A New Cycloartane-Type Triterpenoid Saponin Xanthine Oxidase Inhibitor from Homonoia riparia Lour
Abstract
:1. Introduction
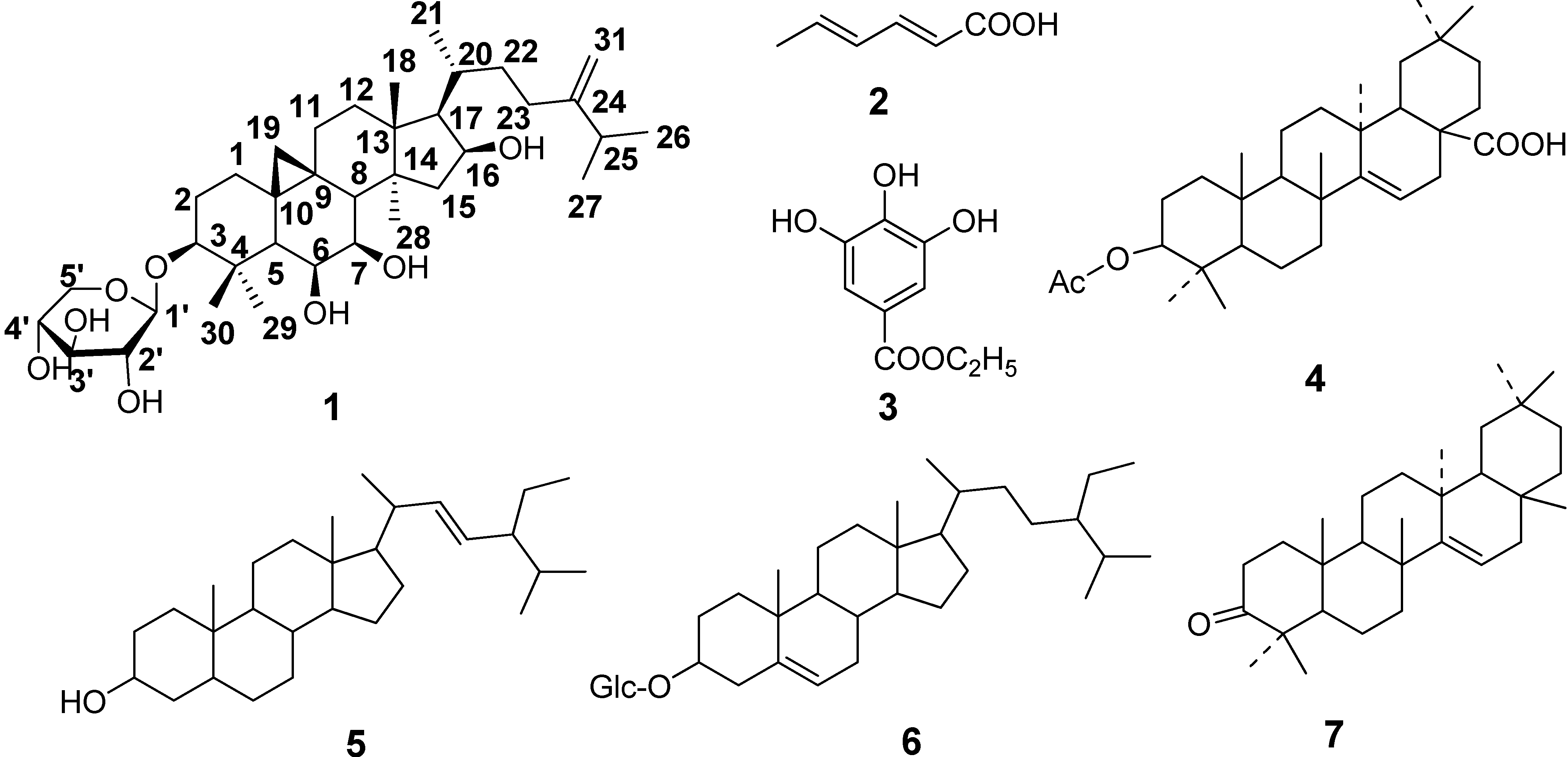
2. Results and Discussion
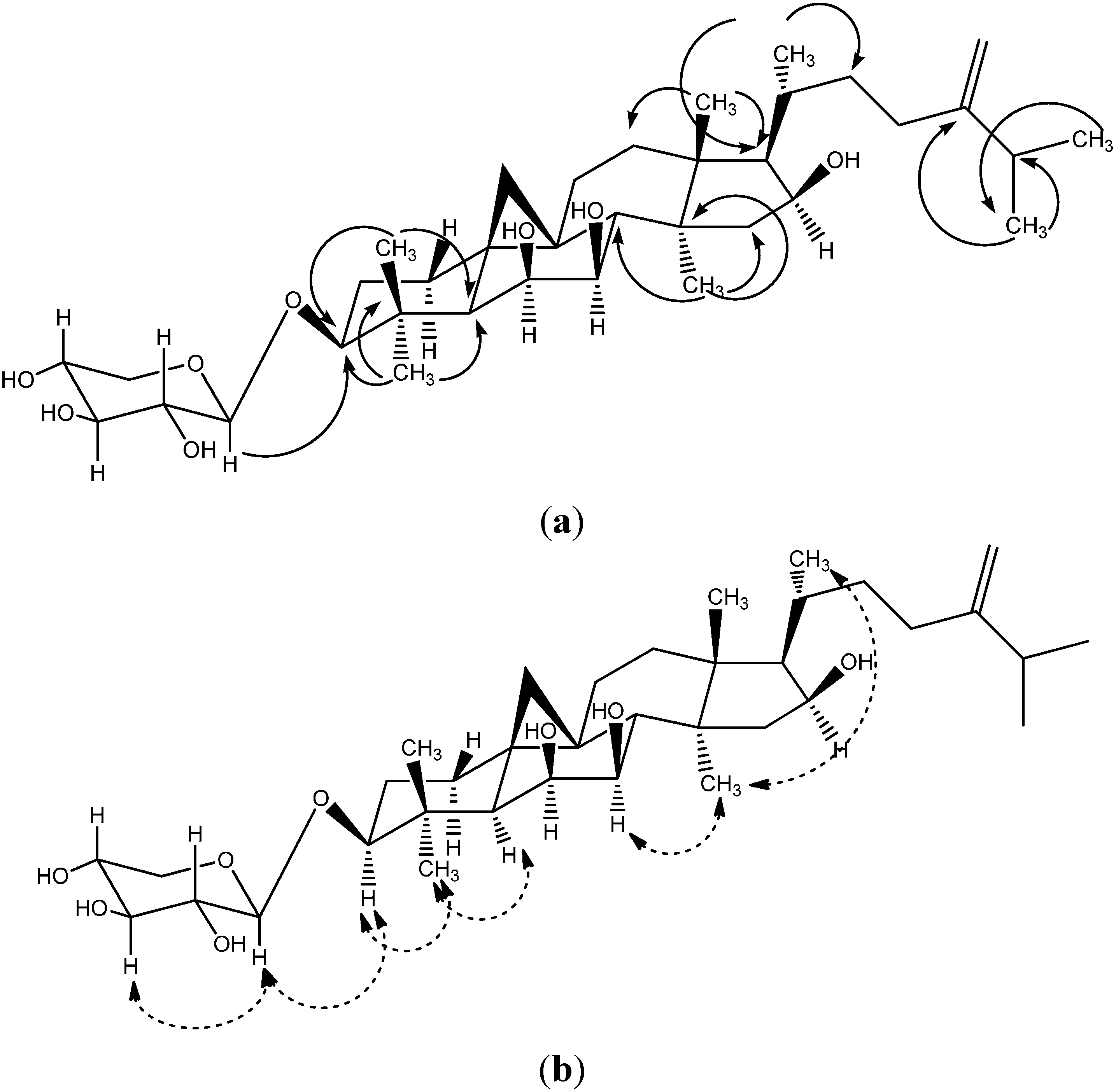
2.1. Identification of the Riparsaponin

| δC | δH | δC | δH | ||
|---|---|---|---|---|---|
| 1 | 32.62 t | 1.53 (2H, m) | 19 | 31.41 t | 0.99 (1H, m) |
| 0.30 (1H, s) | |||||
| 2 | 29.16 t | 1.72 (1H, m) | 20 | 29.77 d | 1.77 (1H, m) |
| 1.53 (1H, m) | |||||
| 3 | 87.52 d | 3.00 (1H, m) | 21 | 17.92 q | 0.87 (3H, d, J = 8.6 Hz) |
| 4 | 40.82 s | 22 | 34.54 t | 1.80 (1H, m) | |
| 1.08 (1H, m) | |||||
| 5 | 49.02 d | 1.22 (1H, s) | 23 | 31.35 t | 2.07 (1H, m) |
| 1.88 (1H, m) | |||||
| 6 | 71.38 d | 3.74 (1H, brs) | 24 | 156.22 s | |
| 7 | 72.74 d | 3.21 (1H, m) | 25 | 33.15 d | 2.22 (1H, m) |
| 8 | 45.90 d | 1.88 (1H, d, J = 11.2 Hz) | 26 | 21.76 q | 0.96 (3H, s) |
| 9 | 24.04 s | 27 | 21.81 q | 0.98 (3H, s) | |
| 10 | 18.72 s | 28 | 19.43 q | 0.88 (3H, s) | |
| 11 | 25.43 t | 1.91 (1H, m) | 29 | 24.07 q | 1.05 (3H, s) |
| 0.92 (1H, m) | |||||
| 12 | 32.27 t | 1.36 (1H, m) | 30 | 16.40 q | 1.08 (3H, s) |
| 1.08 (1H, m) | |||||
| 13 | 45.64 s | 31 | 106.13 t | 4.65 (2H, brs) | |
| 14 | 45.41 s | 1' | 105.90 d | 4.09 (1H, d, J = 7.5 Hz) | |
| 15 | 51.66 t | 2.16 (1H, m) | 2' | 73.82 d | 2.95 (1H, m) |
| 1.41 (1H, m) | |||||
| 16 | 70.72 d | 4.14 (1H, m) | 3' | 76.79 d | 3.04 (1H, m) |
| 17 | 55.44 d | 1.45 (1H, m) | 4' | 69.66 d | 3.24 (1H, m) |
| 18 | 19.39 q | 1.12 (3H, s) | 5' | 65.62 t | 3.61(1H, m) |
| 2.97 (1H, m) |
| Identification code | 070516a |
| Empirical formula | C36H57O8 |
| Formula weight | 617.82 |
| Temperature | 298 (2) K |
| Wave length | 0.71073 A |
| Crystal system, space group | Orthorhombic, P2(1)2(1)2(1) |
| Unit cell dimensions | a = 6.3405(9)Aalpha = 90 deg. |
| b = 12.7265(17)Abeta = 90 deg. | |
| c = 41.573(6)Agamma = 90 deg. | |
| Volume | 3354.6(8) A3 |
| Z, Calculated density | 4, 1.223 Mg/m3 |
| Absorption coefficient | 0.085 mm−1 |
| F (000) | 1348 |
| Crystalsize | 0.26 × 0.22 × 0.08 mm |
| The tarange for data collection | 1.67 to 28.31 deg. |
| Limiting indices | −8 ≤ h ≤ 8,−16 ≤ k ≤ 16,−55 ≤ l ≤ 53 |
| Reflections collected/unique | 29112/8026[R(int) = 0.0893] |
| Completeness to theta = 28.31 | 98.9% |
| Absorption correction | MUTI-SCAN |
| Max. andmin. transmission | 1.000000 and 0.832723 |
| Refinement method | Full-matrixleast-squaresonF2 |
| Data/restraints/parameters | 8026/0/398 |
| Goodness-of-fitonF2 | 0.786 |
| Final Rindices [I > 2sigma(I)] | R1 = 0.0666, wR2 = 0.1877 |
| Rindices (alldata) | R1 = 0.1410, wR2 = 0.2517 |
| Absolute structure parameter | 0.7(17) |
| Extinction coefficient | 0.0040(15) |
| Largest diff. Peak and hole | 0.342 and −0.329 eA−3 |
2.2. Inhibitory Effect of Riparsaponin on Xanthine Oxidase Activity in Vitro
| Group | Concentration (nmol/mL) | Fluorescence Unit | Inhibition Ratio (%) | IC50 Value (nmol/mL) |
|---|---|---|---|---|
| DMSO | 8.291 ± 0.892 | 0.07 | ||
| Riparsaponin | 4.84 | 7.205 ± 0.865 | 13.09 | 11.16 |
| 9.68 | 4.815 ± 0.861 ** | 41.93 | ||
| 19.36 | 3.248 ± 0.912 ** | 60.82 | ||
| 40.32 | 2.362 ± 0.127 ** | 71.52 | ||
| 80.65 | 1.872 ± 0.483 ** | 77.42 | ||
| 161.29 | 1.474 ± 0.131 ** | 82.22 | ||
| Allopurinol | 2.20 | 6.796 ± 0.696 * | 18.03 | 11.84 |
| 4.41 | 6.212 ± 0.580 ** | 25.08 | ||
| 8.82 | 4.879 ± 0.410 ** | 40.93 | ||
| 17.63 | 2.776 ± 0.217 ** | 66.52 | ||
| 35.27 | 2.062 ± 0.370 ** | 75.13 | ||
| 70.53 | 1.617 ± 0.220 ** | 80.49 |
3. Experimental Section
3.1. General Information
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Xanthine Oxidase Inhibitory Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chinese Academy of Medical Sciences Research Institute. Higher Plant Figure of China; Beijing Science Press: Beijing, China, 1972; Volume 2, pp. 606–608. [Google Scholar]
- State Administration of Traditional Chinese Medicine. Chinese Material Medica; Science and Technology Press of Shanghai: Shanghai, China, 1999; Volume 6, pp. 824–825. [Google Scholar]
- Lee, I.; Kim, J.; Kim, Y.S.; Yoo, N.H.; Kim, C.S.; Jo, K.; Kim, J.H.; Bach, T.T.; Kim, J.S. Cycloartane-type triterpenes from the leaves of Homonoia Riparia with VEGF-induced angiogenesis inhibitor activity. J. Nat. Prod. 2012, 75, 1312–1318. [Google Scholar] [CrossRef]
- Parver, N.; Pal-Singh, M.; Khan, N.U. Chemical examination of the leaves of Homonoia riparia Lour ( Euphorbiaceae). J. Indian Chem. Soc. 1988, 65, 815–816. [Google Scholar]
- Yang, S.M.; Liu, X.K.; Qing, C.; Wu, D.G.; Zhu, D.Y. Chemical constituents from the roots of Homonoia riparia. Acta Pharm. Sin. 2007, 42, 292–296. [Google Scholar]
- Wu, L.J. Natural Pharmaceutical Chemistry, 5th ed; People’s Medical Publishing House: Beijing, China, 2004; pp. 93–103. [Google Scholar]
- Sowndhararajan, K.; Joseph, J.M.; Rajendrakumaran, D. In vitro xanthine oxidase inhibitory activity of methanol extracts of Erythrina indica Lam. leaves and stem bark. Asian Pac. J. Trop. Biomed. 2012, 2, S1415–S1417. [Google Scholar] [CrossRef]
- Masuoka, N.; Nihei, K.; Maeta, A.; Yamagiwa, Y.; Kubo, I. Inhibitory effects of cardols and related compounds on superoxide anion generation by xanthine oxidase. Food Chem. 2014, 166, 270–274. [Google Scholar]
- Sweeney, A.P.; Wyllie, S.G.; Shalliker, R.A.; Markham, J.L. Xanthine oxidase inhibitory activity of selected Australian native plants. J. Ethnopharmacol. 2001, 75, 273–277. [Google Scholar] [CrossRef]
- Peng, W.; Ming, Q.L.; Han, P.; Zhang, Q.Y.; Jiang, Y.P.; Zheng, C.J.; Han, T.; Qin, L.P. Anti-allergic rhinitis effect of caffeoylxanthiazonoside isolated fromfruits of Xanthium strumarium L. in rodent animals. Phytomedicine 2014, 21, 824–829. [Google Scholar] [CrossRef]
- Karki, R.; Park, C.H.; Kim, D.W. Extract of buckwheat sprouts scavenges oxidation and inhibits pro-inflammatory mediators in lipopolysaccharide-stimulated macrophages (RAW264.7). J. Integr. Med. 2013, 11, 246–252. [Google Scholar] [CrossRef]
- Nyasse, B.; Ngantchou, I.; Nono, J.J.; Schneider, B. Antifilarial activity in vitro of polycarpol and 3-O-acetyl aleuritolic acid from cameroonian medicinal plants against Onchocerca gutturosa. Nat. Prod. Res. 2006, 20, 391–397. [Google Scholar] [CrossRef]
- Villasenor, I.M.; Domingo, P.D. Antieareinogenicity potential of spinasterol isolated from squash flowers. Teratog. Carcinog. Mutagen. 2000, 20, 99–105. [Google Scholar] [CrossRef]
- Wang, G.K.; Lin, B.B.; Qin, M.J. Study on chemical constituents from leaf of Bombax ceiba (II). J. Chin. Med. Mater. 2014, 37, 240–242. [Google Scholar]
- Tsai, S.F.; Lee, S.S. Neolignans as xanthine oxidase inhibitors from Hyptis rhomboids. Phytochemistry 2014, 101, 121–127. [Google Scholar] [CrossRef]
- Sample Availability: Contact the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Xu, F.; Zhao, X.; Yang, L.; Wang, X.; Zhao, J. A New Cycloartane-Type Triterpenoid Saponin Xanthine Oxidase Inhibitor from Homonoia riparia Lour. Molecules 2014, 19, 13422-13431. https://doi.org/10.3390/molecules190913422
Xu F, Zhao X, Yang L, Wang X, Zhao J. A New Cycloartane-Type Triterpenoid Saponin Xanthine Oxidase Inhibitor from Homonoia riparia Lour. Molecules. 2014; 19(9):13422-13431. https://doi.org/10.3390/molecules190913422
Chicago/Turabian StyleXu, Fan, Xueqian Zhao, Lingli Yang, Xiuhua Wang, and Jing Zhao. 2014. "A New Cycloartane-Type Triterpenoid Saponin Xanthine Oxidase Inhibitor from Homonoia riparia Lour" Molecules 19, no. 9: 13422-13431. https://doi.org/10.3390/molecules190913422
APA StyleXu, F., Zhao, X., Yang, L., Wang, X., & Zhao, J. (2014). A New Cycloartane-Type Triterpenoid Saponin Xanthine Oxidase Inhibitor from Homonoia riparia Lour. Molecules, 19(9), 13422-13431. https://doi.org/10.3390/molecules190913422




