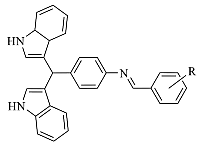
Synthesis of Novel Bisindolylmethane Schiff bases and Their Antibacterial Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
| Reducing Agents | Nickel Acetate | Reaction Time | Yield (%) |
|---|---|---|---|
| Lithium aluminum hydride | + | 2 h | 54 |
| Sodium borohydride | + | 3 h | 95 |
| Lithium aluminum hydride | − | 6 h | 51 |
| Sodium borohydride | − | 4 h | 78 |
| Raney nickel | − | 4 h | 70 |
| Hydrazine hydrate | − | 15 h | 76 |
| Compound | Ar | Compound | Ar |
|---|---|---|---|
| 3 | 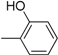 | 15 | 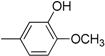 |
| 4 | 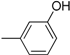 | 16 | 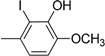 |
| 5 |  | 17 | 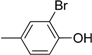 |
| 6 | 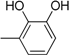 | 18 | 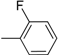 |
| 7 | 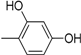 | 19 | 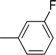 |
| 8 | 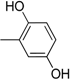 | 20 | 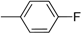 |
| 9 | 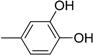 | 21 | 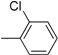 |
| 10 | 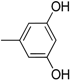 | 22 | 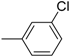 |
| 11 | 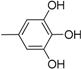 | 23 |  |
| 12 | 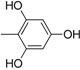 | 24 | 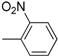 |
| 13 | 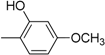 | 25 |  |
| 14 | 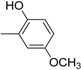 | 26 | 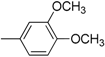 |
2.2. Antibacterial Activity
| Compound | Inhibition Zone (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | |
| 3 | - | - | - | - | - | - | - | - | - |
| 4 | - | - | - | - | - | - | - | - | - |
| 5 | - | - | - | - | - | 11 | - | 09 | - |
| 6 | - | - | - | - | - | - | - | - | - |
| 7 | - | 07 | - | - | - | - | - | - | - |
| 8 | - | - | - | - | - | 10 | - | - | - |
| 9 | - | 09 | - | - | - | - | - | - | - |
| 10 | - | 06 | - | - | - | - | - | - | - |
| 11 | - | - | - | - | - | - | - | - | - |
| 12 | - | - | - | - | - | - | - | - | - |
| 13 | - | - | - | - | - | - | - | - | - |
| 14 | - | - | - | - | - | - | - | - | - |
| 15 | - | - | - | - | - | - | - | - | - |
| 16 | - | - | - | - | - | - | - | - | - |
| 17 | - | - | - | - | - | - | 07 | - | - |
| 18 | - | - | - | - | - | 10 | - | - | - |
| 19 | - | - | 08 | 10 | 15 | - | - | - | - |
| 20 | 10 | - | 12 | 09 | 17 | - | - | 12 | 13 |
| 21 | - | - | 14 | 12 | 16 | - | - | - | 12 |
| 22 | - | - | 11 | 10 | 15 | - | - | - | - |
| 23 | - | - | 10 | - | 16 | - | - | - | 10 |
| 24 | 09 | - | 09 | - | 11 | - | - | - | - |
| 25 | - | - | 10 | - | 09 | - | - | - | - |
| 26 | - | - | 08 | 08 | 10 | - | - | - | - |
| Gentamicin | 29 | 23 | 25 | 25 | 25 | 23 | 28 | 14 | 25 |
| Compound | Inhibition Zone (mm) | |||||
|---|---|---|---|---|---|---|
| A | B | C | D | E | F | |
| 3 | - | - | - | - | - | - |
| 4 | - | - | - | - | - | - |
| 5 | - | - | 10 | - | - | - |
| 6 | - | - | 11 | - | - | - |
| 7 | - | - | 08 | - | - | - |
| 8 | - | - | 09 | 11 | - | 08 |
| 9 | - | - | - | - | - | - |
| 10 | - | - | - | - | - | - |
| 11 | - | - | - | 07 | - | - |
| 12 | - | - | - | - | - | - |
| 13 | - | - | - | - | - | - |
| 14 | - | - | - | - | - | - |
| 15 | - | 08 | 08 | - | - | - |
| 16 | 07 | 07 | NA | 09 | 08 | - |
| 17 | - | - | - | - | 10 | - |
| 18 | 12 | 08 | 09 | 10 | - | - |
| 19 | - | 09 | - | 09 | 12 | 09 |
| 20 | 11 | 10 | 09 | 11 | 11 | - |
| 21 | 09 | 13 | 08 | 12 | 10 | - |
| 22 | 09 | - | 07 | - | 09 | - |
| 23 | - | 07 | 08 | 08 | 12 | - |
| 24 | - | - | - | 07 | 09 | - |
| 25 | - | - | - | 09 | - | - |
| 26 | - | - | - | 08 | 08 | - |
| Gentamicin | 24 | 25 | 25 | 28 | 25 | 20 |
3. Experimental Section
3.1. General Information
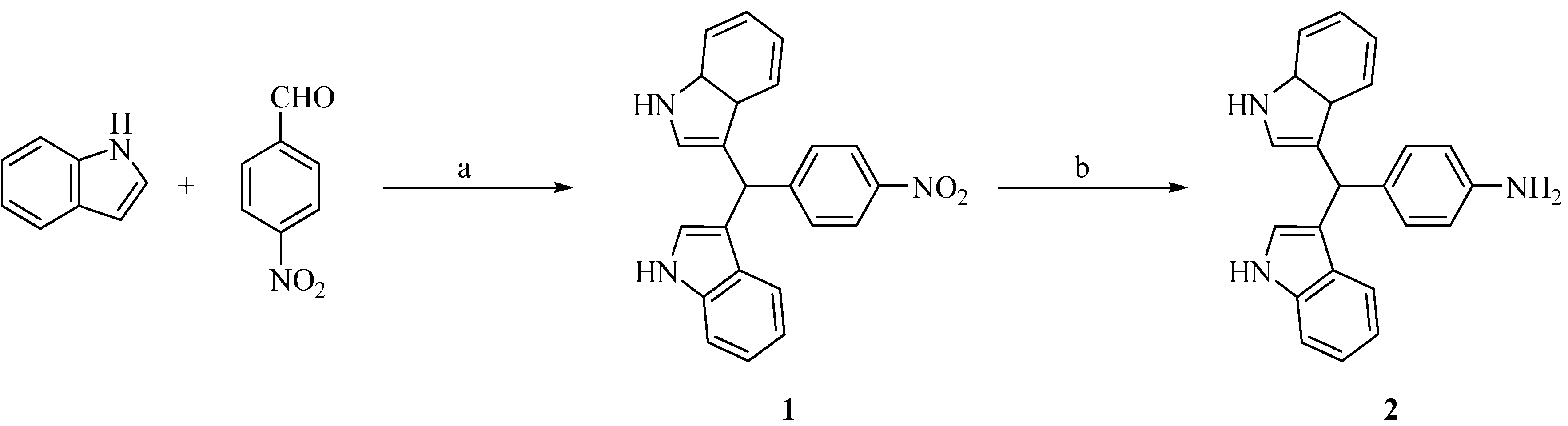
3.2. Synthesis of 3,3'-((4-Nitrophenyl)methylene)bis(1H-indole) (1)
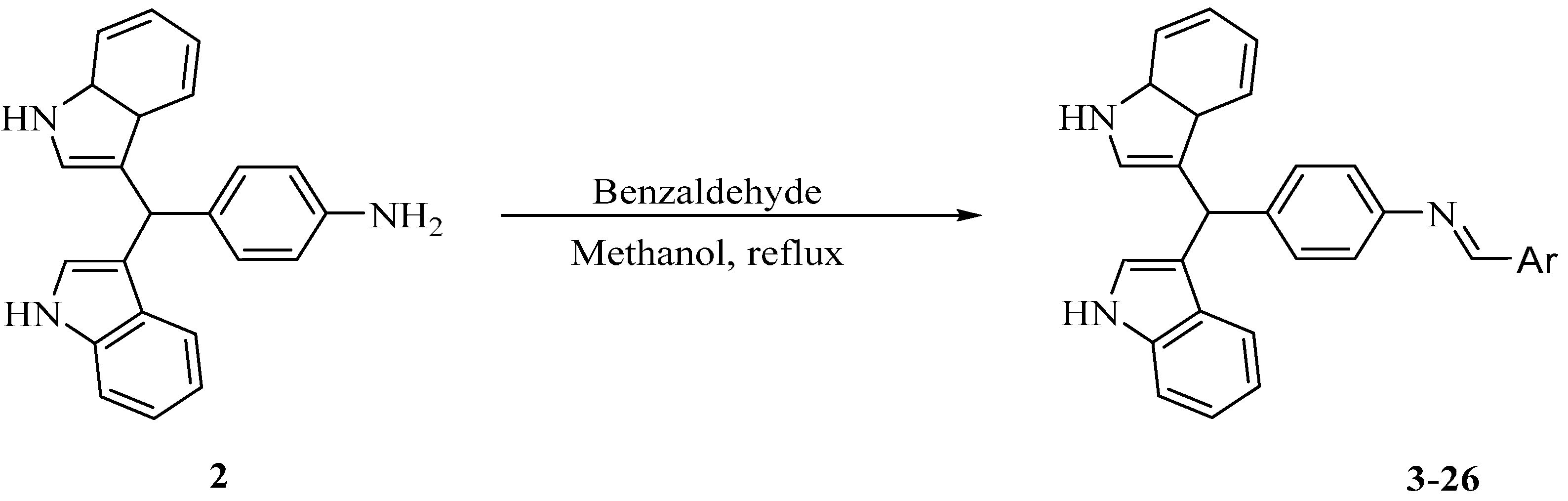
3.3. Synthesis of 4-(di(1H-Indol-3-yl)methyl)aniline (2)
General Procedure for Synthesis of Bisindolylmethane Schiff bases 3–26
3.4. Bioassay
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Butler, M.M.; Williams, J.D.; Peet, N.P.; Moir, D.T.; Panchal, R.G.; Bavari, S.; Bowlin, T.L. Comparative in vitro activity profiles of novel bis-indole antibacterial against gram-positive and gram-negative clinical isolates. Antimicrob. Agents Chemother. 2010, 54, 3974–3977. [Google Scholar]
- Bhatia, N.M.; Mahadik, K.R.; Bhatia, M.S. QSAR analysis of 1,3-diaryl-2-propen-1-ones and their indole analogs for designing potent antibacterial agents. Chem. Pap. 2009, 63, 456–463. [Google Scholar] [CrossRef]
- Leeb, M. Antibiotics: A shot in the arm. Nature 2004, 431, 892–893. [Google Scholar] [CrossRef]
- Iqbal, A.; Siddiqui, H.L.; Ashraf, C.M.; Ahmad, M.; Weaver, G.W. Synthesis, characterization and antibacterial activity of azomethine derivatives derived from 2-formylphenoxyacetic acid. Molecules 2007, 12, 245–254. [Google Scholar] [CrossRef]
- Malladi, S.; Isloor, A.M.; Isloor, S.; Akhila, D.S.; Fun, H.K. Synthesis, characterization and antibacterial activity of some new pyrazole based Schiff bases. Arab. J. Chem. 2013, 6, 335–340. [Google Scholar] [CrossRef]
- Bharti, S.K.; Nath, G.; Tilak, R.; Singh, S.K. Synthesis, anti-bacterial and anti-fungal activities of some novel Schiff bases containing 2,4-disubstituted thiazole ring. Eur. J. Med. Chem. 2010, 45, 651–660. [Google Scholar] [CrossRef]
- Makawana, J.A.; Sangani, C.B.; Lin, L.; Zhu, H.L. Schiff’s base derivatives bearing nitroimidazole and quinoline nuclei: New class of anticancer agents and potential EGFR tyrosine kinase inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 1734–1736. [Google Scholar] [CrossRef]
- Aslam, M.A.S.; Mahmood, S.U.; Shahid, M.; Saeed, A.; Iqbal, J. Synthesis, biological assay in vitro and molecular docking studies of new Schiff base derivatives as potential urease inhibitors. Eur. J. Med. Chem. 2011, 46, 5473–5479. [Google Scholar] [CrossRef]
- Taha, M.; Ismail, N.H.; Jamil, W.; Yousuf, S.; Jaafar, F.M.; Ali, M.I.; Hussain, E. Synthesis, evaluation of antioxidant activity and crystal structure of 2,4-dimethylbenzoylhydrazones. Molecules 2013, 18, 10912–10929. [Google Scholar]
- Anouar, E.H.; Raweh, S.; Bayach, I.; Taha, M.; Baharudin, M.S.; Meo, F.D.; Hasan, M.H.; Adam, A.; Ismail, N.H.; Weber, J.F.; et al. Antioxidant properties of phenolic Schiff bases: Structure-activity relationship and mechanism of action. J. Comput. Aided Mol. Des. 2013, 27, 951–964. [Google Scholar] [CrossRef]
- Khan, K.M.; Shah, Z.; Ahmad, V.U.; Khan, M.; Taha, M.; Ali, S.; Perveen, S.; Choudhary, M.I.; Voelter, W. 2,4,6-Trichlorophenylhydrazine Schiff bases as DPPH radical and super oxide anion scavengers. Med. Chem. 2012, 8, 452–461. [Google Scholar] [CrossRef]
- Khan, K.M.; Taha, M.; Naz, F.; Ali, S.; Perveen, S.; Choudhary, M.I. Synthesis of acylhydrazide Schiff bases and their anti-oxidant activity. Med. Chem. 2012, 8, 705–710. [Google Scholar] [CrossRef]
- Aziz, A.N.; Taha, M.; Ismail, N.H.; Anouar, E.H.; Yousuf, S.; Jamil, W.; Awang, K.; Ahmat, K.A.N.; Khan, K.M.; Kashif, S.M. Synthesis, crystal structure, DFT studies and evaluation of the antioxidant activity of 3,4-dimethoxybenzenamine Schiff bases. Molecules 2014, 19, 8414–8433. [Google Scholar] [CrossRef]
- Taha, M.; Naz, H.; Rasheed, S.; Ismail, N.H.; Rahman, A.A.; Yousuf, S.; Choudhary, M.I. Synthesis of 4-methoxybenzoylhydrazones and evaluation of their antiglycation activity. Molecules 2014, 19, 1286–1301. [Google Scholar] [CrossRef]
- Khan, K.M.; Shah, Z.; Ahmad, V.U.; Khan, M.; Taha, M.; Rahim, F.; Jahun, H.; Perveen, S.; Choudhary, M.I. Synthesis of 2,4,6-trichlorophenyl hydrazones and their inhibitory potential against glycation of protein. Med. Chem. 2011, 7, 572–580. [Google Scholar] [CrossRef]
- Khan, K.M.; Rahim, F.; Ambreen, N.; Taha, M.; Khan, M.; Jahan, H.; Najeebullah, U.; Shaikh, A.; Iqbal, S.; Perveen, S.; et al. Synthesis of benzophenonehydrazone Schiff bases and their in vitro antiglycating activities. Med. Chem. 2013, 9, 588–595. [Google Scholar] [CrossRef]
- Khan, K.M.; Taha, M.; Rahim, F.; Fakhri, M.I.; Jamil, W.; Khan, M.; Rasheed, S.; Karim, A.; Perveen, S.; Choudhary, M.I. Acylhydrazide Schiff bases: Synthesis and antiglycation activity. J. Pak. Chem. Soc. 2013, 35, 929–937. [Google Scholar]
- Sundberg, R.J. The Chemistry of Indoles; Academic Press: New York, NY, USA, 1996. [Google Scholar]
- Gribble, G.W. Novel chemistry of indole in the synthesis of heterocycles. Pure Appl. Chem. 2013, 75, 1417–1432. [Google Scholar]
- Kaishap, P.P.; Dohutia, C. Synthetic approaches for bis(indolyl)methanes. Int. J. Pharm. Sci. Res. 2013, 4, 1312–1322. [Google Scholar]
- Rozhkov, V.V. Synthesis of 6-nitro-4-sulfanyl-1H-indole derivatives from 2,4,6-trinitrotoluene. Tetrahedron 2014, 70, 1–6. [Google Scholar] [CrossRef]
- Popp, F.D. Potential anticonvulsants. VIII. Some hydrazones of indole-3-carboxaldehyde. J. Heterocycl. Chem. 1984, 21, 617–619. [Google Scholar] [CrossRef]
- Radwan, M.A.; Ragab, E.A.; Sabry, N.M.; El-Shenawy, S.M. Synthesis and biological evaluation of new 3-substituted indole derivatives as potential anti-inflammatory and analgesic agents. Bioorg. Med. Chem. 2007, 15, 3832–3841. [Google Scholar] [CrossRef]
- Sivaprasad, G.; Perumal, P.T.; Prabavathy, V.R.; Mathivanan, N. Synthesis and anti-microbial activity of pyrazolylbisindoles-promising anti-fungal compounds. Bioorg. Med. Chem. Lett. 2006, 16, 6302–6305. [Google Scholar] [CrossRef]
- Bell, R.; Carmeli, S.; Sar, N. Vibrindole A, a metabolite of the marine bacterium, Vibrio parahaemolyticus, isolated from the toxic mucus of the boxfish ostracioncubicus. J. Nat. Prod. 1994, 57, 1587–1590. [Google Scholar]
- Sujatha, K.; Perumal, P.T.; Muralidharan, D.; Rajendra, M. Synthesis, analgesic and anti-inflammatory activities of bis(indolyl)methanes. Indian J. Chem. 2009, 48, 267–272. [Google Scholar]
- Gunasekera, S.P.; Peter, J.M.; Michelle, K. Hamacanthin A and B, new antifungal bisindole alkaloids from the deep-water marine sponge, Hamacantha sp. J. Nat. Prod. 1994, 57, 1437–1441. [Google Scholar] [CrossRef]
- Bao, B.; Sun, Q.; Yao, X.; Hong, J.; Lee, C.O.; Sim, C.J.; Im, K.S.; Jung, J.H. Cytotoxic bisindole alkaloids from a marine sponge Spongosorites sp. J. Nat. Prod. 2005, 68, 711–715. [Google Scholar] [CrossRef]
- McDougal, A.; Gupta, M.S.; Morrow, D.; Ramamoorthy, K.; Lee, J.E.; Safe, S.H. Methyl-substituted diindolylmethanes as inhibitors of estrogen-induced growth of T47D cells and mammary tumors in rats. Breast Cancer Res. Tr. 2001, 66, 147–157. [Google Scholar] [CrossRef]
- Safe, S.; Papineni, S.; Chintharlapalli, S. Cancer chemotherapy with indole-3-carbinol, bis(indolyl)methane and synthetic analogs. Cancer Lett. 2008, 269, 326–338. [Google Scholar] [CrossRef]
- Micheal, A.Z. Diet and estrogen status: The cruciferous connection. J. Med. Food 1998, 1, 67–82. [Google Scholar] [CrossRef]
- Hong, C.; Firestone, G.L.; Bjeldanes, L. Bcl-2 family-mediated apoptotic effects of 3,3'-diindolylmethane (DIM) in human breast cancer cells. Biochem. Pharmacol. 2002, 63, 1085–1097. [Google Scholar] [CrossRef]
- Nachshon-Kedmi, M.; Yannai, S.; Haj, A.; Fares, F.A. Indole-3-carbinol and 3,3'-diindolylmethane induce apoptosis in human prostate cancer cells. Food Chem. Toxicol. 2003, 41, 745–752. [Google Scholar] [CrossRef]
- Bhowmik, A.; Das, N.; Pal, U.; Mandal, M.; Bhattacharya, S.; Sarkar, M.; Ghosh, M.K. 2,2'-Diphenyl-3,3'-diindolylmethane: A potent compound induces apoptosis in breast cancer cells by inhibiting EGFR pathway. PLoS One 2013, 8, e59798. [Google Scholar]
- Bharate, S.B.; Bharate, J.B.; Khan, S.I.; Tekwani, B.L.; Jacob, M.R.; Mudududdla, R.; Vishwakarma, R.A. Discovery of 3,3'-diindolylmethanes as potent antileishmanial agents. Eur. J. Med. Chem. 2013, 63, 435–443. [Google Scholar] [CrossRef]
- Olyaei, A.; Vaziri, M.; Razeghi, R.; Shams, B.; Bagheri, H. A novel approach to bis(indolyl)methanes using nickel nanoparticles as a reusable catalyst under solvent-free conditions. J. Serb. Chem. Soc. 2013, 78, 463–468. [Google Scholar]
- Zhao, R.; Tan, C.; Xie, Y.; Gao, C.; Liu, H.; Jiang, Y. One step synthesis of azo compounds from nitroaromatics and anilines. Tetrahedron Lett. 2011, 52, 3805–3809. [Google Scholar] [CrossRef]
- Khan, K.M.; Naz, F.; Taha, M.; Khan, A; Perveen, S.; Choudhary, M.I.; Voelter, W. Synthesis and in vitro urease inhibitory activity of N,N'-disubsitutedthioureas. Eur. J. Med. Chem. 2014, 74, 314–323. [Google Scholar] [CrossRef]
- Taha, M.; Ismail, N.H.; Imran, S.; Khan, K.M. 4-[5-(2-Methoxyphenyl)-1,3,4-oxadiazol-2-yl]benzohydrazide. Molbank 2014, M826. [Google Scholar] [CrossRef]
- Khan, K.M.; Rahim, F.; Wadood, A.; Taha, M.; Khan, M.; Naureen, S.; Ambreen, N.; Hussain, S.; Perveen, S.; Choudhary., M.I. Evaluation of bisindole as potent β-glucuronidase inhibitors: Synthesis and in silico based studies. Bioorg. Med. Chem. Lett. 2014, 24, 1825–1829. [Google Scholar]
- Khan, K.M.; Ambreen, N.; Taha, M.; Halim, S.A.; Zaheer-ul-Haq; Naureen, S.; Rasheed, S.; Perveen, S.; Ali, S.; Choudhary, M.I. Structure-based design, synthesis and biological evaluation of β-glucuronidase inhibitors. J. Comput. Aided Mol. Des. 2014, 28, 577–585. [Google Scholar] [CrossRef]
- Khan, K.M.; Rahim, F.; Wadood, A.; Kosar, N.; Taha, M.; Khan, A.; Fakhri, M.I.; Junaid, M.; Rehman, W.; Khan, M.; et al. Synthesis and molecular docking studies of potent α-glucosidase inhibitors based on biscoumarin skeleton. Eur. J. Med. Chem. 2014, 81, 245–252. [Google Scholar]
- Jamil, W.; Perveen, S.; Shah, S.A.A.; Taha, M.; Ismail, N.H.; Perveen, S.; Ambreen, N.; Khan, K.; Choudhary, M.I. Phenoxyacetohydrazide Schiff bases: β-Glucuronidase inhibitors. Molecules 2014, 19, 8788–8802. [Google Scholar] [CrossRef]
- Venkatesan, P.; Maruthavanan, T. Synthesis of substituted flavone derivatives as potent antimicrobial agents. Bull. Chem. Soc. Ethiop. 2011, 25, 419–425. [Google Scholar]
- Završnik, D.; Selma, Š.; Dženita, S. Synthesis, structure and antibacterial activity of 3-substituted derivatives of 4-hydroxycoumarin. Period. Biol. 2011, 113, 93–97. [Google Scholar]
- Vagdevi, H.M.; Jayanna, N.D.; Latha, K.P. Synthesis, characterization and evaluation of antibacterial activity of some 3-substitutedphenylquinazoline-2,4-diones. Pharm. Chem. 2012, 4, 1754–1758. [Google Scholar]
- Sample Availability: Samples of the compounds 1–26 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Imran, S.; Taha, M.; Ismail, N.H.; Khan, K.M.; Naz, F.; Hussain, M.; Tauseef, S. Synthesis of Novel Bisindolylmethane Schiff bases and Their Antibacterial Activity. Molecules 2014, 19, 11722-11740. https://doi.org/10.3390/molecules190811722
Imran S, Taha M, Ismail NH, Khan KM, Naz F, Hussain M, Tauseef S. Synthesis of Novel Bisindolylmethane Schiff bases and Their Antibacterial Activity. Molecules. 2014; 19(8):11722-11740. https://doi.org/10.3390/molecules190811722
Chicago/Turabian StyleImran, Syahrul, Muhammad Taha, Nor Hadiani Ismail, Khalid Mohammed Khan, Farzana Naz, Memona Hussain, and Saima Tauseef. 2014. "Synthesis of Novel Bisindolylmethane Schiff bases and Their Antibacterial Activity" Molecules 19, no. 8: 11722-11740. https://doi.org/10.3390/molecules190811722
APA StyleImran, S., Taha, M., Ismail, N. H., Khan, K. M., Naz, F., Hussain, M., & Tauseef, S. (2014). Synthesis of Novel Bisindolylmethane Schiff bases and Their Antibacterial Activity. Molecules, 19(8), 11722-11740. https://doi.org/10.3390/molecules190811722