Characterization of Ambrette Seed Oil and Its Mode of Action in Bacteria
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Bioactive Compounds
| Peak No | Components | Class of Compound | Retention Time | SI | Area % |
|---|---|---|---|---|---|
| 1 | Butanoicacid,octyl ester | Lipid | 26.37 | 816 | 0.24 |
| 2 | Acetic acid, decyl ester | Lipid | 27.04 | 961 | 6.53 |
| 3 | Ethanone, 1,1'-(1,3-phenylene)bis- | Aromatic diketone | 27.71 | 838 | 0.18 |
| 4 | Butanoic acid, 2-methyl-, octyl ester | Lipid | 38.68 | 758 | 0.15 |
| 5 | 5,9-Undecadien-2-one, 6,10-dimethyl- | Lipid | 28.37 | 891 | 0.29 |
| 6 | Cis-a-Farnesene | Sesquiterpene | 28.51 | 857 | 0.36 |
| 7 | Aromandendrene | Sesquiterpene | 28.72 | 833 | 0.10 |
| 8 | 1-Dodecanol | Lipid | 29.00 | 856 | 0.23 |
| 9 | Phenol, 2,5-bis(1-methylethyl)- | Phenol | 29.07 | 724 | 0.12 |
| 10 | Bicyclo[3.1.1]hept-2-ene, 2,6-dimethyl-6(4-methyl-3-pentenyl)- | Sesquiterpene | 29.68 | 828 | 0.17 |
| 11 | E-10-Dodecen-1-ol propionate | Lipid | 29.86 | 779 | 0.34 |
| 12 | a-Farnesene | Sesquiterpene | 30.09 | 881 | 0.86 |
| 13 | Phenol, 3,5-bis(1-methylethyl)- | Phenol | 30.19 | 824 | 0.09 |
| 14 | 1,6,10-Dodecatrien-1-ol,3,7,11-trimethyl-, (E)- | Sesquiterpene | 31.72 | 906 | 2.66 |
| 15 | 5-Dodecen-1-ol, acetate, (Z)- | Lipid | 32.45 | 931 | 2.09 |
| 16 | Lauryl acetate | Lipid | 32.02 | 913 | 7.80 |
| 17 | (Z)6-Pentadecen-1-ol | Lipid | 35.37 | 840 | 0.22 |
| 18 | 9,12-Octadecadienoyl chloride, (Z,Z)- | Lipid (acid chloride) | 35.53 | 771 | 0.42 |
| 19 | Nerolidyl acetate | Sesquiterpene | 35.94 | 812 | 0.30 |
| 20 | 17-Octadecynoic acid | Lipid | 36.79 | 792 | 0.16 |
| 21 | 9,12-Tetradecadien-1-ol, acetate, (Z,E) | Lipid | 37.58 | 848 | 0.67 |
| 22 | 5-Tetradecen-1-ol, acetate (Z)- | Lipid | 37.80 | 942 | 3.74 |
| 23 | 8-Hexadecenal, 14-methyl-(Z)- | Lipid | 38.40 | 783 | 0.53 |
| 24 | Pentacyclo [9.1.0(2,4).0.(5,7).8(8,10)]dodecane | Lipid (Cyclic) | 38.75 | 797 | 0.86 |
| 25 | Farnesol acetate | Sesquiterpene | 39.37 | 947 | 51.45 |
| 26 | a-Guaiene | Sesquiterpene | 39.55 | 759 | 1.16 |
| 27 | 1,3,6,10-Cyclotetradecatetraene, 3,7,11-trimethyl-14-(1-methylethyl) | Sesquiterpene | 40.97 | 833 | 0.31 |
| 28 | Cyclopropanebutanoic acid | Lipid | 41.30 | 780 | 0.11 |
| 29 | Propanoic acid, 2,2-dimethyl | Lipid | 41.43 | 842 | 1.45 |
| 30 | 9-Hexadecenoic acid | Lipid | 41.55 | 817 | 0.10 |
| 31 | Ambrettolide | Lactone | 41.78 | 848 | 12.96 |
| 32 | Caryophyllene oxide | Oxygenated Sesquiterpene | 42.11 | 824 | 0.37 |
| 33 | 5,8,11,14-Eicosatetraenoic acid, methyl ester | Lipid | 43.30 | 807 | 0.18 |
| 34 | Nerolidyl acetate | Sesquiterpene | 44.44 | 816 | 0.56 |
| 35 | 9,12-Octadecadienoic acid | Lipid | 46.48 | 841 | 2.03 |
2.2. Antibacterial Assay of Ambrette Seed Oil
| Bacterial Strain | Zone of Inhibition (mm) | |
|---|---|---|
| Ambrette Seed Oil (18 mg/mL) | Streptomycin (30 µg/mL) | |
| B. subtilis | 12 ± 0.5 | 28 ± 1.2 |
| S. aureus | 13 ± 0.5 | 20 ± 1.4 |
| E. faecalis | 13 ± 0.8 | 15 ± 1.6 |
| P. aeruginosa | 09 ± 0.7 | 15 ± 1.5 |
2.3. ALP Quantification Assay
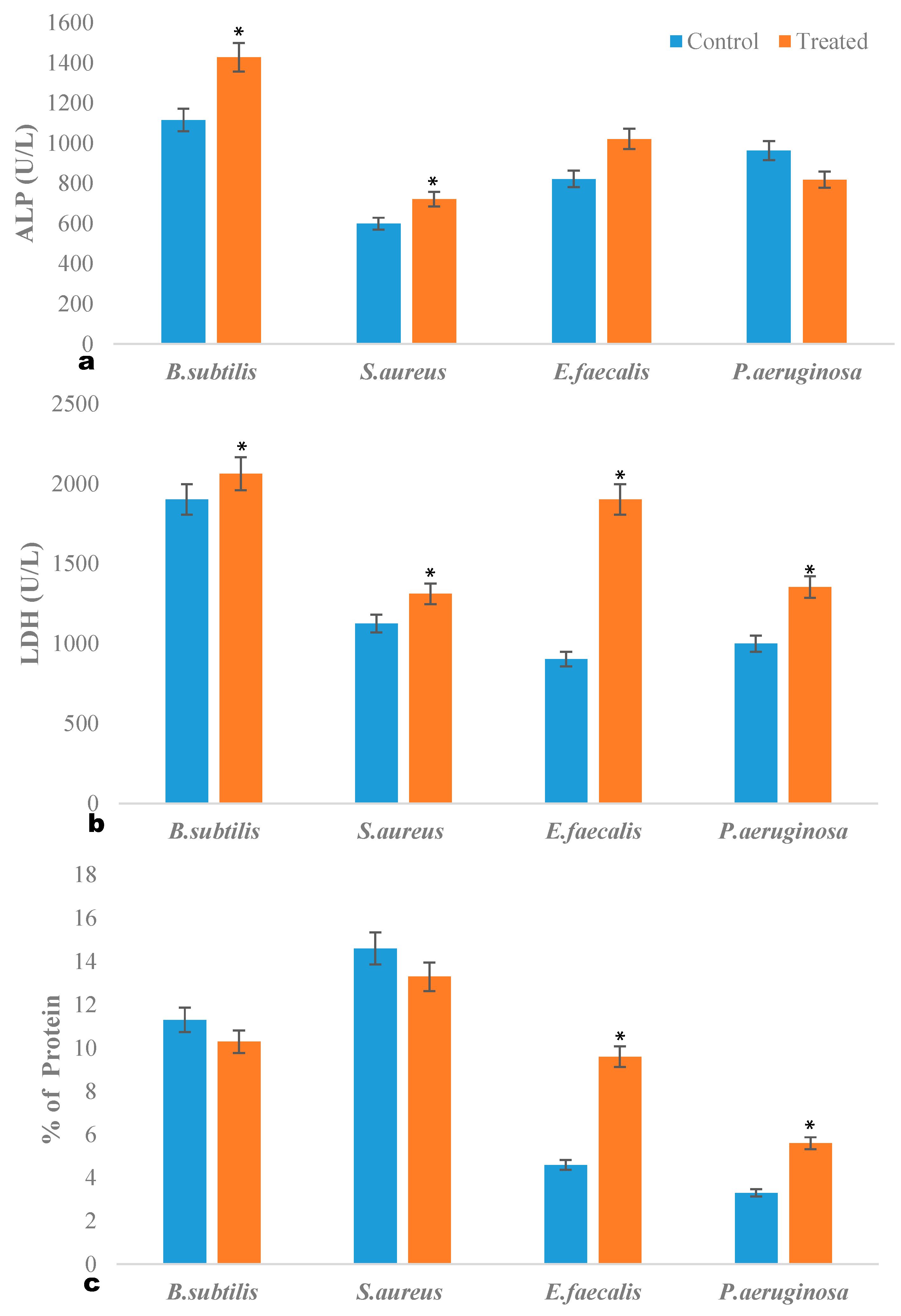
2.4. LDH Quantification Assay
2.5. Intracellular Protein Leakage
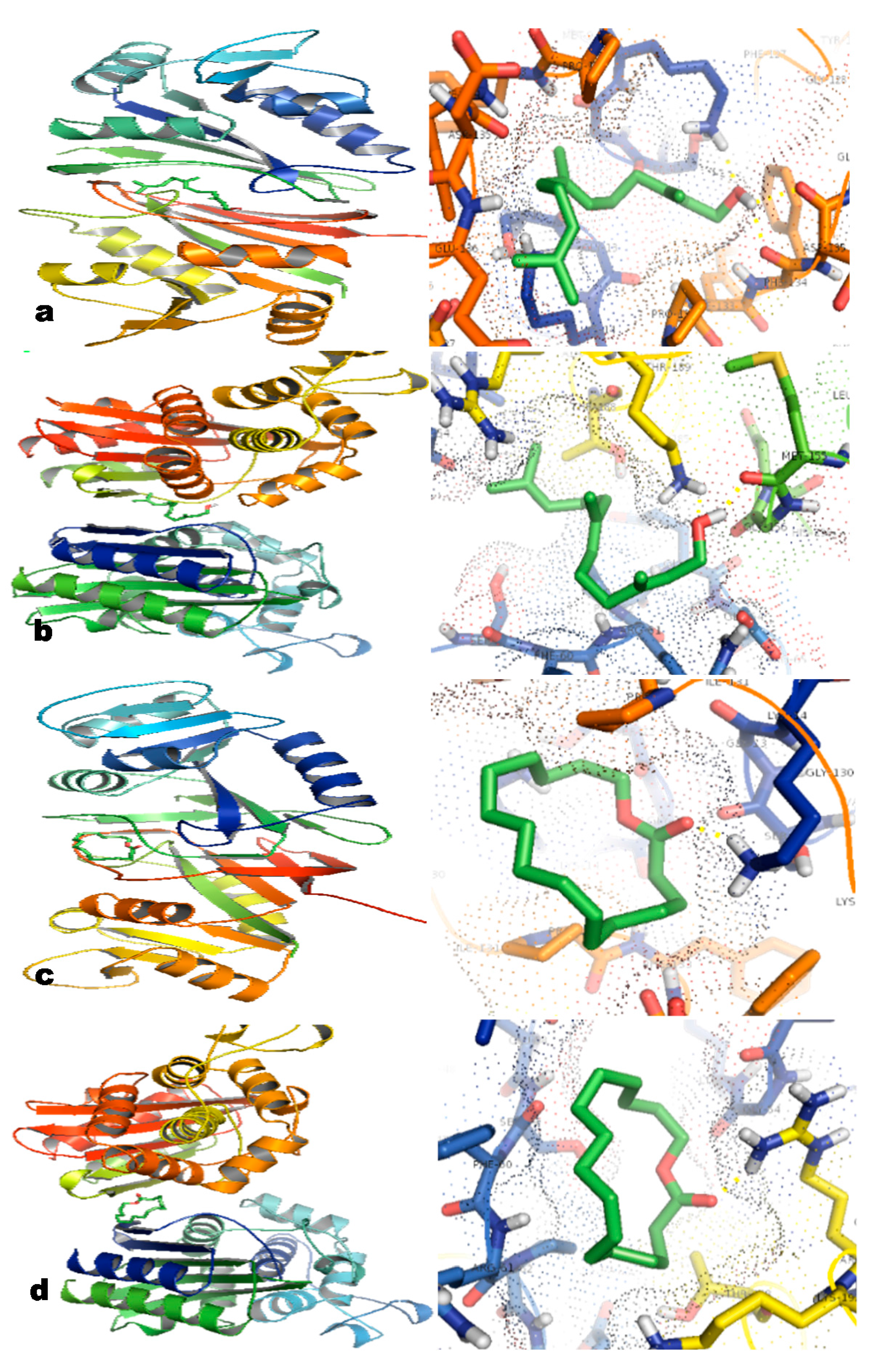
2.6. In Silico Studies
3. Experimental Section
3.1. GC-MS Analysis
3.2. Microorganisms
3.3. Antibacterial Assay
3.4. ALP Quantification (Alkaline Phosphatase)
3.5. Lactate Dehydrogenase Quantification (LDH)
3.6. Protein Leakage
3.7. Ligand and Target Protein Preparation
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Centers for Disease Control and Prevention (CDC). Antibiotic resistance threats in the United States. Available online: http://www.cdc.gov/features/antibioticresistancethreats/ (accessed on 16 September 2013).
- Livermoore, D.M. Multiple mechanisms of antimicrobial resistance P. aeruginosa, our worst nightmare. Clin. Infect. Dis. 2002, 34, 634–640. [Google Scholar] [CrossRef]
- Wimmerstedt, A.; Kahlmeler, G. Associated antimicrobial resistance in Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pneumonia and streptococcus pyogenes. Clin. Microbiol. Infect. 2008, 14, 315–321. [Google Scholar] [CrossRef]
- Ramzi, A.M.; Mansour, S.A.; Mohammed, A.A.; Adnan, J.A.; Jamal, M.K. GC and GC/MS analysis of essential oil composition of the endemic Soqotraen Leucas virgat Balf.f. and its antimicrobial and antioxidant activities. Int. J. Mol. Sci. 2013, 14, 23129–23139. [Google Scholar]
- Pereira, V.; Dias, C.; Vasconcelos, M.C.; Rosa, E.; Saavedra, M.J. Antibacterial activity and synergistic effects between Eucalyptus globulus leaf residues (Essential oils and extracts) and antibiotics against several isolates of respiratory tract infections (Pseudomonas aeruginosa). Ind. Crops Prod. 2014, 52, 1–7. [Google Scholar] [CrossRef]
- Xiong, L.; Peng, C.; Zhou, Q.M.; Wan, F.; Xie, X.F.; Guo, L.; Li, X.H.; He, C.J.; Dai, O. Chemical composition and antibacterial activity of essential oils from different parts of Leonurus japonicus Houtt. Molecules 2013, 18, 963–973. [Google Scholar] [CrossRef]
- Djabou, N.; Lorenzi, V.; Guinoiseau, E.; Andreani, S.; Giuliani, M.-C.; Desjobert, J.-M.; Bolla, J.-M.; Costa, J.; BertiL, L.; Luciani, A. Phytochemical composition of Teucrium essential oils and antibacterial activity against foodborne or toxi-infectious pathogens. Food Cont. 2013, 30, 354–363. [Google Scholar]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol. 1999, 86, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Bown, D. Encyclopedia of Herbs and Their Uses; Dorling Kindersley: London, UK, 1984. [Google Scholar]
- Gul, M.Z.; Bhakshu, L.M.; Ahmad, F.; Kondapi, A.K.; Qureshi, I.A.; Ghazi, I.A. Evaluation of Abelmoschus moschatus extracts for antioxidant, free radical scavenging, antimicrobial and antiproliferative activities using in vitro assays. BMC Complement. Altern. Med. 2011, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, P.; Kumar, A. Antimicrobial activity of Abelmoschus moschatus leaf extracts. Curr. Trends Biotechnol. Pharm. 2009, 3, 260–266. [Google Scholar]
- Cravo, L.; Perineau, F.; Gaset, A.; Bessiere, J.M. Study of the chemical composition ofthe essential oil, oleoresin and its volatile product obtained from Ambrette (Abelmoschus moschatus Moench) seeds. Flavour Fragr. J. 1992, 7, 65–67. [Google Scholar] [CrossRef]
- Molfetta, I.; Ceccarini, L.; Macchia, M.; Flamini, G.; Cioni, P.L. Abelmoschus esculentus (L.) Moench. and Abelmoschus moschatus Medik: Seeds production and analysis of the volatile compounds. Food Chem. 2013, 141, 34–40. [Google Scholar]
- Nautiyal, O.H.; Tiwari, K.K. Extraction of ambrette seed oil and isolation of ambrettolide with its characterization by 1H NMR. J. Nat. Prod. 2011, 4, 75–80. [Google Scholar]
- Jabra-Rizk, M.A.; Meiller, T.F.; James, C.E.; Shirtliff, M.E. Effect of Farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob. Agents Chemother. 2006, 50, 1463–1469. [Google Scholar] [CrossRef] [PubMed]
- Rhayour, K.; Bouchikhi, T.; Tantaoui-Elaraki, A.; Sendice, K.; Remmal, A. The mechanism of bactericidal action of oregano and clove essential oils and of their phenolic major components on Escherichia coli and Bacillus subtilis. J. Essent. Oil Res. 2003, 15, 286–292. [Google Scholar] [CrossRef]
- Lambert, R.J.; Skandamis, P.N.; Coote, P.J.; Nychas, G.J. A study of the minimum inhibitory concentration and mode of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001, 91, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, M.; Ammerman, J.W. The alkaline phosphatase PhoX is more widely distributed in marine bacteria than the classical PhoA. ISME J. 2009, 3, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Cabiscol, E.; Tamarit, J.; Ros, J. Oxidative stress in bacteria and protein damage by reactive oxygen species. Int. Microbiol. 2000, 3, 3–8. [Google Scholar] [PubMed]
- Chesnut, R.S.; Bookstein, C.; Hulett, F.M. Separate promoters direct expression of phoAIII, a member of the Bacillus subtilis alkaline phosphatase multigene family, during phosphate starvation and sporulation. Mol. Microbiol. 1991, 5, 2181–2190. [Google Scholar] [CrossRef] [PubMed]
- Tsai, G.J.; Su, W.H. Antibacterial activity of shrimp chitosan against Escherichia coli. J. Food Prot. 1999, 62, 239–243. [Google Scholar] [PubMed]
- Henie, E.F.P.; Zaiton, H.; Suhaila, M. Bacterial membrane disruption in food pathogens by Psidium guajava leaf extracts. Int. Food Res. J. 2009, 16, 297–311. [Google Scholar]
- Randhawa, V.; Jamwal, R. Molecular modeling and virtual screening studies of NDM-1 beta lactamase for identification of a series of potent inhibitors. Int. Res. J. Biochem. Bioinform. 2011, 1, 95–102. [Google Scholar]
- Irobi, O.N.; Young, M.M.; Daramola, S.O. Antimicrobial activity of Annatto (Bixa orellana) extract. Pharm. Biol. 1996, 34, 87–90. [Google Scholar] [CrossRef]
- Hood, J.R.; Wilkinson, J.M.; Cavanagh, H.M.A. Evaluation of common bacterial screening methods utilised in essential oil research. J. Essent. Oil Res. 2003, 15, 428–433. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.R. Extended-spectrum and inhibitor-resistant tem type β-lactamases: Mutation, speficity and three-dimentional structure. Antimicrob. Agents Chemother. 1995, 39, 2593–2601. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, X.; Yang, S.-P.; Liu, W.-Q. Evaluation of the antibacterial activity of patchouli oil. Iran. J. Pharm. Res. 2013, 12, 307–316. [Google Scholar] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arokiyaraj, S.; Choi, S.H.; Lee, Y.; Bharanidharan, R.; Hairul-Islam, V.I.; Vijayakumar, B.; Oh, Y.K.; Dinesh-Kumar, V.; Vincent, S.; Kim, K.H. Characterization of Ambrette Seed Oil and Its Mode of Action in Bacteria. Molecules 2015, 20, 384-395. https://doi.org/10.3390/molecules20010384
Arokiyaraj S, Choi SH, Lee Y, Bharanidharan R, Hairul-Islam VI, Vijayakumar B, Oh YK, Dinesh-Kumar V, Vincent S, Kim KH. Characterization of Ambrette Seed Oil and Its Mode of Action in Bacteria. Molecules. 2015; 20(1):384-395. https://doi.org/10.3390/molecules20010384
Chicago/Turabian StyleArokiyaraj, Selvaraj, Seong Ho Choi, Yoonseok Lee, Rajaraman Bharanidharan, Villianur Ibrahim Hairul-Islam, Badathala Vijayakumar, Young Kyoon Oh, Vannam Dinesh-Kumar, Savariar Vincent, and Kyoung Hoon Kim. 2015. "Characterization of Ambrette Seed Oil and Its Mode of Action in Bacteria" Molecules 20, no. 1: 384-395. https://doi.org/10.3390/molecules20010384
APA StyleArokiyaraj, S., Choi, S. H., Lee, Y., Bharanidharan, R., Hairul-Islam, V. I., Vijayakumar, B., Oh, Y. K., Dinesh-Kumar, V., Vincent, S., & Kim, K. H. (2015). Characterization of Ambrette Seed Oil and Its Mode of Action in Bacteria. Molecules, 20(1), 384-395. https://doi.org/10.3390/molecules20010384







