Antioxidant Properties of Essential Oil Extracted from Pinus morrisonicola Hay Needles by Supercritical Fluid and Identification of Possible Active Compounds by GC/MS
Abstract
:1. Introduction
2. Results
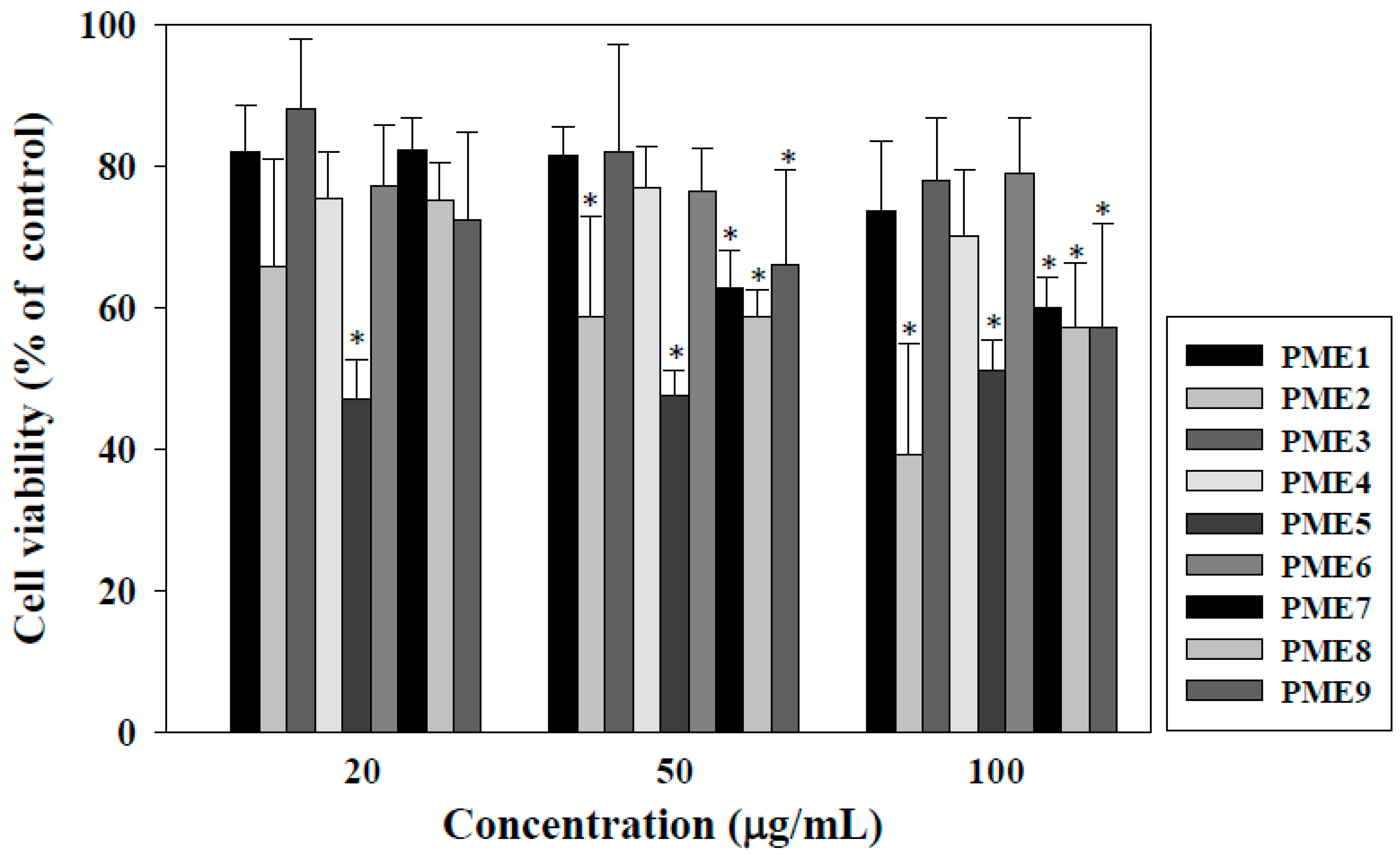
2.1. Effect of PME on Cell Viability of Macrophages
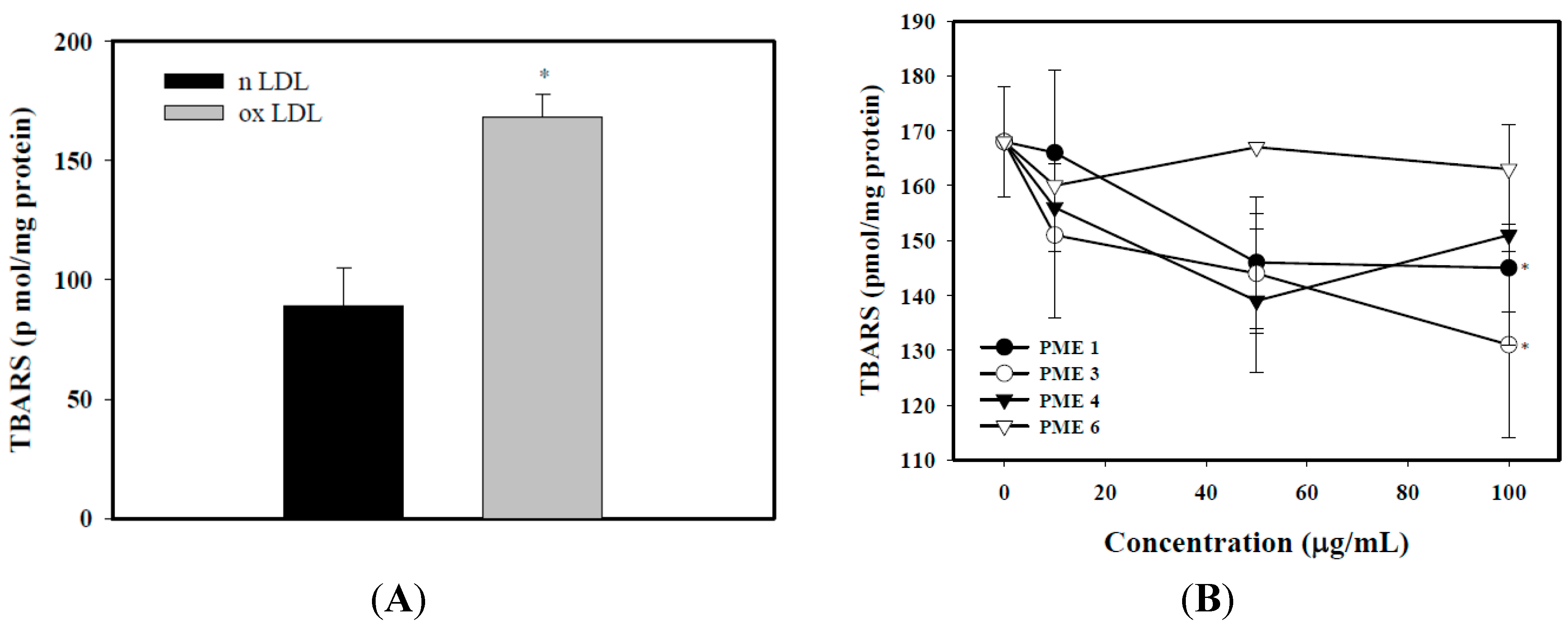
2.2. Effect of PME on Lipid Peroxidation in Macrophages Treated with ox-LDL
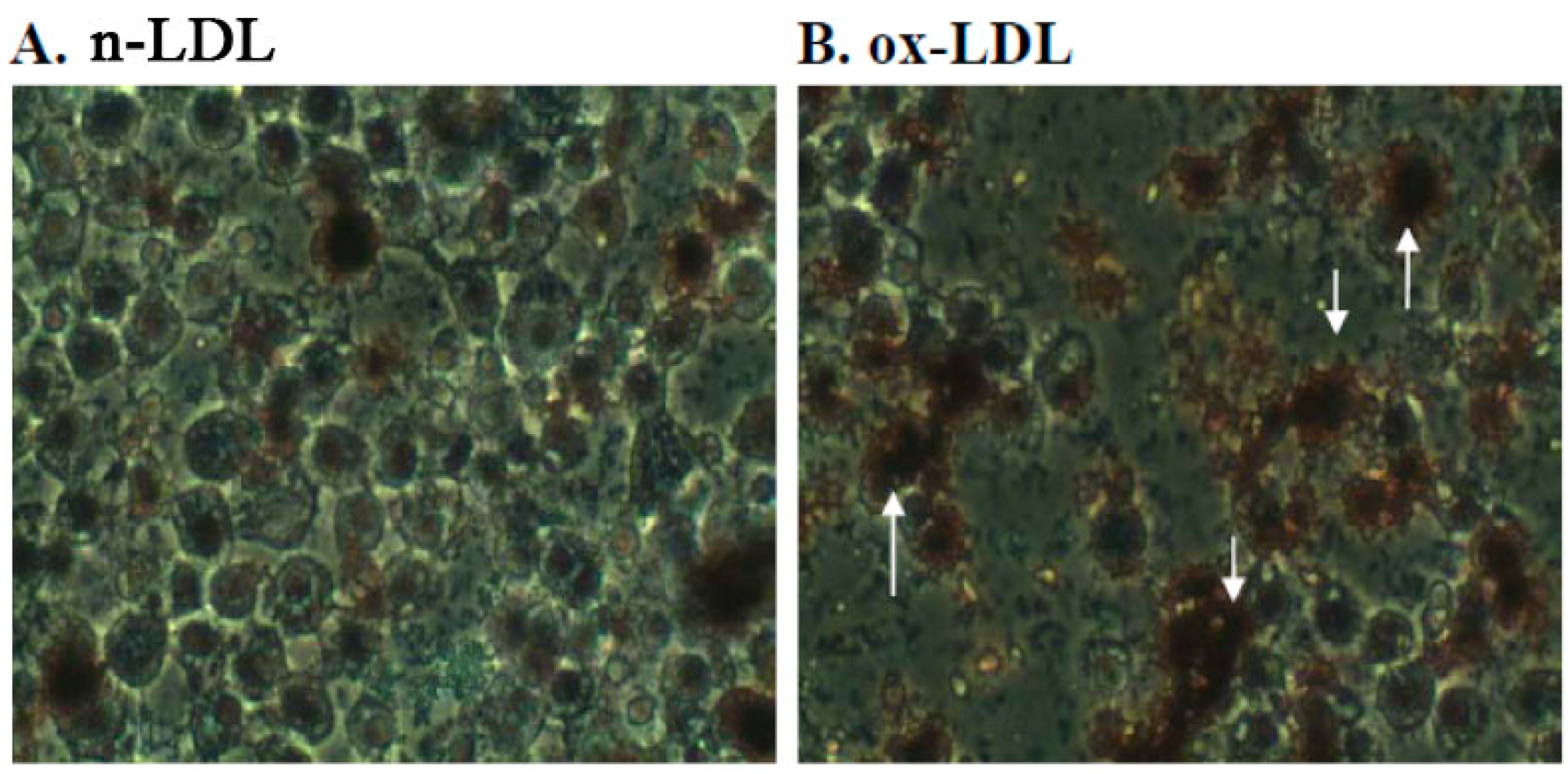
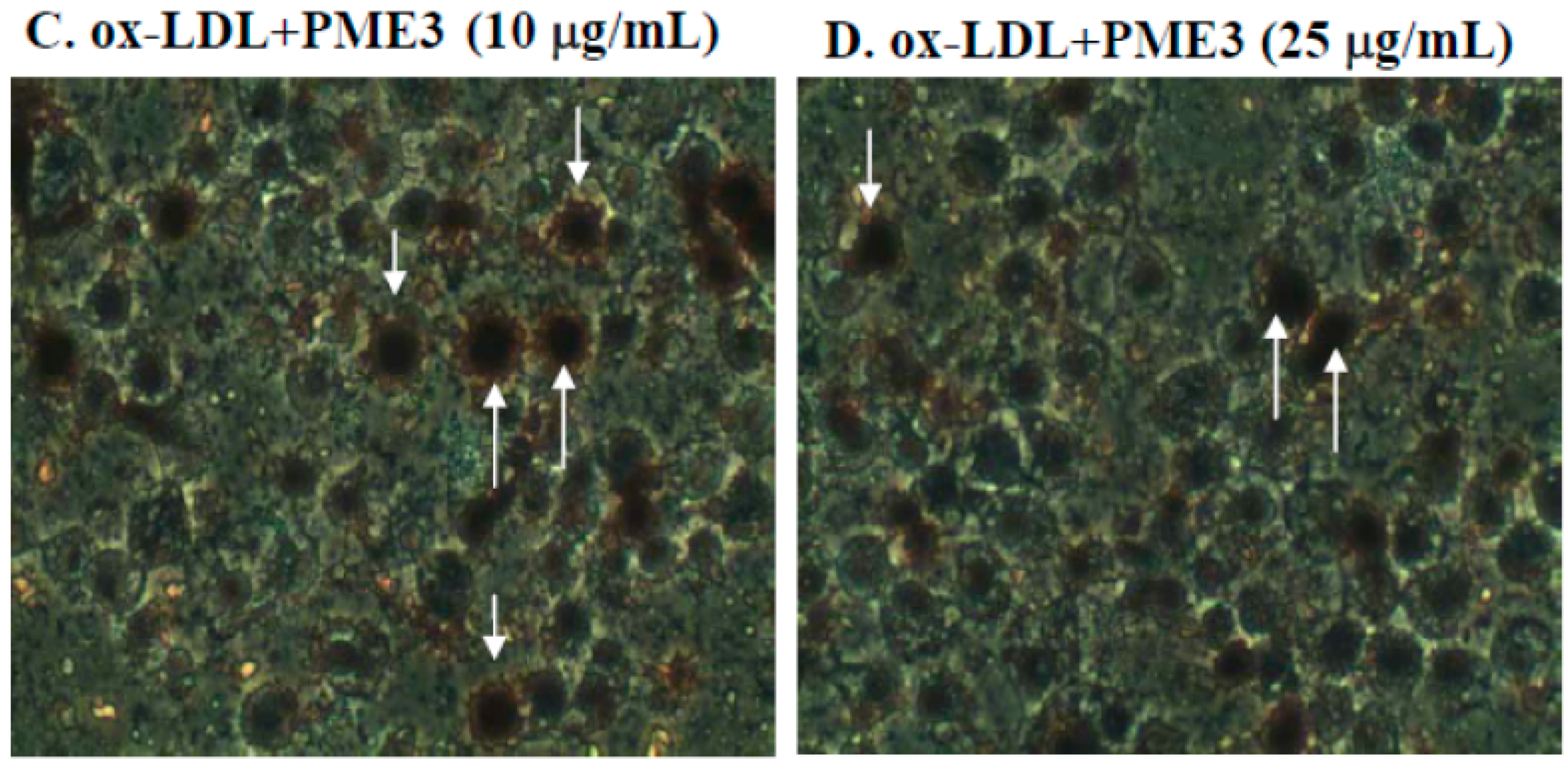
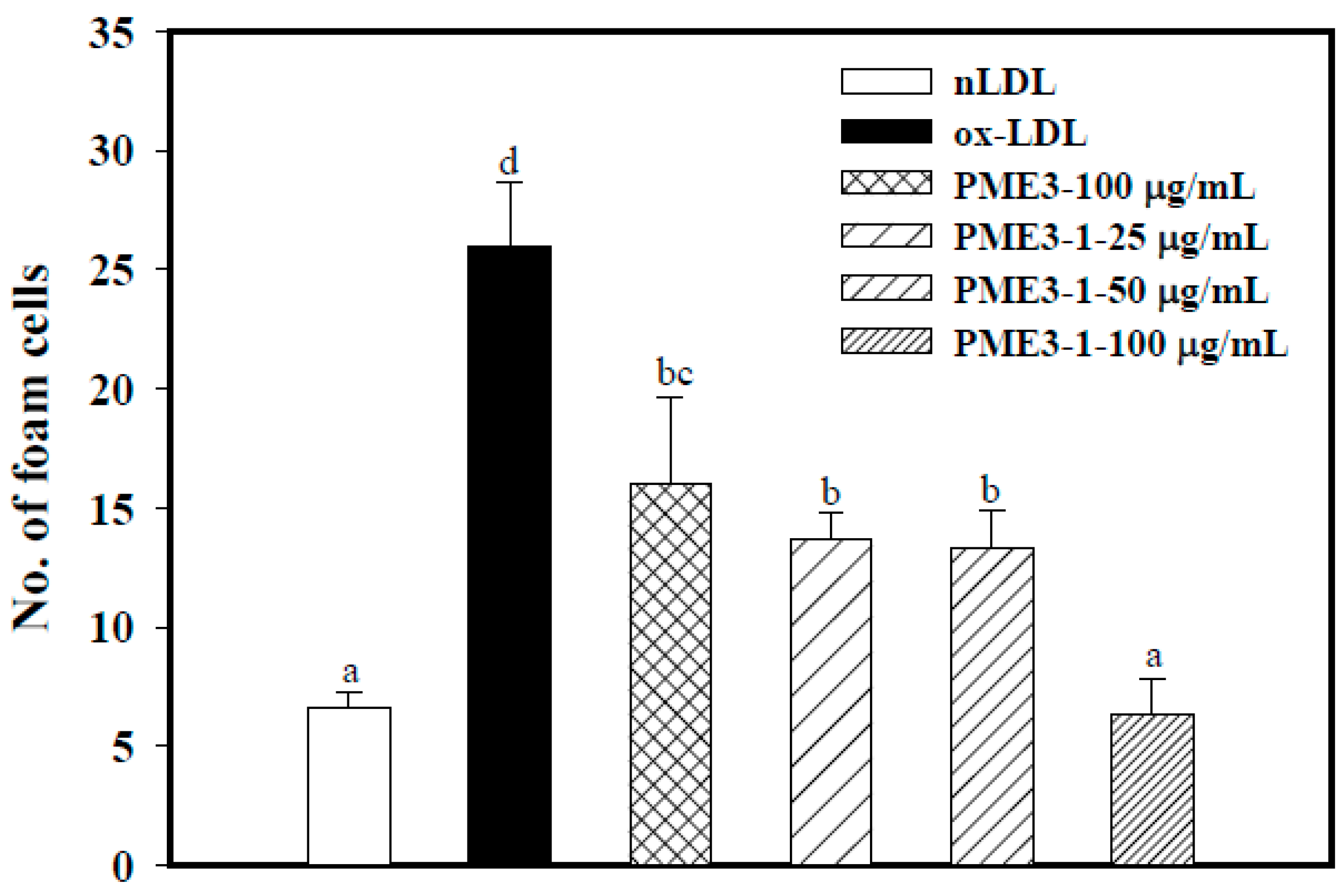
2.3. Effect of PME3 on Foam cell Formation in Macrophages Treated with ox-LDL
2.4. Fractionation of PME3 by Column Chromatography and Thin-Layer Chromatography
| PME3 Fractions | Tube Number | Rf Value | Yields (%) |
|---|---|---|---|
| PME3-1 | 1–6 * | 0.91 | 60 |
| PME3-2 | 8–14 | 0.50 | 28 |
| PME3-3 | 20–32 | 0.26 | 3 |
| PME3-4 | 251–270 | 0.10 | 0.5 |
2.5. Inhibitory Effects of PME3-1 on Conjugated Diene in Cell-Free n-LDL Oxidized by Cu2+
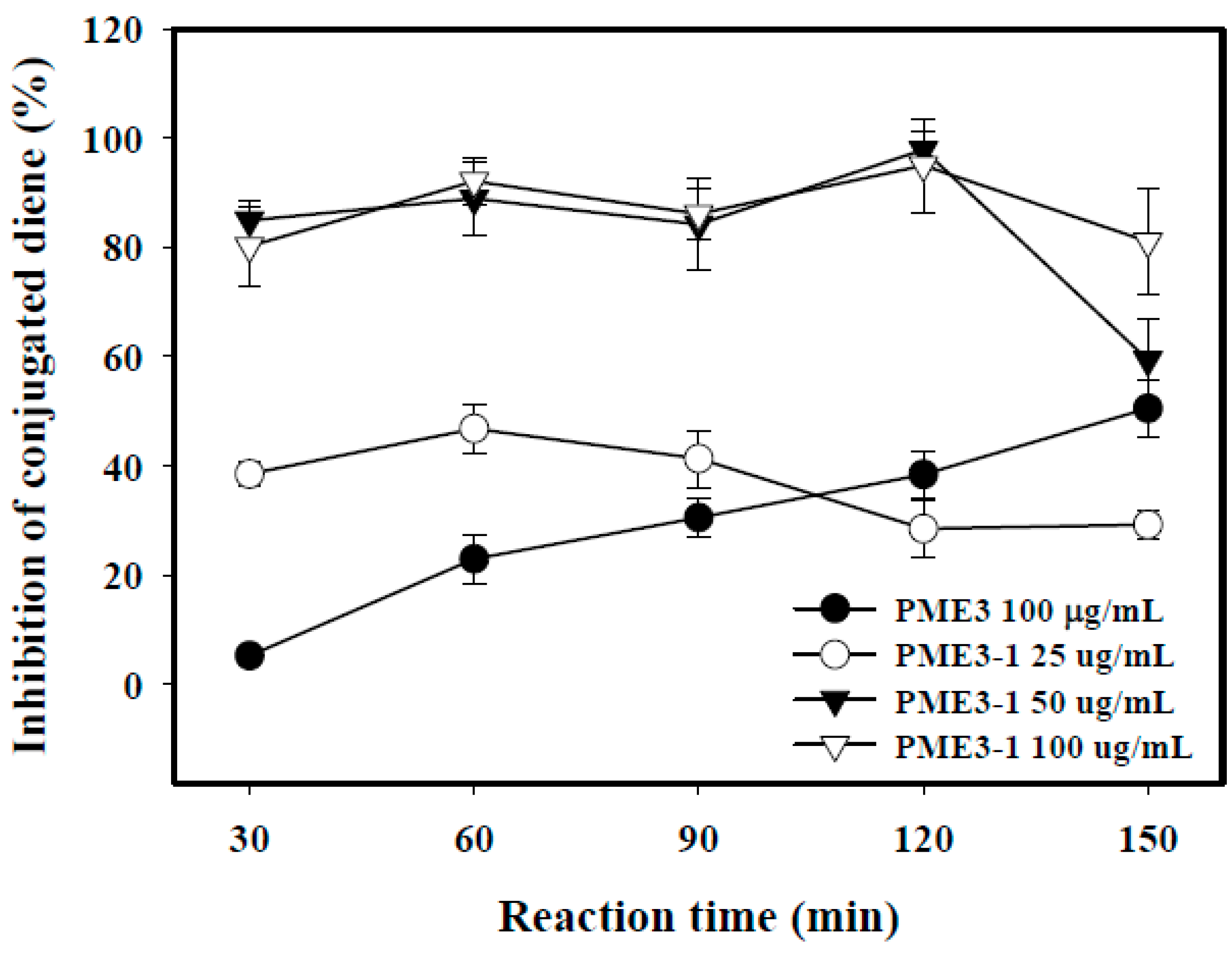
2.6. Inhibitory Effects of PME3-1 on Foam Cell Formation in Macrophages Treated with ox-LDL
2.7. GC/MS Analysis of PME3 and PME3-1
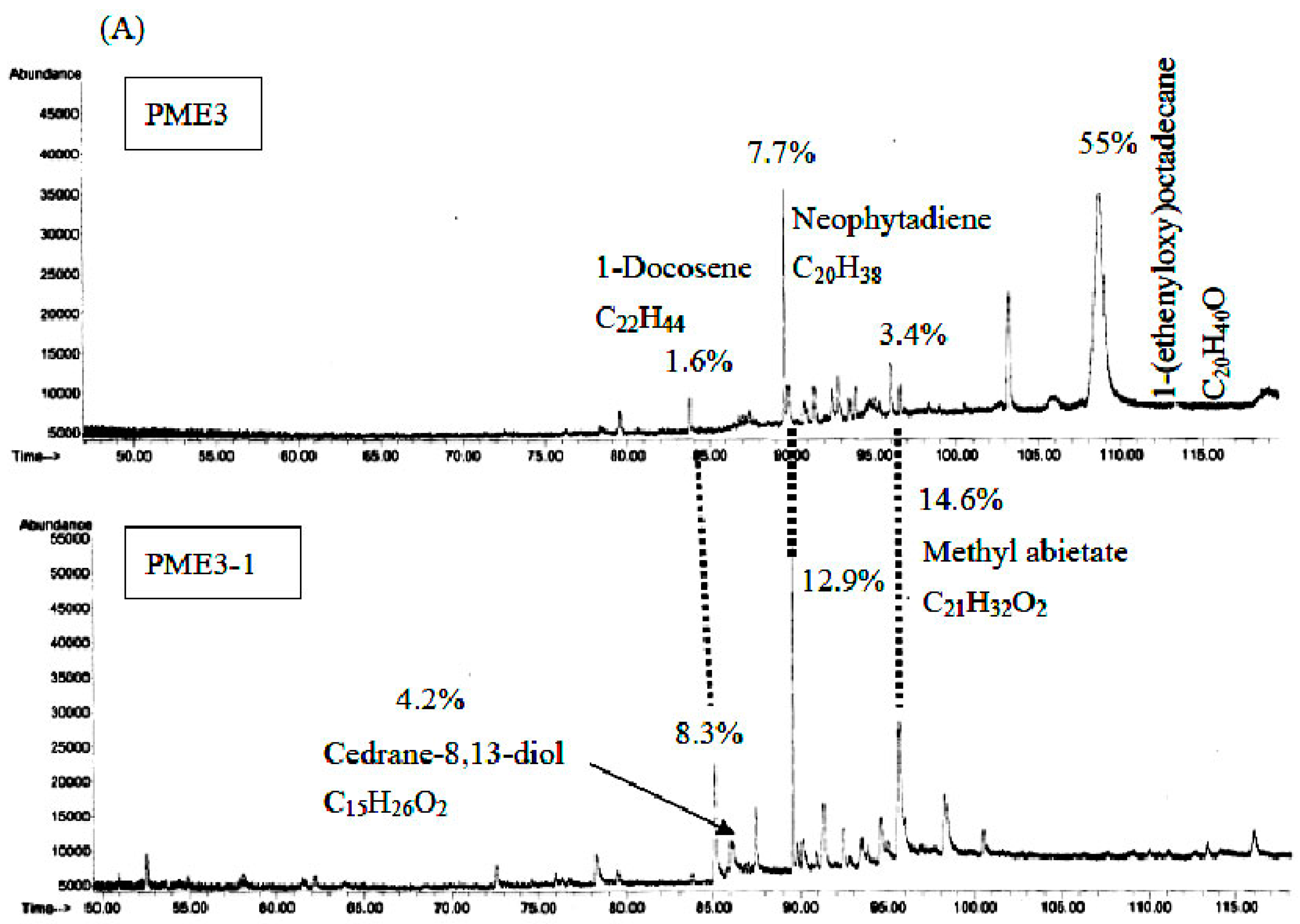

3. Discussion
4. Experimental Section
4.1. Chemical and Reagents
4.2. Sample Preparation
4.3. Supercritical Fluid Extraction
| Extract | Temperature (°C) | Pressure (MPa) | Time (min) | Adjuvant (mL) | Yield (%) |
|---|---|---|---|---|---|
| PME 1 | 40 | 15 | 5 | 1 | 1.1 ± 0.04 * |
| PME 2 | 40 | 20 | 10 | 2 | 3.2 ± 0.52 |
| PME 3 | 40 | 25 | 15 | 3 | 3.9 ± 1.07 |
| PME 4 | 50 | 15 | 20 | 3 | 3.7 ± 0.90 |
| PME 5 | 50 | 20 | 25 | 1 | 3.4 ± 0.02 |
| PME 6 | 50 | 25 | 15 | 2 | 2.7 ± 0.27 |
| PME 7 | 60 | 15 | 25 | 2 | 2.8 ± 0.50 |
| PME 8 | 60 | 20 | 15 | 3 | 3.7 ± 0.05 |
| PME 9 | 60 | 25 | 20 | 1 | 2.5 ± 0.06 |
4.4. Separation and Characterization of PME3
4.5. GC/MS Analysis of Chemical Components in PME3 and PME3-1
4.6. Lipoprotein Separation
4.7. Cell Culture and Treatment with Oxidized LDL
4.8. Determination of Cell Viability
4.9. Determination of Thiobarbituric Acid-Reactive Substances (TBARS) in Macrophages Treated with Oxidized LDL
4.10. Determination of Foam cell Formation in Macrophages Treated with Ox-LDL
4.11. Determination of Conjugated Diene Formation in Cell-Free LDL Oxidized by Cu2+
4.12. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Steinberg, D. Low density lipoprotein oxidation and its pathobiological significance. J. Biol. Chem. 1997, 272, 20963–20966. [Google Scholar] [CrossRef] [PubMed]
- Young, I.S.; McEneny, J. Lipoprotein oxidation and atherosclerosis. Biochem. Soc. Trans. Pt. 2001, 2, 358–362. [Google Scholar] [CrossRef]
- Isner, J.M.; Kearney, M.; Bortman, S.; Passeri, J. Apoptosis in human atherosclerosis and restenosis. Circulation 1995, 91, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Kolodgie, F.D.; Narula, J.; Guillo, P.; Virmani, R. Apoptosis in human atherosclerotic plaques. Apoptosis 1999, 4, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Fuhrman, B.; Aviram, M. Flavonoids protect LDL from oxidation and attenuate atherosclerosis. Curr. Opin. Lipidol. 2001, 12, 41–48. [Google Scholar]
- Ness, A.R.; Powles, J.W. Fruit and vegetables, and cardiovascular disease: A review. Int. J. Epidemiol. 1997, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hartley, L.; Igbinedion, E.; Thorogood, M.; Clarke, A.; Stranges, S.; Hooper, L.; Rees, K. Increased consumption of fruit and vegetables for the primary prevention of cardiovascular diseases. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef]
- Kim, K.Y.; Chung, H.J. Flavor compounds of pine sprout tea and pine needle tea. J. Agric. Food Chem. 2000, 48, 1269–1272. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Hsieh, P.C.; Mau, J.L.; Sheu, S.C. Antioxidant properties and mutagenicity of Pinus morrisonicola and its vinegar preparation. LTW-Food Sci. Technol. 2011, 44, 1477–1481. [Google Scholar]
- Rohdewald, P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int. J. Clin. Pharmacol. Ther. 2002, 40, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Schafer, A.; Chovanova, Z.; Muchova, J.; Sumegova, K.; Liptakova, A.; Durackova, Z.; Högger, P. Inhibition of COX-1 and COX-2 activity by plasma of human volunteers after ingestion of French maritime pine bark extract (Pycnogenol). Biomed. Pharmacother. 2005, 60, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.Y.; Sheu, S.C.; Liaw, E.T.; Wang, T.C.; Lin, C.C. Antioxidant activity and effect of Pinus morrisonicola Hay on the survival of leukemia cell line U937. Phytomedicine 2005, 12, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Tourino, S.; Selga, A.; Jimenez, A.; Julia, L.; Lozano, C.; Lizarraga, D.; Cascante, M.; Torres, J.L. Procyanidin fractions from pine (Pinus pinaster) bark: Radical scavenging power in solution, antioxidant activity in emulsion, and antiproliferative effect in melanoma cells. J. Agric. Food Chem. 2005, 53, 4728–4735. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Duh, P.; Huang, D.W.; Hsu, C.L.; Fu, T.Y. Protective effect of pine (Pinus morrisonicola Hay) needle on LDL oxidation and anti-inflammatory action by modulation of iNOS and COX-2 expression in LPS-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2008, 46, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Modey, W.K.; Mulholl, D.A.; Raynor, M.W. Analytical Supercritical Fluid Extraction of Natural Products. Phytochem. Anal. 1996, 7, 1–15. [Google Scholar] [CrossRef]
- Babovic, N.; Djilas, S.; Jadranin, M.; Vajs, V.; Ivanovic, J.; Petrovic, S.; Zizovic, I. Supercritical carbon dioxide extraction of antioxidant fractions from selected Lamiaceae herbs and their antioxidant capacity. Innov. Food Sci. Emerg. Technol. 2010, 11, 98–107. [Google Scholar]
- Chen, Z.; Mei, X.; Jin, Y.; Kim, E.H.; Yang, Z.; Tu, Y. Optimization of supercritical carbon dioxide extraction of essential oil of flowers of tea (Camellia sinensis L.) plants and its antioxidative activity. J. Sci. Food Agric. 2014, 94, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.C.; Lin, L.Y.; Yu, T.H.; Peng, R.Y. Hypolipidemic and antioxidant activity of mountain celery (Cryptotaenia japonica Hassk) seed essential oils. J. Agric. Food Chem. 2008, 56, 3997–4003. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.C.; Zhu, R.X.; Jia, L.R.; Gao, H.; Zheng, Y.; Sun, Q. Chemical composition, antimicrobial and antioxidant activities of essential oil from Gnaphlium affine. Food Chem. Toxicol. 2011, 49, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.C.; Zhang, Z.; Gao, H.; Jia, L.R.; He, Q. Chemical composition, antioxidant, and antimicrobial activities of essential oil from pine needle (Cedrus deodara). J. Food Sci. 2012, 77, C824–C829. [Google Scholar] [CrossRef] [PubMed]
- Kabara, J.J. Phenols and Chelators. In Food Preservatives, 2nd ed.; Russell, N.J., Gould, J.W., Eds.; Blackie, Glasgow: London, UK, 1991; pp. 200–214. [Google Scholar]
- Lin, M.C.; Tsai, M.J.; Wen, K.C. Supercritical fluid extraction of flavonoids from Scutellariae radix. J. Chromatogr A 1999, 830, 387–398. [Google Scholar] [CrossRef]
- Ibañez, E.; Señoráns, F.J. Tuning of mobile and stationary phase polarity for the separation of polar compounds by SFC. J. Biochem. Biophys. Methods 2000, 43, 25–43. [Google Scholar]
- Machana, S.; Weerapreeyakul, N.; Barusrux, S.; Thumanu, K.; Tanthanuch, W. Synergistic anticancer effect of the extracts from Polyalthia evecta caused apoptosis in human hepatoma (HepG2) cells. Asian Pac. J. Trop. Biomed. 2012, 2, 589–596. [Google Scholar] [CrossRef]
- Fang, J.M.; Chang, V.F.; Chang, Y.S. Flavonoids from P. morrisonicola. Phytochemistry 1987, 26, 2559–2561. [Google Scholar] [CrossRef]
- Jung, M.J.; Jung, H.A.; Kang, S.S.; Hwang, G.S.; Choi, J.S. A new abietic acid-type diterpene glucoside from the needles of Pinus densiflora. Arch. Pharm. Res. 2009, 32, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Grassmann, J.; Hippeli, S.; Vollmann, R.; Elstner, E.F. Antioxidative properties of the essential oil from Pinus mugo. J. Agric. Food Chem. 2003, 51, 7576–7582. [Google Scholar] [CrossRef] [PubMed]
- Carretero, M.E.; López-Pérez, J.L.; Abad, M.J.; Bermejo, P.; Tillet, S.; Israel, A.; Noguera, P.B. Preliminary study of the anti-inflammatory activity of hexane extract and fractions from Bursera simaruba (Linneo) Sarg. (Burseraceae) leaves. J. Ethnopharmacol. 2008, 116, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Schonburg, G.; Dielmann, G. Identification by means of retention parameters. J. Chromatogr. Sci. 1973, 11, 151–159. [Google Scholar]
- Ou, H.C.; Lee, W.J.; Lee, S.D.; Huang, C.Y.; Chiu, T.H.; Tsai, K.L.; Hus, W.C.; Sheu, W.H.H. Ellagic acid protects endothelial cells from oxidized low-density lipoprotein-induced apoptosis by modulating the PI3K/Akt/eNOS pathway. Toxicol. Appl. Pharmacol. 2010, 248, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Denizot, F.; Wilson, A.; Battye, F.; Berke, G.; Shortman, K. Clonal expansion of T cells: A cytotoxic T-cell response in vivo that involves precursor cell proliferation. Proc. Natl. Acad. Sci. USA 1986, 83, 6089–6092. [Google Scholar] [CrossRef] [PubMed]
- Buege, A.J.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [PubMed]
- Halvorsen, B.; Waehre, T.; Scholz, H.; Clausen, O.P.; von der Thusen, J.H.; Muller, F.; Heimli, H.; Tonstad, S.; Hall, C.; Frøland, S.S.; et al. Interluukin-10 enhances the oxidized LDL-induced foam cell formation of macrophages by antiapoptotic mechanisms. J. Lipid Res. 2005, 46, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds PME3 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, M.-C.; Chang, W.-H.; Chen, C.-W.; Li, W.-W.; Tseng, C.-Y.; Song, T.-Y. Antioxidant Properties of Essential Oil Extracted from Pinus morrisonicola Hay Needles by Supercritical Fluid and Identification of Possible Active Compounds by GC/MS. Molecules 2015, 20, 19051-19065. https://doi.org/10.3390/molecules201019051
Cheng M-C, Chang W-H, Chen C-W, Li W-W, Tseng C-Y, Song T-Y. Antioxidant Properties of Essential Oil Extracted from Pinus morrisonicola Hay Needles by Supercritical Fluid and Identification of Possible Active Compounds by GC/MS. Molecules. 2015; 20(10):19051-19065. https://doi.org/10.3390/molecules201019051
Chicago/Turabian StyleCheng, Ming-Ching, Wen-Hua Chang, Chih-Wei Chen, Wen-Wing Li, Chin-Yin Tseng, and Tuzz-Ying Song. 2015. "Antioxidant Properties of Essential Oil Extracted from Pinus morrisonicola Hay Needles by Supercritical Fluid and Identification of Possible Active Compounds by GC/MS" Molecules 20, no. 10: 19051-19065. https://doi.org/10.3390/molecules201019051
APA StyleCheng, M.-C., Chang, W.-H., Chen, C.-W., Li, W.-W., Tseng, C.-Y., & Song, T.-Y. (2015). Antioxidant Properties of Essential Oil Extracted from Pinus morrisonicola Hay Needles by Supercritical Fluid and Identification of Possible Active Compounds by GC/MS. Molecules, 20(10), 19051-19065. https://doi.org/10.3390/molecules201019051






