Hinokitiol Exerts Anticancer Activity through Downregulation of MMPs 9/2 and Enhancement of Catalase and SOD Enzymes: In Vivo Augmentation of Lung Histoarchitecture
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Effects of Hinokitiol on MMP-2 and MMP-9 Expression
2.1.2. Effects of Hinokitiol on MMP-9 and MMP-2 Activities in B16-F10 Cells

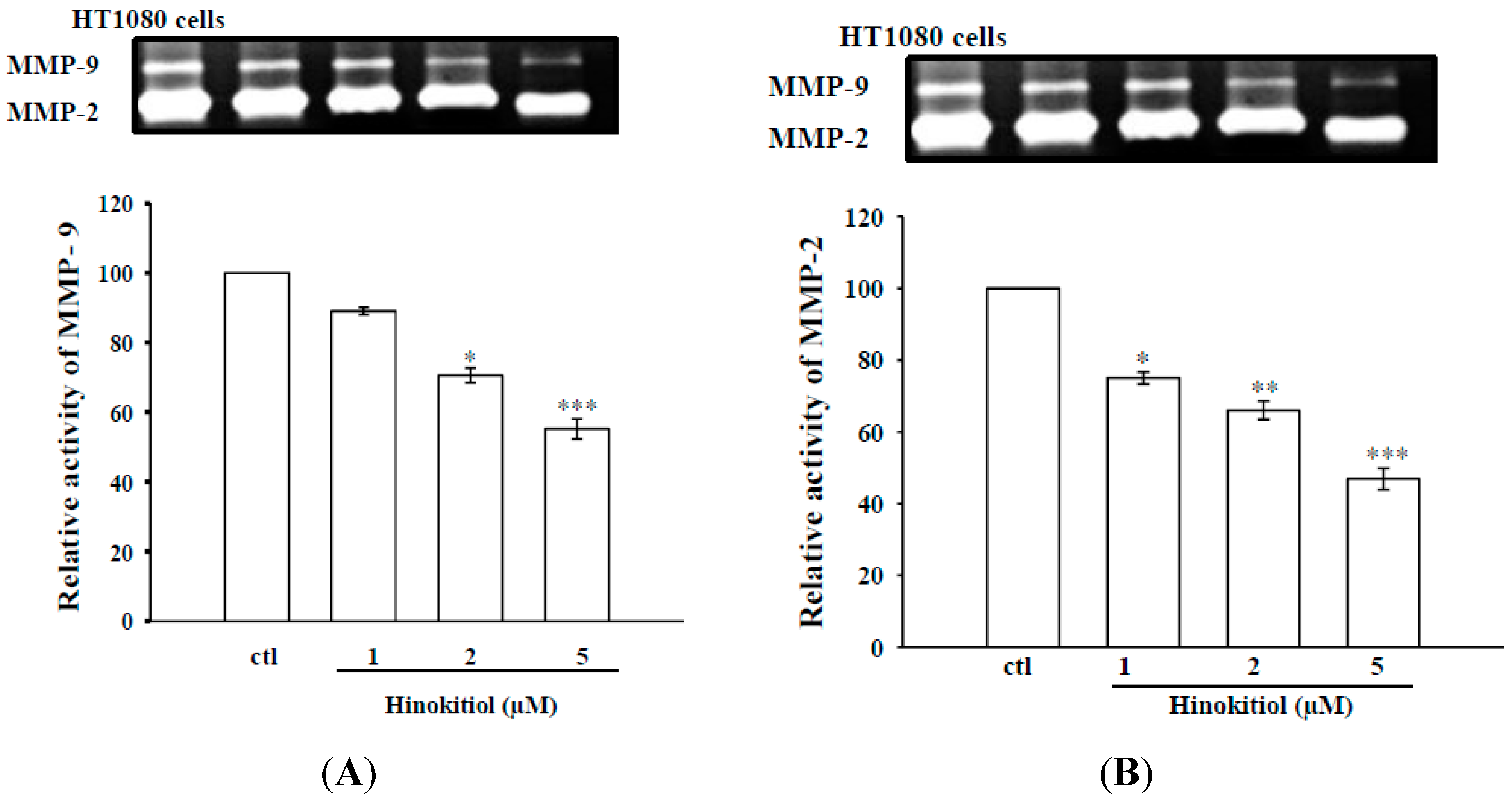
2.1.3. Effects of Hinokitiol on Enzymatic Antioxidants CAT and SOD
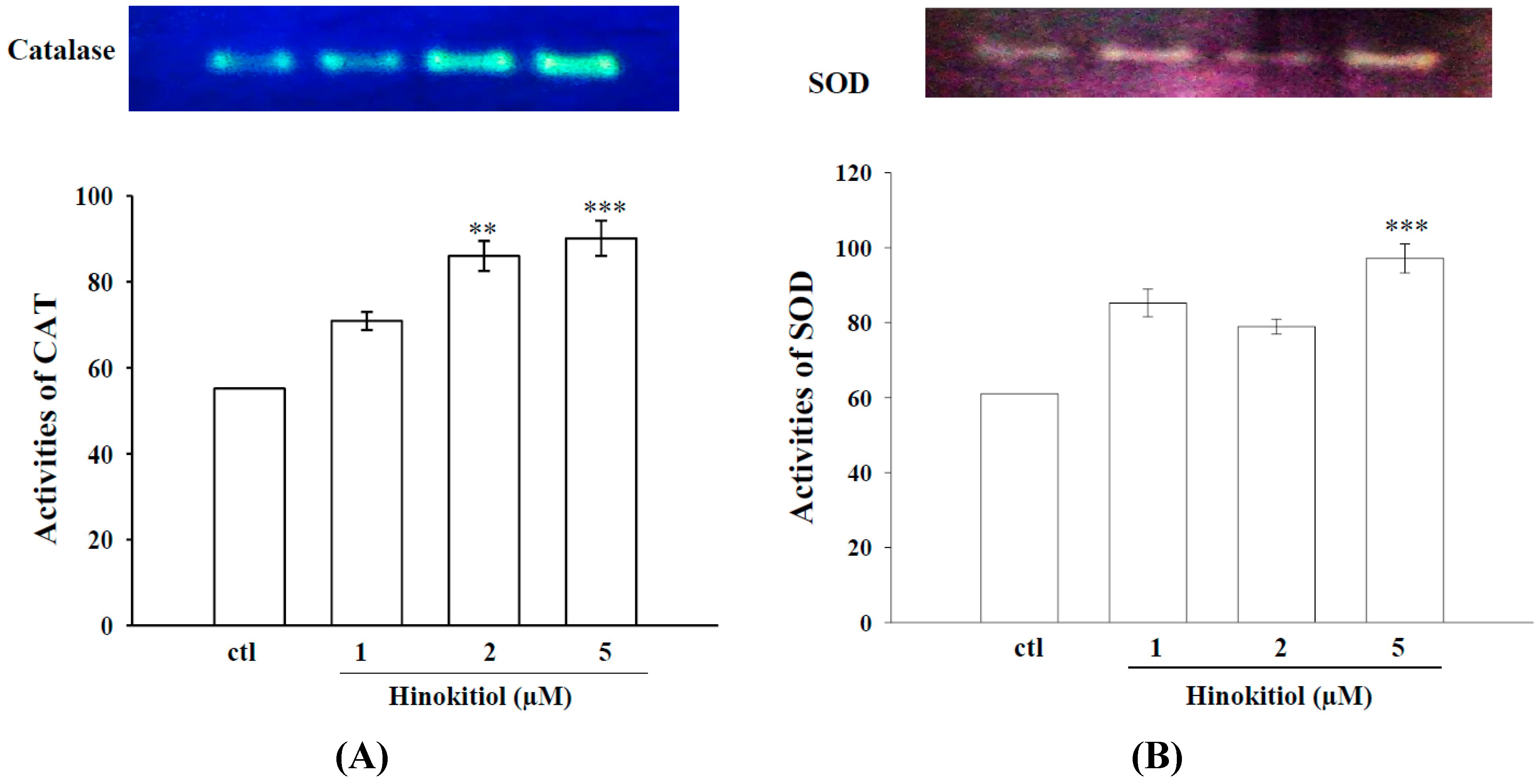
2.1.4. Hinokitiol Dampens OH· Production in Vitro

2.1.5. Histopathological Observation in the Lungs of Hinokitiol Treated Mice
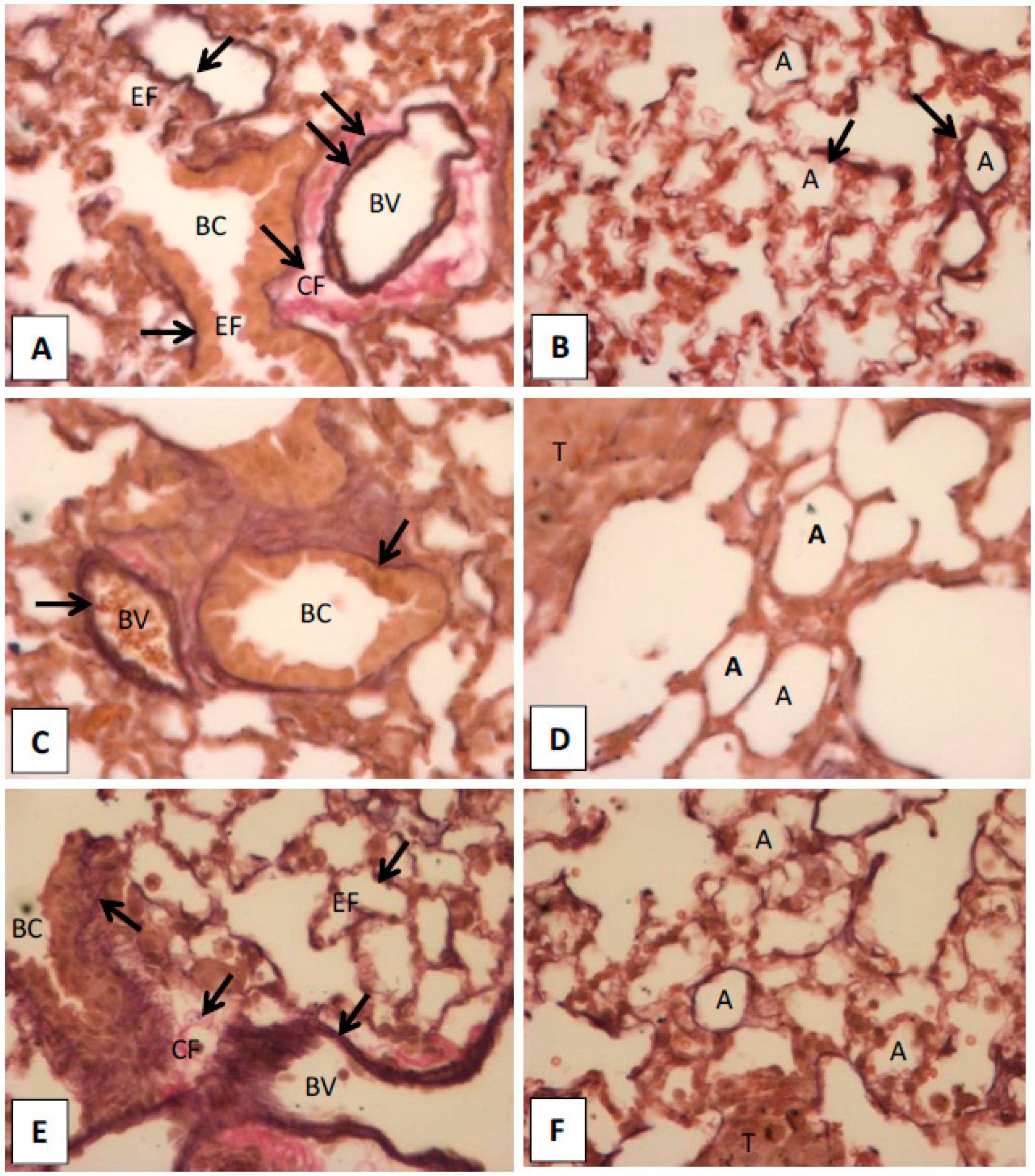
2.2. Discussion
3. Experimental Section
3.1. Materials
3.2. Cell Cultivation and Hinokitiol Administration
3.3. Western Blot Analysis of MMP-9 and MMP-2
3.4. Gelatin Zymographic Assay for MMP-9 and MMP-2
3.5. Non-Denaturing Polyacrylamide Gel Electrophoresis (Native-PAGE) for CAT and SOD Activity Assay
- (i)
- CAT activity was detected by the method of Woodbury et al. [43]. Here, the gel was soaked in 5 mM H2O2 solution for 10 min and then washed with water and stained with a reaction mixture containing 1% potassium ferricyanide (w/v) and 1% ferric chloride. The enzyme appeared as a yellow band superimposed on a dark green background. The reaction was terminated by adding water and the gel was photographed at once.
- (ii)
- SOD activity was identified by the method of Beauchamp and Fridovich [44]. The gel was soaked in 50 mM Tris-HCl buffer (pH 8) containing 10 mg nitroblue tetrazolium (NBT), 1 mg ethylene diamine tetra acetic acid (EDTA), and 2 mg riboflavin (50 mL final volume), and kept in the dark for 30 min. The gel was then placed on an illuminated light box to locate the area of SOD activity, which appeared as a clear zone on a bluish-violet background.
3.6. Detection of Hydroxyl Radical (HO·) Formation Using Electron Spin Resonance (ESR) Spectrometry
3.7. In Vivo Histological Analysis of Lungs to Detect the Elastic Fibers and Collagens
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Authors Contributions
Conflicts of Interest
References
- Kushiro, K.; Chu, R.A.; Verma, A.; Nunez, N.P. Adipocytes promote B16BL6 melanoma cell invasion and the epithelial-to-mesenchymal transition. Cancer Microenviron. 2012, 5, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Fidler, I.J. The pathogenesis of cancer metastasis: The “seed and soil” hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Curran, S.; Murray, G.I. Matrix metalloproteinases: Molecular aspects of their roles in tumour invasion and metastasis. Eur. J. Cancer 2000, 36, 1621–1630. [Google Scholar] [CrossRef]
- Kleiner, D.L.; Stetler-Stevenson, W.G. Matrix metalloproteinases and metastasis. Cancer Chemother. Pharmacol. 1999, 43, S42–S51. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.M.; Stetler-Stevenson, W.G. The role of matrix metalloproteases and their inhibitors in tumour invasion, metastasis and angiogenesis. Eur. Respir. J. 1994, 7, 2062–2072. [Google Scholar] [PubMed]
- Senoir, R.M.; Griffin, G.L.; Fliszar, C.J.; Shapiro, S.D.; Goldberg, G.I.; Welgus, H.G. Human 92- and 72-kilodalton type IV collagenases are elastases. J. Biol. Chem. 1991, 266, 7870–7875. [Google Scholar]
- Nishikawa, M.; Hyoudou, K.; Kobayashi, Y.; Umeyama, Y.; Takakura, Y.; Hashida, M. Inhibition of metastatic tumor growth by targeted delivery of antioxidant enzymes. J. Control. Release 2005, 109, 101–107. [Google Scholar] [CrossRef]
- Loganayaki, M.; Manian, S. Antitumor activity of the methanolic extract of Ammannia baccifera L. against Dalton’s ascites lymphoma induced ascetic and solid tumors in mice. J. Ethnopharmacol. 2012, 142, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, K.; Manoharan, S. Anticarcinogenic and antilipidperoxidative effects of Tephrosia purpurea (Linn) pers. In 7,12-dimethyl benz (a) anthracene (DMBA) induced hamster buccal pouch carcinoma. Ind. J. Pharmacol. 2006, 38, 185–189. [Google Scholar]
- Thornthwaite, J.T.; Shah, H.R.; Shah, P.; Peeples, W.C.; Respess, H. The formulation for cancer prevention & therapy. Adv. Biol. Chem. 2013, 3, 356–387. [Google Scholar]
- Garbisa, S.; Sartor, L.; Biggin, S.; Salvato, B.; Benelli, R.; Albini, A. Tumor gelatinases and invasion inhibited by the green tea flavanol epigallocatechin-3-gallate. Cancer 2001, 9, 822–832. [Google Scholar] [CrossRef]
- Bloomston, M.; Zervos, E.E.; Rosemurgy, A.S. Matrix metalloproteinases and their role in pancreatic cancer: A review of preclinical studies and clinical trials. Ann. Surg. Oncol. 2002, 9, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.H.; Hu, M.L. l-carnosine inhibits metastasis of SK-Hep-1 cells by inhibition of matrix metaoproteinase-9 expression and induction of an antimetastatic gene, nm23-H1. Nutr. Cancer 2008, 60, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.S.; Kim, Y.A.; Kim, M.M.; Park, J.S.; Kim, J.A.; Kim, S.K.; Lee, B.J.; Nam, T.J.; Seo, Y. Flavonoid glycosides isolated from Salicornia herbacea inhibit matrix metalloproteinase in HT1080 cells. Toxicol. In Vitro 2008, 22, 1742–1748. [Google Scholar] [CrossRef] [PubMed]
- Shabaruddin, F.H.; Chen, L.C.; Elliott, R.A.; Payne, K. A systematic review of utility values for chemotherapy-related adverse events. Pharmacoeconomics 2013, 31, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.K.; Fujita, K.; Sakai, K. Reactive oxygen species, nitric oxide, and their interactions play different roles in Cupressus lusitanica cell death and phytoalexin biosynthesis. New Phytol. 2007, 175, 215–229. [Google Scholar] [CrossRef] [PubMed]
- Saeki, Y.; Ito, Y.; Shibata, M.; Sato, Y.; Okuda, K.; Takazoe, I. Antimicrobial action of natural substances on oral bacteria. Bull. Tokyo Dent. Coll. 1989, 30, 129–135. [Google Scholar] [PubMed]
- Arima, Y.; Nakai, Y.; Hayakawa, R.; Nishino, T. Antibacterial effect of beta-thujaplicin on staphylococci isolated from atopic dermatitis: Relationship between changes in the number of viable bacterial cells and clinical improvement in an eczematous lesion of atopic dermatitis. J. Antimicrob. Chemother. 2003, 51, 113–122. [Google Scholar] [CrossRef]
- Koufaki, M.; Theodorou, E.; Alexi, X.; Nikoloudaki, F.; Alexis, M.N. Synthesis of tropolone derivatives and evaluation of their in vitro neuroprotective activity. Eur. J. Med. Chem. 2010, 45, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Lu, S.H.; Chang, C.C.; Thomas, P.A.; Jayakumar, T.; Sheu, J.R. Hinokitiol, a tropolone derivative, inhibits mouse melanoma (B16-F10) cell migration and in vivo tumor formation. Eur. J. Pharmacol. 2015, 746, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.S.; Wang, M.J.; Jayakumar, T.; Chou, D.S.; Ko, C.Y.; Hsu, M.J.; Hsieh, C.Y. Antiproliferative activity of hinokitiol, a tropolone derivative, is mediated via the inductions of p-JNK and p-PLCγ1 signaling in PDGF-BB-stimulated vascular smooth muscle cells. Molecules 2015, 20, 8198–8212. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, R.J.; Guise, T.A. Molecular mechanisms of bone metastasis and therapeutic implications. Clin. Orthop. Relat. Res. 2003, 415, S100–S104. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.; Du, J.; Liu, J.; Ritchie, J.M.; Oberley, L.W.; Cullen, J.J. Metastatic progression of pancreatic cancer: Changes in antioxidant enzymes and cell growth. Clin. Exp. Metastasis 2005, 22, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fang, P.; Mai, J.; Choi, E.T.; Wang, H.; Yang, X.F. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Mierau, G.W.; Goin, L. Perivascular elastic fibers: A diagnostic feature of ependymoma. Ultrastruct. Pathol. 2007, 31, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Kamino, H.; Tam, S.; Roses, D.; Toussaint, S. Elastic fiber pattern in regressing melanoma: A histochemical and immunohistochemical study. J. Cutan. Pathol. 2010, 37, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Farris, S.D.; Hu, J.H.; Krishnan, R.; Emery, I.; Chu, T.; Du, L.; Kremen, M.; Dichek, H.L.; Gold, E.; Ramsey, S.A.; et al. Mechanisms of urokinase plasminogen activator (uPA)-mediated atherosclerosis: Role of the uPA receptor and S100A8/A9 proteins. J. Biol. Chem. 2011, 286, 22665–22677. [Google Scholar] [CrossRef] [PubMed]
- Chiodoni, C.; Colombo, M.P.; Sangaletti, S. Matricellular proteins: From homeostasis to inflammation, cancer, and metastasis. Cancer Metastasis Rev. 2010, 29, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Pires, I.; Gomes, J.; Prada, J.; Pereira, D.; Queiroga, F.L. MMP-2 and MMP-9 expression in canine cutaneous melanocytic tumours: Evidence of a relationship with tumoural malignancy. Melanoma—From Early Detection to Treatment/Book 2. Intech 2013, 1–29. [Google Scholar] [CrossRef]
- Lai, W.W.; Hsu, S.C.; Chueh, F.S.; Chen, Y.Y.; Yang, J.S.; Lin, J.P.; Lien, J.C.; Tsai, C.H.; Chung, J.G. Quercetin inhibits migration and invasion of SAS human oral cancer cells through inhibition of NF-κB and matrix metalloproteinase-2/-9 signaling pathways. Anticancer Res. 2013, 33, 1941–1950. [Google Scholar] [PubMed]
- Chandrashekar, N.; Selvamani, A.; Subramanian, R.; Pandi, A.; Thiruvengadam, D. Baicalein inhibits pulmonary carcinogenesis-associated inflammation and interferes with COX-2, MMP-2 and MMP-9 expressions in vivo. Toxicol. Appl. Pharmacol. 2012, 261, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.; Venkatesan, B.; Tumala, A.; Vellaichamy, E. Topical application of Gallic acid suppresses the 7,12-DMBA/Croton oil induced two-step skin carcinogenesis by modulating anti-oxidants and MMP-2/MMP-9 in Swiss albino mice. Food Chem. Toxicol. 2014, 66, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.K.; Ranganathan, A.C.; Mansouri, J.; Rodriguez, A.M.; Providence, K.M.; Rutter, J.L.; Pumiglia, K.; Bennett, J.A.; Melendez, J.A. Elevated Sod2 activity augments matrix metalloproteinase expression evidence for the involvement of endogenous hydrogen peroxide in regulating metastasis. Clin. Cancer Res. 2003, 9, 424–432. [Google Scholar] [PubMed]
- Zhang, H.J.; Zhao, W.; Venkataraman, S.; Robbins, M.E.; Buettner, G.R.; Kregel, K.C.; Oberley, L.W. Activation of matrix metalloproteinase-2 by overexpression of manganese superoxide dismutase in human breast cancer MCF-7 cells involves reactive oxygen species. J. Biol. Chem. 2002, 277, 20919–20926. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M.; Hashida, M. Inhibition of tumour metastasis by targeted delivery of antioxidant enzymes. Expert. Opin. Drug. Deliv. 2006, 3, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Onoda, J.M.; Piechocki, M.P.; Honn, K.V. Radiation-induced increase in expression of the alpha IIb beta 3 integrin in melanoma cells: Effects on metastatic potential. Radiat. Res. 1992, 130, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar] [PubMed]
- Gismondi, A.; Canuti, L.; Impei, S.; di Marco, G.; Kenzo, M.; Colizzi, V.; Canini, A. Antioxidant extracts of African medicinal plants induce cell cycle arrest and differentiation in B16F10 melanoma cells. Int. J. Oncol. 2013, 43, 956–964. [Google Scholar] [PubMed]
- Gismondi, A.; Canuti, L.; Grispo, M.; Canini, A. Biochemical composition and antioxidant properties of Lavandula angustifolia Miller essential oil are shielded by propolis against UV radiations. Photochem. Photobiol. 2014, 90, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Kazama, S.; Watanabe, T.; Ajioka, Y. Tumour budding at the deepest invasive margin correlates with lymph node metastasis in submucosal colorectal cancer detected by anticytokeratin antibody CAM5.2. Br. J. Cancer 2006, 94, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Kamino, H.; Toussaint, S.; Tam, S.T.; Tapia, B. Distinctive patterns of elastic fibers in melanocytic nevi and malignant melanoma. A histochemical and immunohistochemical study. Lab. Investig. 2000, 3, 63A. [Google Scholar]
- Lu, Y.C.; Jayakumar, T.; Duann, Y.F.; Chou, Y.C.; Hsieh, C.Y.; Yu, S.Y.; Sheu, J.R.; Hsiao, G. Chondroprotective role of sesamol by inhibiting MMPs expression via retaining NF-κB signaling in activated SW1353 cells. J. Agric. Food Chem. 2011, 59, 4969–4978. [Google Scholar] [CrossRef] [PubMed]
- Woodbury, W.; Speacer, A.K.; Stahman, M.A. An improved procedure using ferricyanide for detecting catalase isozymes. Anal. Biochem. 1971, 44, 301–305. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Chou, D.D.; Hsiao, G.; Shen, M.Y.; Tsai, Y.J.; Chen, T.F.; Sheu, J.R. ESR spin trapping of a carbon-centered free radical from agonist-stimulated human platelets. Free Radic. Biol. Med. 2005, 39, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Gopal, K.; Kishor, K.R.; Nandini, R.; Jahan, P.; Kumar, M.J. High fat diet containing cholesterol induces aortic aneurysm through recruitment and proliferation of circulating agranulocytes in apoE knockout mice model. J. Thromb. Thrombolysis 2010, 30, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-H.; Jayakumar, T.; Chang, C.-C.; Fong, T.-H.; Lu, S.-H.; Thomas, P.A.; Choy, C.-S.; Sheu, J.-R. Hinokitiol Exerts Anticancer Activity through Downregulation of MMPs 9/2 and Enhancement of Catalase and SOD Enzymes: In Vivo Augmentation of Lung Histoarchitecture. Molecules 2015, 20, 17720-17734. https://doi.org/10.3390/molecules201017720
Huang C-H, Jayakumar T, Chang C-C, Fong T-H, Lu S-H, Thomas PA, Choy C-S, Sheu J-R. Hinokitiol Exerts Anticancer Activity through Downregulation of MMPs 9/2 and Enhancement of Catalase and SOD Enzymes: In Vivo Augmentation of Lung Histoarchitecture. Molecules. 2015; 20(10):17720-17734. https://doi.org/10.3390/molecules201017720
Chicago/Turabian StyleHuang, Chien-Hsun, Thanasekaran Jayakumar, Chao-Chien Chang, Tsorng-Harn Fong, Shing-Hwa Lu, Philip Aloysius Thomas, Cheuk-Sing Choy, and Joen-Rong Sheu. 2015. "Hinokitiol Exerts Anticancer Activity through Downregulation of MMPs 9/2 and Enhancement of Catalase and SOD Enzymes: In Vivo Augmentation of Lung Histoarchitecture" Molecules 20, no. 10: 17720-17734. https://doi.org/10.3390/molecules201017720
APA StyleHuang, C.-H., Jayakumar, T., Chang, C.-C., Fong, T.-H., Lu, S.-H., Thomas, P. A., Choy, C.-S., & Sheu, J.-R. (2015). Hinokitiol Exerts Anticancer Activity through Downregulation of MMPs 9/2 and Enhancement of Catalase and SOD Enzymes: In Vivo Augmentation of Lung Histoarchitecture. Molecules, 20(10), 17720-17734. https://doi.org/10.3390/molecules201017720






