 48, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lei, M.; Hu, L.H. Synthesis of 1-aryl indoles via coupling reaction of indoles and aryl halides catalyzed by CuI/metformin. Tetrahedron 2014, 70, 5626–5631. [Google Scholar] [CrossRef]
- Ghorbani-Vaghei, R.; Hemmati, S.; Veisi, H. An in situ generated CuI/metformin complex as a novel and efficient catalyst for C–N and C–O cross-coupling reactions. Tetrahedron Lett. 2013, 54, 7095–7099. [Google Scholar] [CrossRef]
- Yang, S.L.; Wu, C.Q.; Ruan, M.B.; Yang, Y.Q.; Zhao, Y.X.; Niu, J.J.; Yang, W.; Xu, J.W. Metal- and ligand-free Ullmann-type C–O and C–N coupling reactions promoted by potassium tert-butoxide. Tetrahedron Lett. 2012, 53, 4288–4292. [Google Scholar] [CrossRef]
- Yuan, Y.; Thome, I.; Kim, S.H.; Chen, D.; Beyer, A.; Bonnamour, J.; Zuidema, E.; Chang, S.; Bolm, C. Dimethyl sulfoxide/potassium hydroxide: A superbase for the transition metal-free preparation of cross-coupling products. Adv. Synth. Catal. 2010, 352, 2892–2898. [Google Scholar] [CrossRef]
- Cano, R.; Ramon, D.J.; Yus, M. Transition-metal-free O-, S-, and N-arylation of alcohols, thiols, amides, amines, and related heterocycles. J. Org. Chem. 2011, 76, 654–660. [Google Scholar] [CrossRef] [PubMed]
- Creutz, S.E.; Lotito, K.J.; Fu, G.C.; Peters, J.C. Photoinduced Ullmann C–N coupling: Demonstrating the viability of a radical pathway. Science 2012, 338, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, D.T.; Choi, J.; Munoz-Molina, J.M.; Bissember, A.C.; Peters, J.C.; Fu, G.C. A versatile approach to Ullmann C–N couplings at room temperature: New families of nucleophiles and electrophiles for photoinduced, copper-catalyzed processes. J. Am. Chem. Soc. 2013, 135, 13107–13112. [Google Scholar] [CrossRef] [PubMed]
- Majek, M.; von Jacobi Wangelin, A. Ambient-light-mediated copper-catalyzed C–C and C–N bond formation. Angew. Chem. Int. Ed. 2013, 52, 5919–5921. [Google Scholar] [CrossRef] [PubMed]
- Strieter, E.R.; Bhayana, B.; Buchwald, S.L. Mechanistic studies on the copper-catalyzed N-arylation of amides. J. Am. Chem. Soc. 2009, 131, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Z.; Jiang, Y.Y.; Fu, Y.; Liu, L. Alternative mechanistic explanation for ligand-dependent selectivities in copper-catalyzed N- and O-arylation reactions. J. Am. Chem. Soc. 2010, 132, 18078–18091. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.O.; Liu, P.; Houk, K.N.; Buchwald, S.L. Computational explorations of mechanisms and ligand-directed selectivities of copper-catalyzed Ullmann-type reactions. J. Am. Chem. Soc. 2010, 132, 6205–6213. [Google Scholar] [CrossRef] [PubMed]
- Satish, G.; Reddy, K.H.V.; Ramesh, K.; Kumar, B.S.P.A.; Nageswar, Y.V.D. An elegant protocol for the synthesis of N-substituted pyrroles through C–N cross coupling/aromatization process using CuFe2O4 nanoparticles as catalyst under ligand-free conditions. Tetrahedron Lett. 2014, 55, 2596–2599. [Google Scholar] [CrossRef]
- Costa, M.V.; Viana, G.M.; de Souza, T.M.; Malta, L.F.B.; Aguiar, L.C.S. Copper-catalyzed C–N cross-coupling reactions for the preparation of aryl diamines applying mild conditions. Tetrahedron Lett. 2013, 54, 2332–2335. [Google Scholar] [CrossRef]
- Reddy, K.H.V.; Satish, G.; Ramesh, K.; Karnakar, K.; Nageswar, Y.V.D. An efficient synthesis of N–substituted indoles from indoline/indoline carboxylic acid via aromatization followed by C–N cross-coupling reaction by using nano copper oxide as a recyclable catalyst. Tetrahedron Lett. 2012, 53, 3061–3065. [Google Scholar] [CrossRef]
- Zhang, P.F.; Yuan, J.Y.; Li, H.R.; Liu, X.F.; Xu, X.; Antonietti, M.; Wang, Y. Mesoporous nitrogen-doped carbon for copper mediated Ullmann-type C–O/–N/–S cross-coupling reactions. RSC Adv. 2013, 3, 1890–1895. [Google Scholar] [CrossRef]
- Xiao, R.; Zhao, H.; Cai, M. MCM-41-immobilized bidentate nitrogen copper(I) complex: A highly efficient and recyclable catalyst for Buchwald N-arylation of indoles. Tetrahedron 2013, 69, 5444–5450. [Google Scholar] [CrossRef]
- Likhar, P.R.; Roy, S.; Roy, M.; Kantam, M.L.; De, R.L. Silica immobilized copper complexes: Efficient and reusable catalysts for N-arylation of N(H)-heterocycles and benzyl amines with aryl halides and arylboronic acids. J. Mol. Catal. A: Chem. 2007, 271, 57–62. [Google Scholar] [CrossRef]
- Choudary, B.M.; Sridhar, C.; Kantam, M.L.; Venkanna, G.T.; Sreedhar, B. Design and evolution of copper apatite catalysts for N-arylation of heterocycles with chloro- and fluoroarenes. J. Am. Chem. Soc. 2005, 127, 9948–9949. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, G.; Wang, D.; Alper, H. Magnetic nanoparticle-supported proline as a recyclable and recoverable ligand for the CuI catalyzed arylation of nitrogen nucleophiles. Chem. Commun. 2007, 45, 4809–4811. [Google Scholar] [CrossRef] [PubMed]
- Mostafalu, R.; Kaboudin, B.; Kazemi, F.; Yokomatsu, T. N-arylation of amines: C–N coupling of amines with arylboronic acids using Fe3O4 magnetic nanoparticles-supported EDTA-Cu(II) complex in water. RSC Adv. 2014, 4, 49273–49279. [Google Scholar] [CrossRef]
- Huang, L.; Yu, R.; Zhu, X.; Wan, Y. A recyclable Cu-catalyzed C–N coupling reaction in water and its application to synthesis of imidazo[1,2-a]quinoxaline. Tetrahedron 2013, 69, 8974–8977. [Google Scholar] [CrossRef]
- Huang, Y.B.; Yang, C.T.; Yi, J.; Deng, X.J.; Fu, Y.; Liu, L. Cu-catalyzed carbon-heteroatom coupling reactions under mild conditions promoted by resin-bound organic ionic bases. J. Org. Chem. 2011, 76, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.M.; Mondal, S.; Mondal, P.; Roy, A.S.; Tuhina, K.; Salam, N.; Mobarak, M. A reusable polymer supported copper catalyst for the C–N and C–O bond cross-coupling reaction of aryl halides as well as arylboronic acids. J. Org. Chem. 2012, 696, 4264–4274. [Google Scholar] [CrossRef]
- Yang, B.; Mao, Z.X.; Zhu, X.H.; Wan, Y.Q. Functionalised chitosan as a green, recyclable, supported catalyst for the copper-catalysed Ullmann C–N coupling reaction in water. Catal. Commun. 2015, 60, 92–95. [Google Scholar] [CrossRef]
- Salam, N.; Kundu, S.K.; Roy, A.S.; Mondal, P.; Roy, S.; Bhaumik, A.; Islam, S.M. Cu-grafted mesoporous organic polymer: A new recyclable nanocatalyst for multi-component, N-arylation and S-arylation reactions. Catal. Sci. Technol. 2013, 3, 3303–3316. [Google Scholar]
- Corma, A.; Garcia, H.; Llabres, I.; Xamena, F.X. Engineering metal organic frameworks for heterogeneous catalysis. Chem. Rev. 2010, 110, 4606–4655. [Google Scholar] [CrossRef] [PubMed]
- Chughtai, A.H.; Ahmad, N.; Younus, H.A.; Laypkov, A.; Verpoort, F. Metal-organic frameworks: Versatile heterogeneous catalysts for efficient catalytic organic transformations. Chem. Soc. Rev. 2015, 44, 6804–6849. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Opanasenko, M.; Cejka, J.; Garcia, H. Metal organic frameworks as heterogeneous catalysts for the production of fine chemicals. Catal. Sci. Technol. 2013, 3, 2509–2540. [Google Scholar] [CrossRef]
- Gomez-Lor, B.; Gutierrez-Puebla, E.; Iglesias, M.; Monge, M.A.; Ruiz-Valero, C.; Snejko, N. In2(OH)3(BDC)1.5 (BDC = 1,4-Benzendicarboxylate): An In(III) supramolecular 3D framework with catalytic activity. Inorg. Chem. 2002, 41, 2429–2432. [Google Scholar] [CrossRef] [PubMed]
- Llabresi Xamena, F.X.; Abad, A.; Corma, A.; Garcia, H. MOFs as catalysts: Activity, reusability and shape-selectivity of a Pd-containing MOF. J. Catal. 2007, 250, 294–298. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Chevreau, H.; Horcajada, P.; Devic, T.; Serre, C.; Garcia, H. Iron(III) metal-organic frameworks as solid Lewis acids for the isomerization of α-pinene oxide. Catal. Sci. Technol. 2012, 2, 324–330. [Google Scholar] [CrossRef]
- Gu, J.M.; Kim, W.S.; Huh, S. Size-dependent catalysis by DABCO-functionalized Zn-MOF with one-dimensional channels. Dalton Trans. 2011, 40, 10826–10829. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.; Wu, P.; He, C.; Xie, Z.; Duan, C. Homochiral metal-organic frameworks for heterogeneous asymmetric catalysis. J. Am. Chem. Soc. 2010, 132, 14321–14323. [Google Scholar] [CrossRef] [PubMed]
- Llabres i Xamena, F.X.; Casanova, O.; Tailleur, R.G.; Garcia, H.; Corma, A. Metal organic frameworks (MOFs) as catalysts: A combination of Cu2+ and Co2+ MOFs as an efficient catalyst for tetralin oxidation. J. Catal. 2008, 255, 220–227. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Garcia, H. Aerobic oxidation of benzylic alcohols catalyzed by metal-organic frameworks assisted by TEMPO. ACS Catal. 2011, 1, 48–53. [Google Scholar] [CrossRef]
- Marx, S.; Kleist, W.; Baiker, A. Synthesis, structural properties, and catalytic behavior of Cu-BTC and mixed-linker Cu-BTC-PyDC in the oxidation of benzene derivatives. J. Catal. 2011, 281, 76–87. [Google Scholar] [CrossRef]
- Fei, H.H.; Shin, J.; Meng, Y.S.; Adelhardt, M.; Sutter, J.; Meyer, K.; Cohen, S.M. Reusable oxidation catalysis using metal-monocatecholato species in a robust metal-organic framework. J. Am. Chem. Soc. 2014, 136, 4965–4973. [Google Scholar] [CrossRef] [PubMed]
- Maksimchuk, N.V.; Kovalenko, K.A.; Fedin, V.P.; Kholdeeva, O.A. Cyclohexane selective oxidation over metal-organic frameworks of MIL-101 family: Superior catalytic activity and selectivity. Chem. Commun. 2012, 48, 6812–6814. [Google Scholar] [CrossRef] [PubMed]
- Laurier, K.G.M.; Vermoortele, F.; Ameloot, R.; de Vos, D.E.; Hofkens, J.; Roeffaers, M.B.J. Iron(III)-based metal-organic frameworks as visible light photocatalysts. J. Am. Chem. Soc. 2013, 135, 14488–14491. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.K.; Huang, R.K.; Liu, W.J.; Sun, D.R.; Li, Z.H. Fe-based MOFs for photocatalytic CO2 reduction: Role of coordination unsaturated sites and dual excitation pathways. ACS Catal. 2014, 4, 4254–4260. [Google Scholar] [CrossRef]
- Deng, M.; Ling, Y.; Xia, B.; Chen, Z.; Zhou, Y.; Liu, X.; Yue, B.; He, H. Synthesis of isoreticular zinc(II)-phosphonocarboxylate frameworks and their application in the Friedel-Crafts benzylation reaction. Chem. Eur. J. 2011, 17, 10323–10328. [Google Scholar] [CrossRef] [PubMed]
- Phan, N.T.S.; Le, K.K.A.; Phan, T.D. MOF-5 as an efficient heterogeneous catalyst for Friedel-Crafts alkylation reactions. Appl. Catal. A Gen. 2010, 382, 246–253. [Google Scholar] [CrossRef]
- Ravon, U.; Savonnet, M.; Aguado, S.; Domine, M.E.; Janneau, E.; Farrusseng, D. Engineering of coordination polymers for shape selective alkylation of large aromatics and the role of defects. Microporous Mesoporous Mater. 2010, 129, 319–329. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Opanasenko, M.; Cejka, J.; Garcia, H. Metal organic frameworks as solid catalysts in condensation reactions of carbonyl groups. Adv. Synth. Catal. 2013, 355, 247–268. [Google Scholar] [CrossRef]
- Phan, N.T.S.; Nguyen, T.T.; Nguyen, C.V.; Nguyen, T.T. Ullmann-type coupling reaction using metal-organic framework MOF-199 as an efficient recyclable solid catalyst. Appl. Catal. A: Gen. 2013, 457, 69–77. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, B.Z.; Ma, T.M.; Jiang, H.F.; Li, Y.W. Ligand-free coupling of phenols and alcohols with aryl halides by arecyclable heterogeneous copper catalyst. RSC Adv. 2012, 2, 5528–5530. [Google Scholar] [CrossRef]
- Dang, T.T.; Zhu, Y.H.; Ghosh, S.C.; Chen, A.Q.; Chai, C.L.L.; Seayad, A.M. Atmospheric pressure aminocarbonylation of aryl iodides using palladium nanoparticles supported on MOF-5. Chem. Commun. 2012, 48, 1805–1807. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Alvaro, M.; Concepcion, P.; Garcia, H. Chemical instability of Cu3(BTC)2 by reaction with thiols. Catal. Commun. 2011, 12, 1018–1021. [Google Scholar] [CrossRef]
- Li, B.Y.; Zhang, Z.J.; Li, Y.; Yao, K.X.; Zhu, Y.H.; Deng, Z.Y.; Yang, F.; Zhou, X.J.; Li, G.H.; Wu, H.H.; et al. Enhanced binding affinity, remarkable selectivity, and high capacity of CO2 by dual functionalization of a rht-type metal-organic framework. Angew. Chem. Int. Ed. 2012, 51, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Gant, T.G.; Sarshar, S. New Substituted N-Arylpyridone Derivatives Useful for Treating e.g., Idiopathic Pulmonary Fibrosis, Cirrhosis, Diffuse Parenchymal Lung Disease, Mediastinal Fibrosis, Tuberculosis, and Rheumatoid Arthritis. U.S. Patent 0,319,026 A1, 20 June 2008. [Google Scholar]
- Okazaki, A.; Ohkura, N.; Fujimura, M.; Katayama, N.; Kasahara, K. Effects of pirfenidone on increased cough reflex sensitivity in guinea pigs. Pulm. Pharmacol. Ther. 2013, 26, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.K.T.; Rajak, S.S.; Bhanage, B.M. N-arylation of indoles with aryl halides using copper/glycerolas a mild and highly efficient recyclable catalytic system. Tetrahedron Lett. 2014, 55, 931–935. [Google Scholar] [CrossRef]
- Ricordi, V.G.; Freitas, C.S.; Perin, G.; Lenardao, E.J.; Jacob, R.G.; Savegnago, L.; Alves, D. Glycerol as a recyclable solvent for copper-catalyzed cross-coupling reactionsof diaryldiselenides with aryl boronic acids. Green Chem. 2012, 14, 1030–1034. [Google Scholar] [CrossRef]
- Frank, J.; Katritzky, A.R. Tautornericpyridines. Part XV. Pyridone-hydroxypyridine equilibriain solvents of differing polarity. J. Chem. Soc. Perkin Trans. 1976, 2, 1428–1431. [Google Scholar] [CrossRef]
- Alaerts, L.; Seguin, E.; Poelman, H.; Thibault-Starzyk, F.; Jacobs, P.A.; de Vos, D.E. Probing the Lewis acidity and catalytic activity of the metal-organic framework [Cu3(btc)2] (BTC = Benzene-1,3,5-tricarboxylate). Chem. Eur. J. 2006, 12, 7353–7363. [Google Scholar] [CrossRef] [PubMed]
- Schlichte, K.; Kratzke, T.; Kaskel, S. Improved synthesis, thermal stability and catalytic properties of the metal-organic framework compound Cu3(BTC)2. Microporous Mesoporous Mater. 2004, 73, 81–88. [Google Scholar] [CrossRef]
- Priyadarshini, S.; Amal Joseph, P.J.; Lakshmi Kantam, M.; Sreedhar, B. Copper MOF: Scope and limitation in catalytic hydroxylation and nitration of aryl halides. Tetrahedron 2013, 69, 6409–6414. [Google Scholar] [CrossRef]
- Wee, L.H.; Bajpe, S.R.; Janssens, N.; Hermans, I.; Houthoofd, K.; Kirschhock, C.E. A.; Martens, J.A. Convenient synthesis of Cu3(BTC)2 encapsulated Keggin heteropolyacid nanomaterial for application in catalysis. Chem. Commun. 2010, 46, 8186–8188. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Q.; Qiu, W.G.; Long, W.; Deng, F.; Bai, G.M.; Zhang, G.Z.; Zi, X.H.; He, H. Synthesis of porphyrin@MOFs type catalysts through “one-pot” self-assembly. J. Mol. Catal. A Chem. 2014, 393, 166–170. [Google Scholar] [CrossRef]
- Yin, J.; Buchwald, S.L. Palladium-catalyzed intermolecular coupling of aryl halides and amides. Org. Lett. 2000, 2, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Quan, Z.J.; Xia, H.D.; Zhang, Z.; Da, Y.X.; Wang, X.C. An efficient copper-catalyzed N-arylation of amides: Synthesis of N-arylacrylamides and 4-amido-N-phenylbenzamides. Tetrahedron 2013, 69, 8368–8374. [Google Scholar] [CrossRef]
- Sperotto, E.; Klink, G.P. M.V.; Koten, G.V.; Vries, J.G. D. The mechanism of the modified Ullmann reaction. Dalton Trans. 2010, 39, 10338–10351. [Google Scholar] [CrossRef] [PubMed]
- Sambiagio, C.; Marsden, S.P.; John Blacker, A.; McGowan, P.C. Copper catalyzed Ullmann type chemistry: From mechanistic aspects to modern development. Chem. Soc. Rev. 2014, 43, 3525–3550. [Google Scholar] [CrossRef] [PubMed]
- Mkami, H.E.L.; Mohideen, M.I.H.; Pal, C.; McKinlay, A.; Scheimann, O.; Morris, R.E. EPR and magnetic studies of a novel copper metal organic framework (STAM-I). Chem. Phys. Lett. 2012, 544, 17–21. [Google Scholar] [CrossRef]
- Simenas, M.; Kobalz, M.; Mendt, M.; Eckold, P.; Krautscheid, H.; Banys, J.; Poppl, A. Synthesis, structure, and electron paramagnetic resonance study of a mixed valent metal-organic framework containing Cu2 paddle-wheel units. J. Phys. Chem. C 2015, 119, 4898–4907. [Google Scholar] [CrossRef]
- Sample Availability: Sample of the compound Cu-TDPAT is available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).