A Novel Fungal Metabolite with Beneficial Properties for Agricultural Applications
Abstract
:1. Introduction
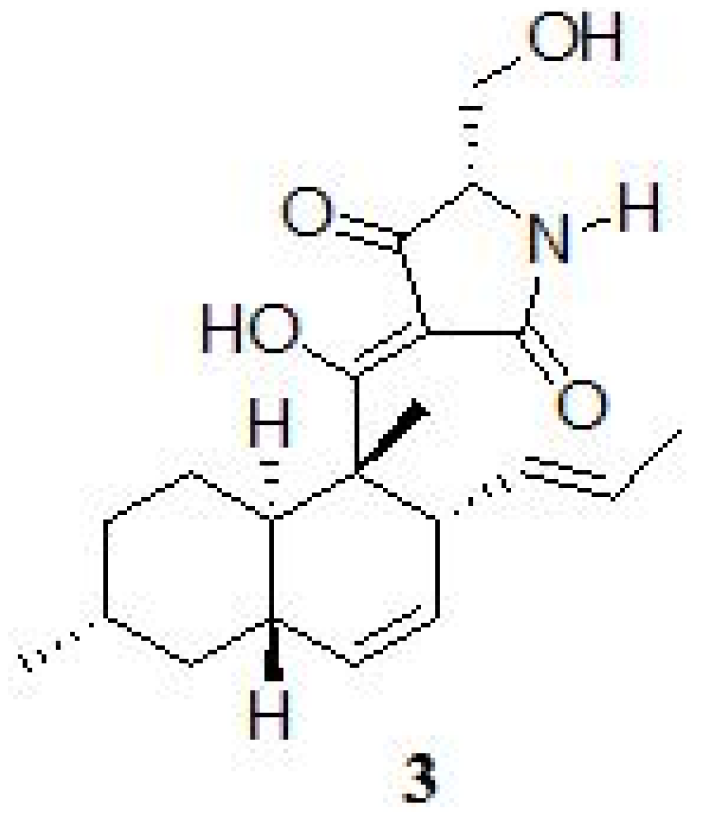
2. Results and Discussion
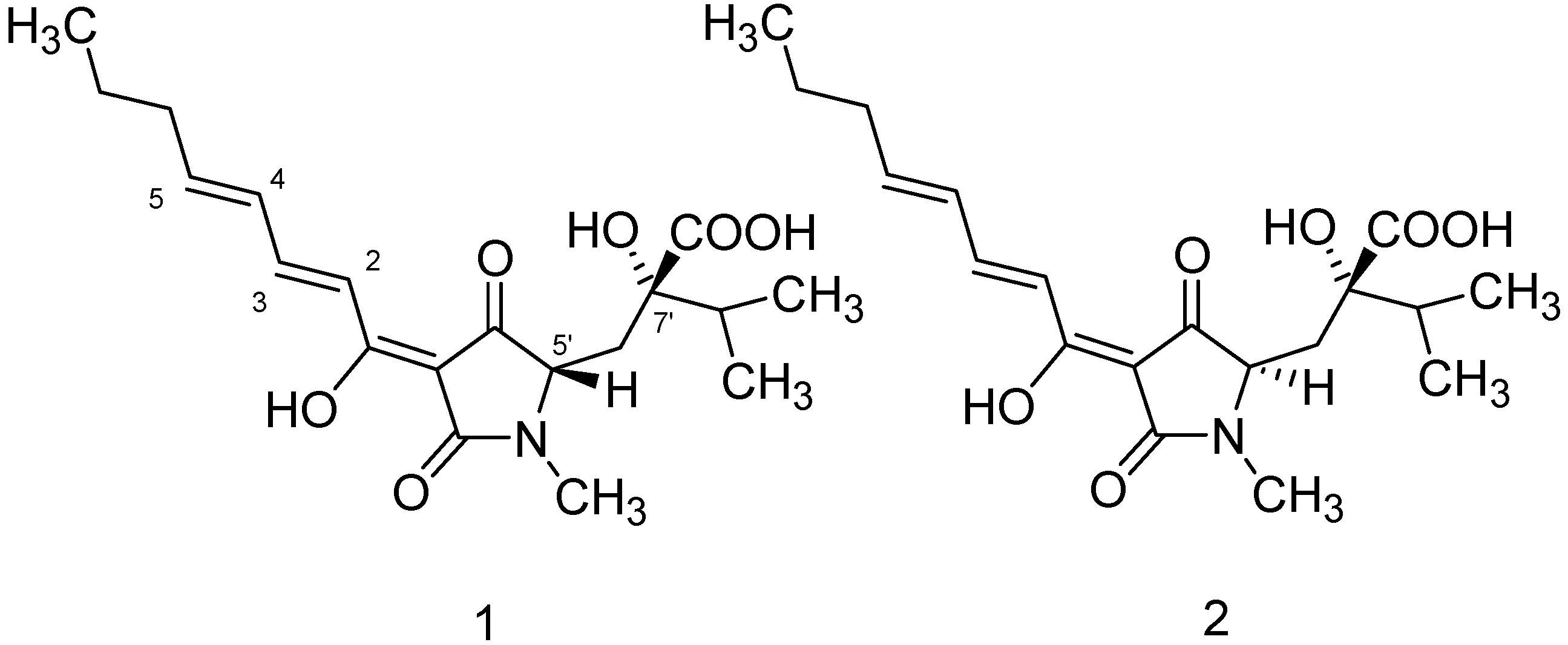
| Position | HA (1) | iso-HA (2) | ||||||
|---|---|---|---|---|---|---|---|---|
| δ 13C | δ 1H | Multi | J (Hz) | δ 13C | δ 1H | Multi | J (Hz) | |
| 1 | 174.0 | _ | _ | 174.0 | _ | _ | ||
| 2 | 119.3 | 7.0 | d | 15.6 | 119.4 | 7.05 | d | 15.25 |
| 3 | 146.2 | 7.57 | m | _ | 146.2 | 7.47 | dd | 10.17; 5.4 |
| 4 | 129.7 | 6.35 | m | _ | 129.7 | 6.35 | m | _ |
| 5 | 148.5 | 6.30 | m | _ | 148.5 | 6.30 | m | _ |
| 6 | 35.4 | 2.19 | m | _ | 35.4 | 2.19 | m | _ |
| 7 | 21.8 | 1.50 | m | _ | 21.8 | 1.50 | m | _ |
| 8 | 13.7 | 0.93 | m | _ | 13.7 | 0.93 | m | _ |
| 2' | 174.0 | _ | _ | 174.0 | _ | _ | ||
| 3' | 99.6 | _ | q | _ | 99.5 | _ | q | _ |
| 4' | 195.0 | _ | _ | 195.1 | _ | _ | ||
| 5' | 63.7 | 3.62 | dd | 1.17, 9.3 | 63.6 | 3.80 | dd | 2.7, 4.6 |
| 6'a | 34.9 | 2.20 | dd | 34.8 | 2.2 | c | ||
| 6'b | 2.51 | 2.2 | ||||||
| 7' | 78.1 | _ | q | _ | 78.0 | _ | q | _ |
| 8' | 35.9 | 2.02 | m | _ | 35.8 | 2.02 | m | _ |
| 9' | 17.2 | 0.98 | m | _ | 17.2 | 0.98 | m | _ |
| 10' | 16.4 | 0.99 | m | _ | 16.4 | 0.99 | m | _ |
| 11' | 27.4 | 2.99 | s | _ | 27.3 | 2.98 | s | _ |
| 12' | 176.8 | _ | _ | 176.7 | _ | _ | ||
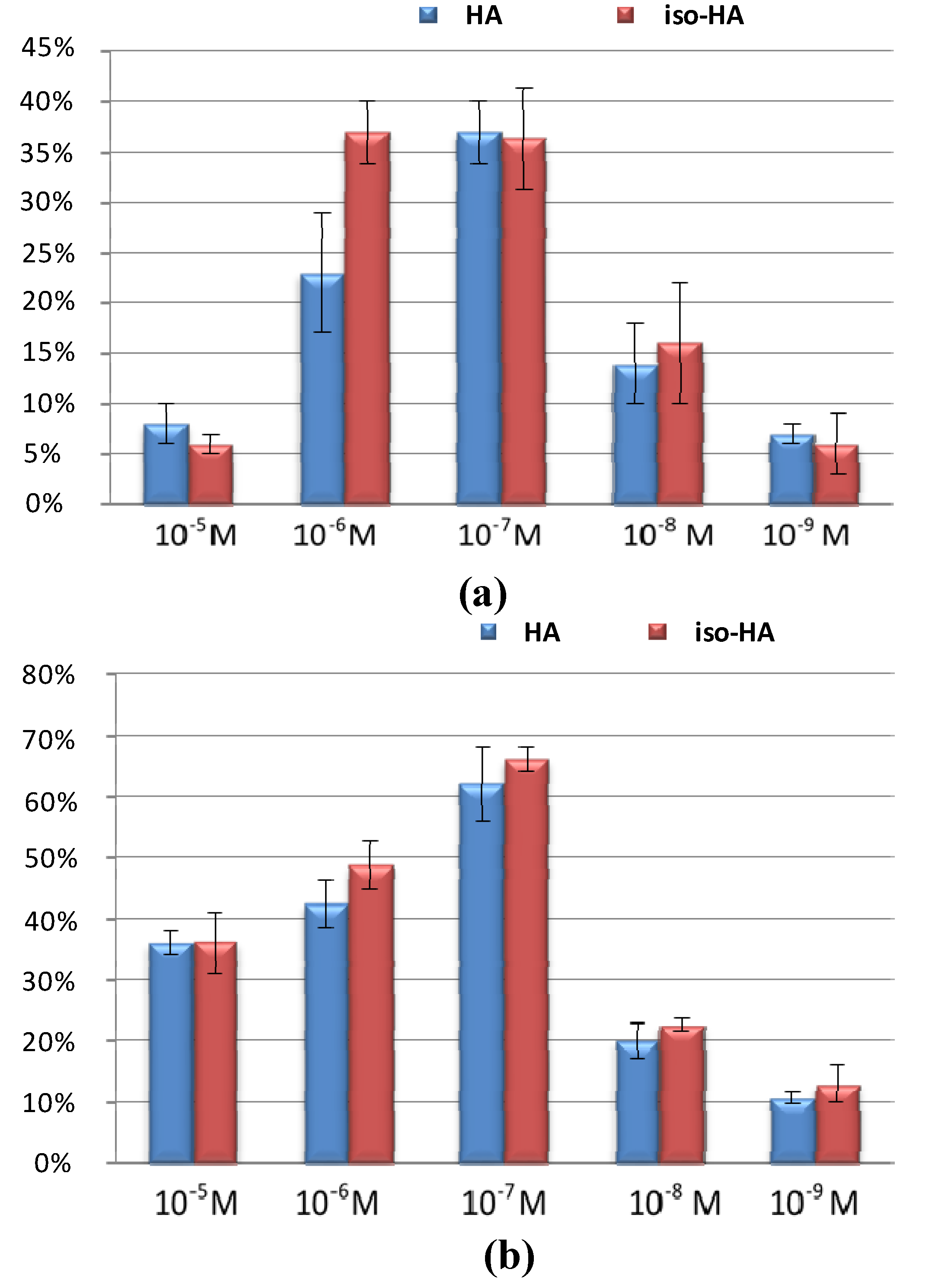
| % of Germination | ||||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | 12 h | DS | 24 h | DS | 36 h | DS | 48 h | DS |
| Control | 0% | 0% | 0% | 0% | 55% | 3.6% | 100% | 0% |
| HA 10−5 M | 0% | 0% | 50% | 3.9% | 100% | 0% | 100% | 0% |
| HA 10−6 M | 0% | 0% | 72% | 4.1% | 88% | 3.4% | 100% | 0% |
| HA 10−7 M | 0% | 0% | 55% | 3.1% | 100% | 0% | 100% | 0% |
| HA 10−8 M | 0% | 0% | 61% | 7.3% | 76% | 12.3% | 100% | 0% |
| HA 10−9 M | 0% | 0% | 56% | 7.9% | 63% | 11.1% | 100% | 0% |
| iso-HA 10−5 M | 0% | 0% | 72% | 3.9% | 100% | 0% | 100% | 0% |
| iso-HA 10−6 M | 0% | 0% | 66% | 5.2% | 100% | 0% | 100% | 0% |
| iso-HA 10−7 M | 0% | 0% | 88% | 5.6% | 100% | 0% | 100% | 0% |
| iso-HA 10−8 M | 0% | 0% | 62% | 2.6% | 83% | 11.8% | 100% | 0% |
| iso-HA 10−9 M | 0% | 0% | 56% | 2.8% | 67% | 3.8% | 100% | 0% |



3. Experimental Section
3.1. General Information
3.2. Fungal Strains
3.3. Production and Isolation of Trichoderma Secondary Metabolites
3.4. Antifungal Assay
3.5. In Vitro Plant Growth Promotion
3.6. Plant Growth Promotion and Induction of Resistance
3.7. Production of HA and Iso HA in Presence of Plant Tissue
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harman, G.E. Myths and dogmas of biocontrol: Changes in perceptions derived from research on Trichoderma harzianum T-22. Plant Dis. 2000, 84, 377–393. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma-plant-pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Hanson, J.R. Natural Products: The Secondary Metabolites; Royal Society of Chemistry: Cambridge, UK, 2003. [Google Scholar]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical Overview of Natural Products in Drug Discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Chiang, Y.; Lee, K.; Sanchez, J.F.; Keller, N.P.; Wang, C.C.C. Unlocking fungal cryptic natural products. Nat. Prod. Commun. 2009, 4, 1505–1510. [Google Scholar]
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism—From biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [Google Scholar] [CrossRef]
- Sivasithamparam, K.; Ghisalberti, E.L. Secondary metabolism in Trichoderma and Gliocladium. In Trichoderma and Gliocladium; Harman, G.E., Kubicek, C.P., Eds.; Taylor and Francis Ltd.: London, UK, 1998; Volume 1, pp. 139–191. [Google Scholar]
- Reino, J.L.; Guerrero, R.F.; Hernández-Galán, R.; Collado, I.G. Secondary metabolites from species of the biocontrol agent Trichoderma. Phytochem. Rev. 2008, 7, 89–123. [Google Scholar]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Woo, S.L.; Nigro, M.; Marra, R.; Lombardi, N.; Pascale, A.; Ruocco, M.; Lanzuise, S.; et al. Trichoderma secondary metabolites active on plants and fungal pathogens. Open Mycol. J. 2014, submitted. [Google Scholar]
- Vinale, F.; Ghisalberti, E.L.; Sivasithamparam, K.; Marra, R.; Ritieni, A.; Ferracane, R.; Woo, S.L.; Lorito, M. Factors affecting the production of Trichoderma harzianum secondary metabolites during the interaction with different plant pathogens. Lett. Appl. Microbiol. 2009, 48, 705–711. [Google Scholar]
- Sawa, R.; Mori, Y.; Iinuma, H.; Naganawa, H.; Hamada, M.; Yoshida, S.; Furutani, H.; Kajimura, Y.; Fuwa, T.; Takeuchi, T. Harzianic acid, a new antimicrobial antibiotic from a fungus. J. Antibiot. 1994, 47, 731–732. [Google Scholar] [CrossRef]
- Vinale, F.; Flematti, G.; Sivasithamparam, K.; Lorito, M.; Marra, R.; Skelton, B.W.; Ghisalberti, E.L. Harzianic acid, an antifungal and plant growth promoting metabolite from Trichoderma harzianum. J. Nat. Prod. 2009, 72, 2032–2035. [Google Scholar]
- Vinale, F.; Nigro, N.; Sivasithamparam, K.; Flematti, G.; Ghisalberti, E.L.; Ruocco, M.; Varlese, R.; Marra, R.; Lanzuise, S.; Eid, A.; et al. Harzianic acid: A novel siderophore from Trichoderma harzianum. FEMS Microbiol. Lett. 2013, 347, 123–129. [Google Scholar]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Ruocco, M.; Woo, S.; Lorito, M. Trichoderma secondary metabolites that affect plant metabolism. Nat. Prod. Commun. 2012, 7, 1545–1550. [Google Scholar]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Barbetti, M.J.; Li, H.; Woo, S.L.; Lorito, M. A novel role for Trichoderma secondary metabolites in the interactions with plants. Physiol. Mol. Plant Pathol. 2008, 72, 80–86. [Google Scholar] [CrossRef]
- Cai, F.; Yu, G.; Wang, P.; Wei, Z.; Fu, L.; Shen, Q.; Chen, W. Harzianolide, a novel plant growth regulator and systemic resistance elicitor from Trichoderma harzianum. Plant Physiol. Biochem. 2013, 73, 106–113. [Google Scholar] [CrossRef]
- Marfori, E.C.; Kajiyama, S.; Fukusaki, E.; Kobayashi, A. Trichosetin, a novel tetramic acid antibiotic produced in dual culture of Trichoderma harzianum and Catharanthus roseus callus. Z. Naturforsch. 2002, 57c, 465–470. [Google Scholar]
- Marfori, E.C.; Kajiyama, S.; Fukusaki, E.; Kobayashi, A. Phytotoxicity of the tetramic acid metabolite trichosetin. Phytochemistry 2003, 62, 715–721. [Google Scholar] [CrossRef]
- Marco, S.; Rullo, R.; Albino, A.; Masullo, M.; de Vendittis, E.; Amato, M. The thioredoxin system in the dental caries pathogen Streptococcus mutans and the food-industry bacterium Streptococcus thermophilus. Biochimie 2013, 95, 2145–2156. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1 and 2 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinale, F.; Manganiello, G.; Nigro, M.; Mazzei, P.; Piccolo, A.; Pascale, A.; Ruocco, M.; Marra, R.; Lombardi, N.; Lanzuise, S.; et al. A Novel Fungal Metabolite with Beneficial Properties for Agricultural Applications. Molecules 2014, 19, 9760-9772. https://doi.org/10.3390/molecules19079760
Vinale F, Manganiello G, Nigro M, Mazzei P, Piccolo A, Pascale A, Ruocco M, Marra R, Lombardi N, Lanzuise S, et al. A Novel Fungal Metabolite with Beneficial Properties for Agricultural Applications. Molecules. 2014; 19(7):9760-9772. https://doi.org/10.3390/molecules19079760
Chicago/Turabian StyleVinale, Francesco, Gelsomina Manganiello, Marco Nigro, Pierluigi Mazzei, Alessandro Piccolo, Alberto Pascale, Michelina Ruocco, Roberta Marra, Nadia Lombardi, Stefania Lanzuise, and et al. 2014. "A Novel Fungal Metabolite with Beneficial Properties for Agricultural Applications" Molecules 19, no. 7: 9760-9772. https://doi.org/10.3390/molecules19079760
APA StyleVinale, F., Manganiello, G., Nigro, M., Mazzei, P., Piccolo, A., Pascale, A., Ruocco, M., Marra, R., Lombardi, N., Lanzuise, S., Varlese, R., Cavallo, P., Lorito, M., & Woo, S. L. (2014). A Novel Fungal Metabolite with Beneficial Properties for Agricultural Applications. Molecules, 19(7), 9760-9772. https://doi.org/10.3390/molecules19079760





