Synthesis and Photophysical Properties of the 2-(3-(2-Alkyl-6,8-diaryl-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl)propyl)-6,8-diarylquinazolin-4(3H)-ones
Abstract
:1. Introduction
2. Results and Discussion
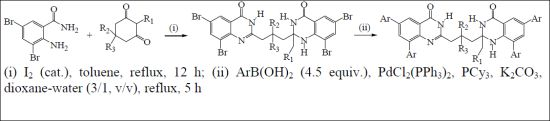
2.1. Synthesis of 2-(3-(2-Alkyl-6,8-dibromo-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl)propyl)-6,8-dibromoquinazolin-4(3H)-ones
| R1 | R2 | R3 | % Yield 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 3a | H | H | H | 85 | |||||
| 3b | -CH3 | H | H | 60 | |||||
| 3c | H | -CH3 | H | 83 | |||||
| 3d | H | -CH3 | -CH3 | 63 | |||||
2.2. Suzuki-Miyaura Cross-coupling of the 2-(3-(2-Alkyl-6,8-dibromo-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl)propyl)-6,8-dibromoquinazolin-4(3H)-ones

| R1 | R2 | R3 | Ar | % Yield | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4a | H | H | H | -C6H5 | 80 | |||||
| 4b | H | H | H | 4-FC6H4- | 81 | |||||
| 4c | H | H | H | 4-MeOC6H4- | 74 | |||||
| 4d | -CH3 | H | H | -C6H5 | 63 | |||||
| 4e | -CH3 | H | H | 4-FC6H4- | 63 | |||||
| 4f | -CH3 | H | H | 4-MeOC6H4- | 60 | |||||
| 4g | H | -CH3 | H | -C6H5 | 75 | |||||
| 4h | H | -CH3 | H | 4-FC6H4- | 70 | |||||
| 4i | H | -CH3 | H | 4-MeOC6H4- | 73 | |||||
| 4j | H | -CH3 | -CH3 | -C6H5 | 76 | |||||
| 4k | H | -CH3 | -CH3 | 4-FC6H4- | 77 | |||||
| 4l | H | -CH3 | -CH3 | 4-MeOC6H4- | 81 | |||||
2.3. Photophysical Property Studies of Compounds 4a–l
2.3.1. UV-Vis Absorption Properties of the 2-(3-(2-Alkyl-6,8-diaryl-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl)propyl)-6,8-diarylquinazolin-4(3H)-ones 4a–l
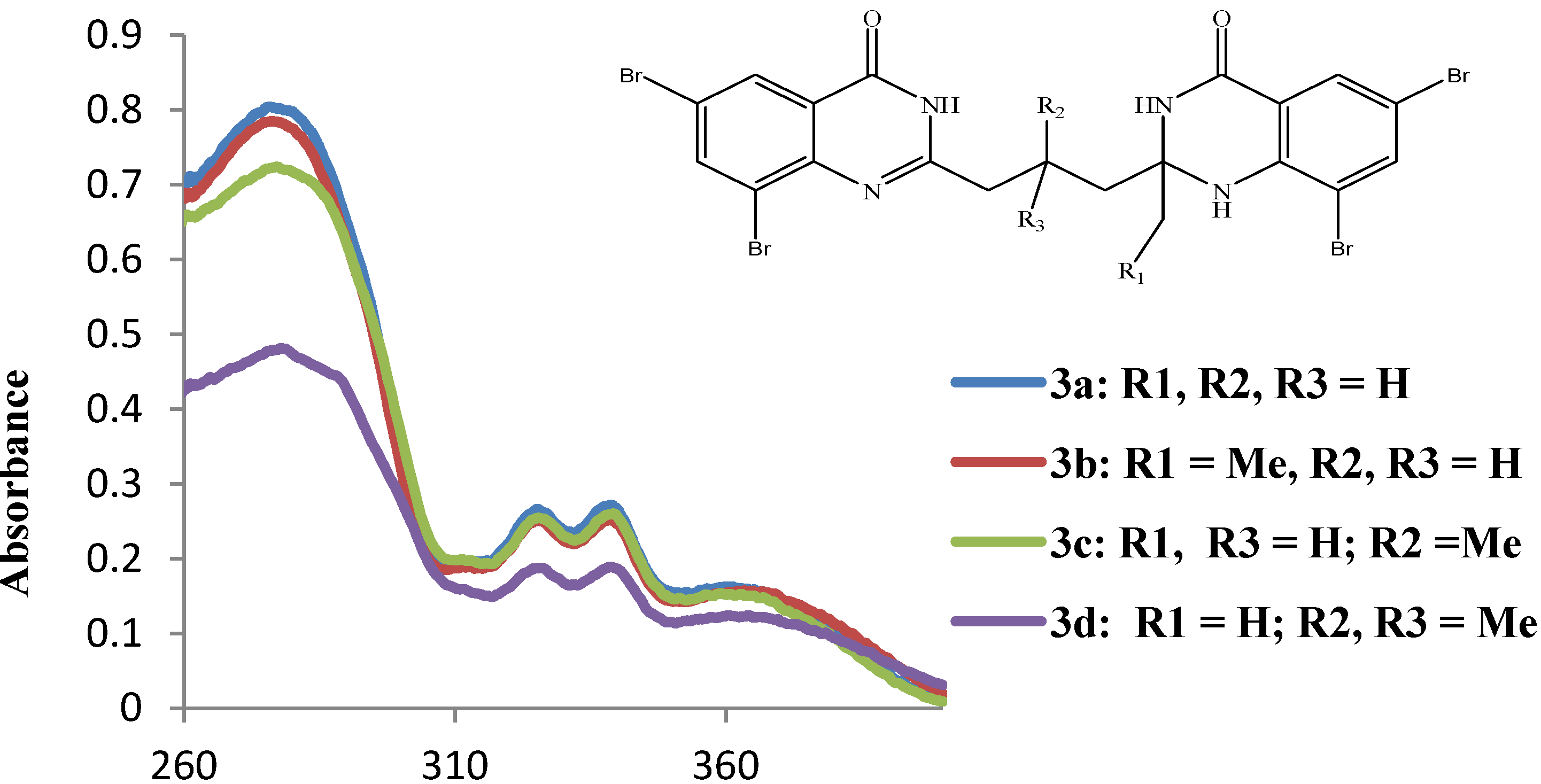
| Compound | λmax (nm) | Molar Extinction Coefficient |
|---|---|---|
| DMSO | (ε) × 104 mol−1 cm−1 | |
| 3a | 275.8, 325.3, 338.8, 360.4 | 1.6 , 0.5, 0.5, 0.3 |
| 3b | 276.1, 325.3, 338.8, 362.2 | 1.5, 0.5, 0.5, 0.3 |
| 3c | 277.0, 325.3, 339.1, 359.5 | 1.4, 0.5, 0.5, 0.3 |
| 3d | 277.9, 325.9, 338.8, 360.4 | 1.0, 0.4, 0.4, 0.2 |
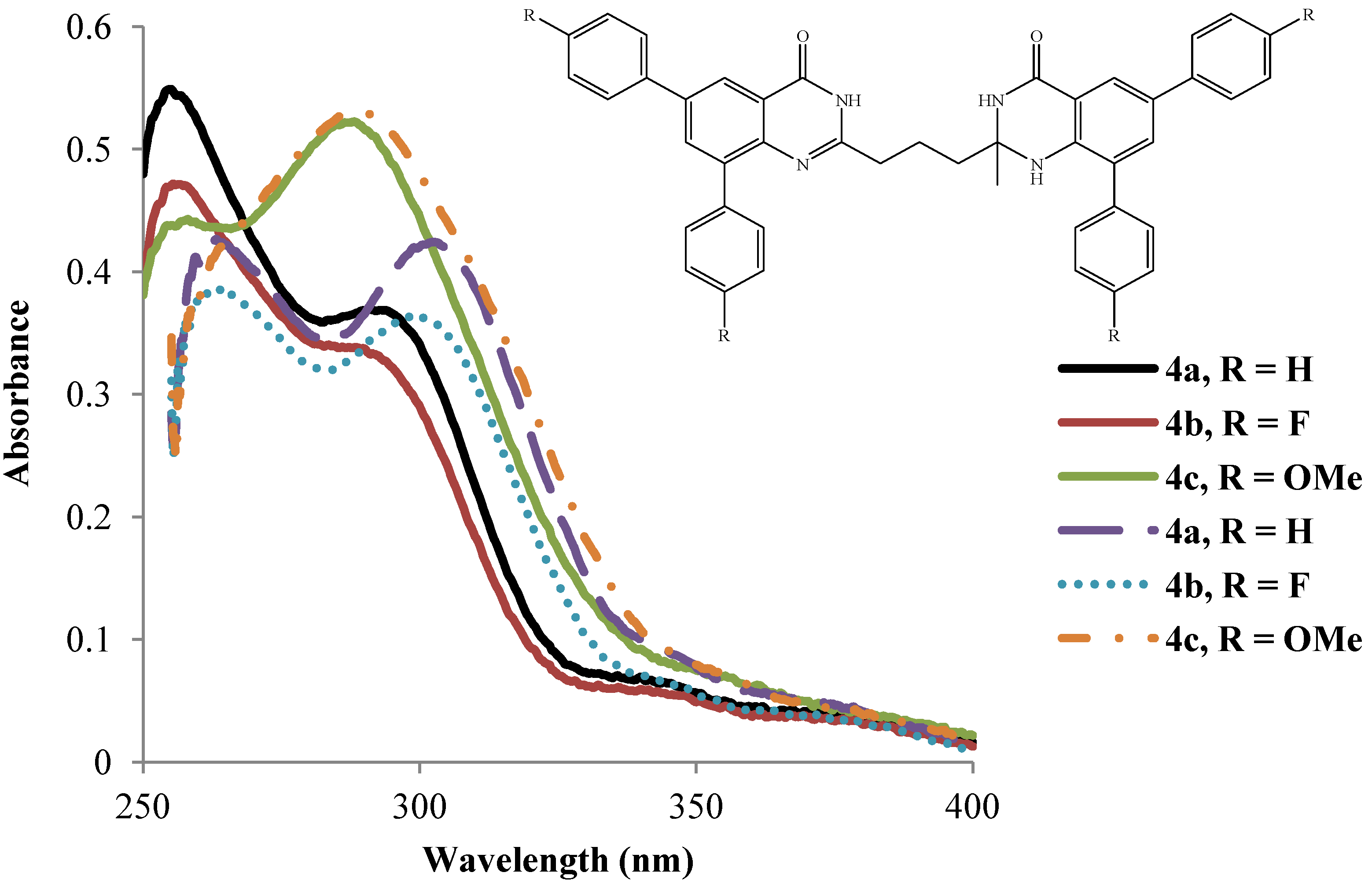
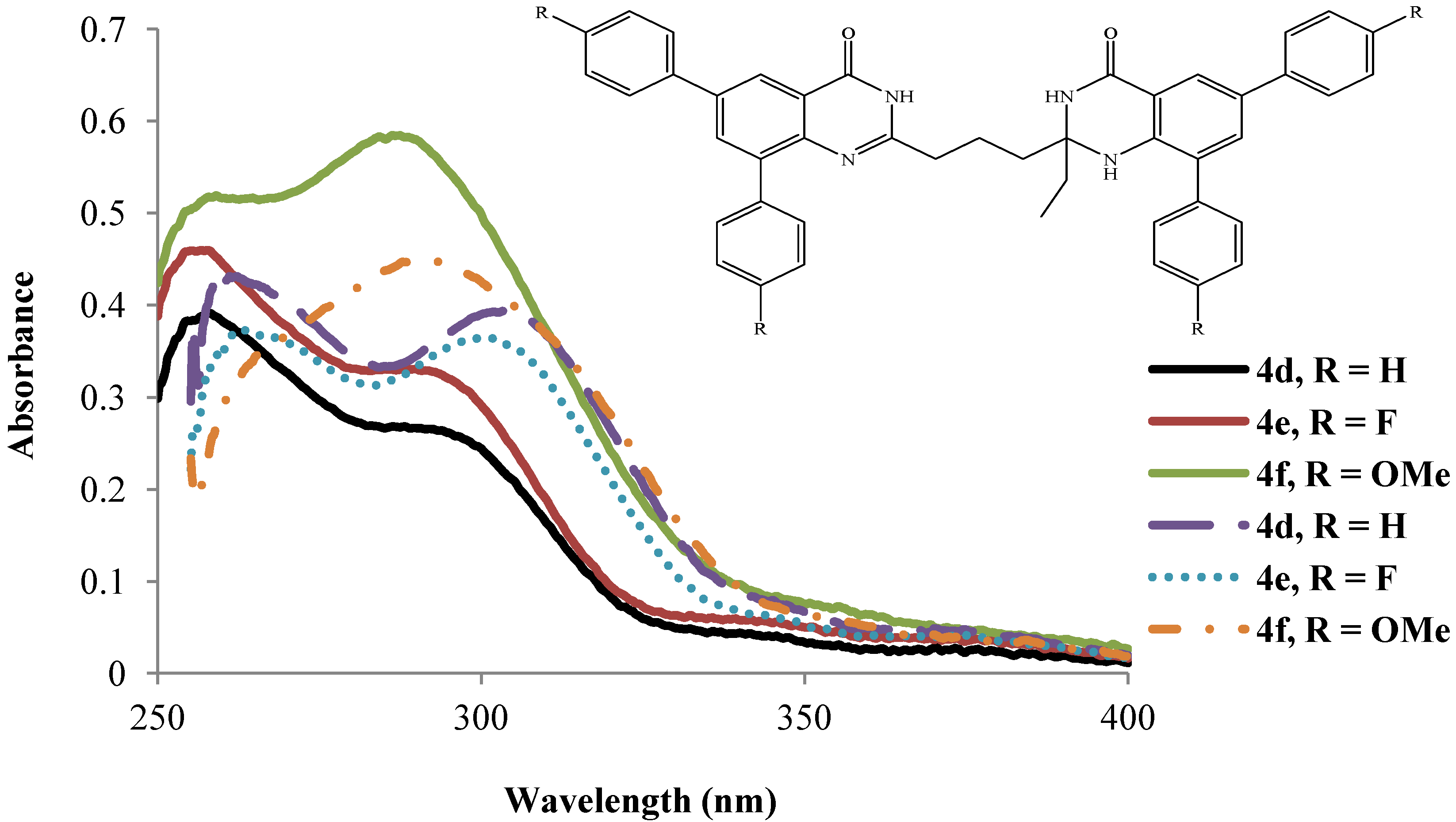


2.3.2. Emission Properties of the 2-(3-(2-Alkyl-6,8-diaryl-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl)propyl)-6,8-diarylquinazolin-4(3H)-ones 4a–l
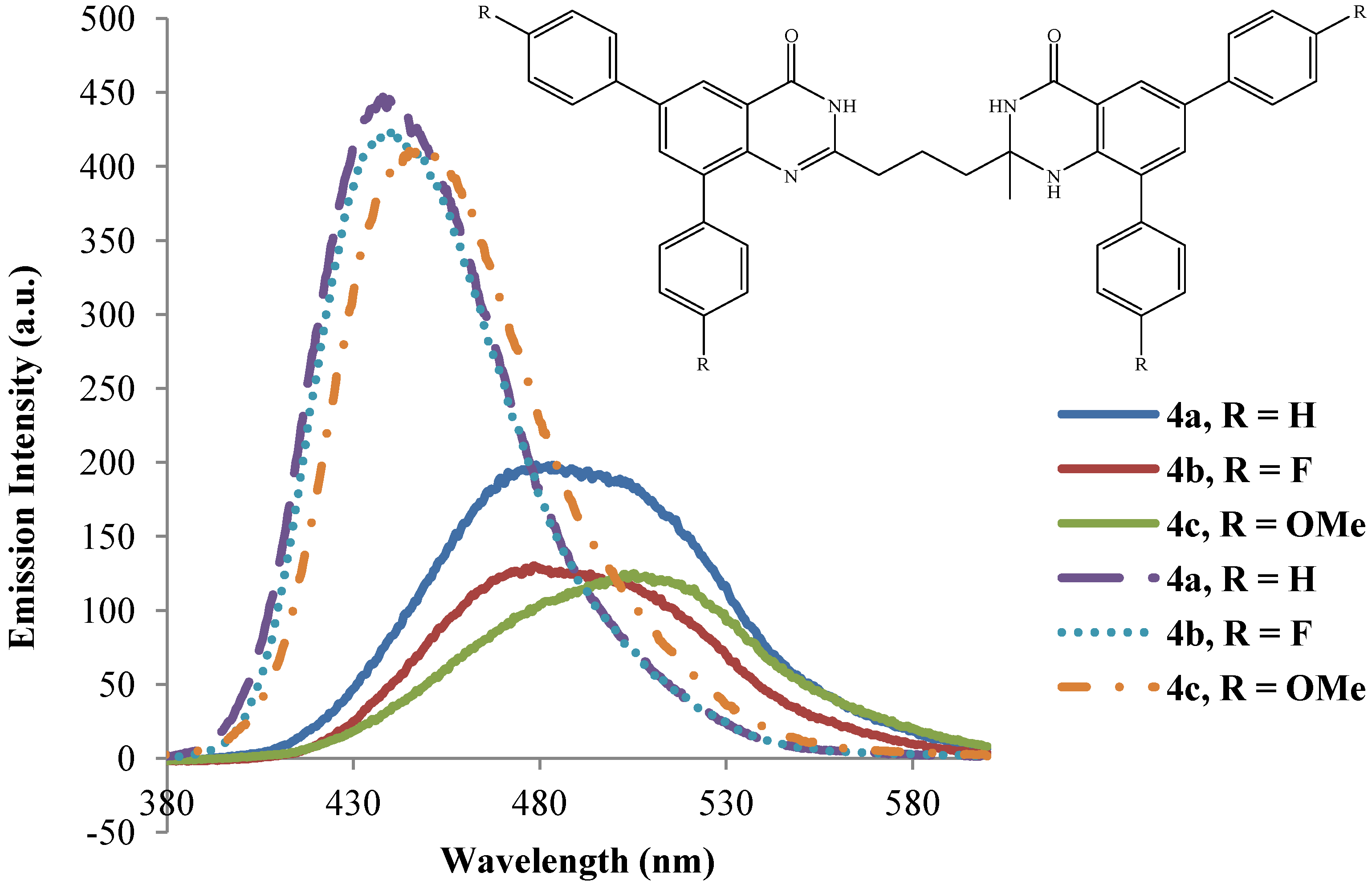
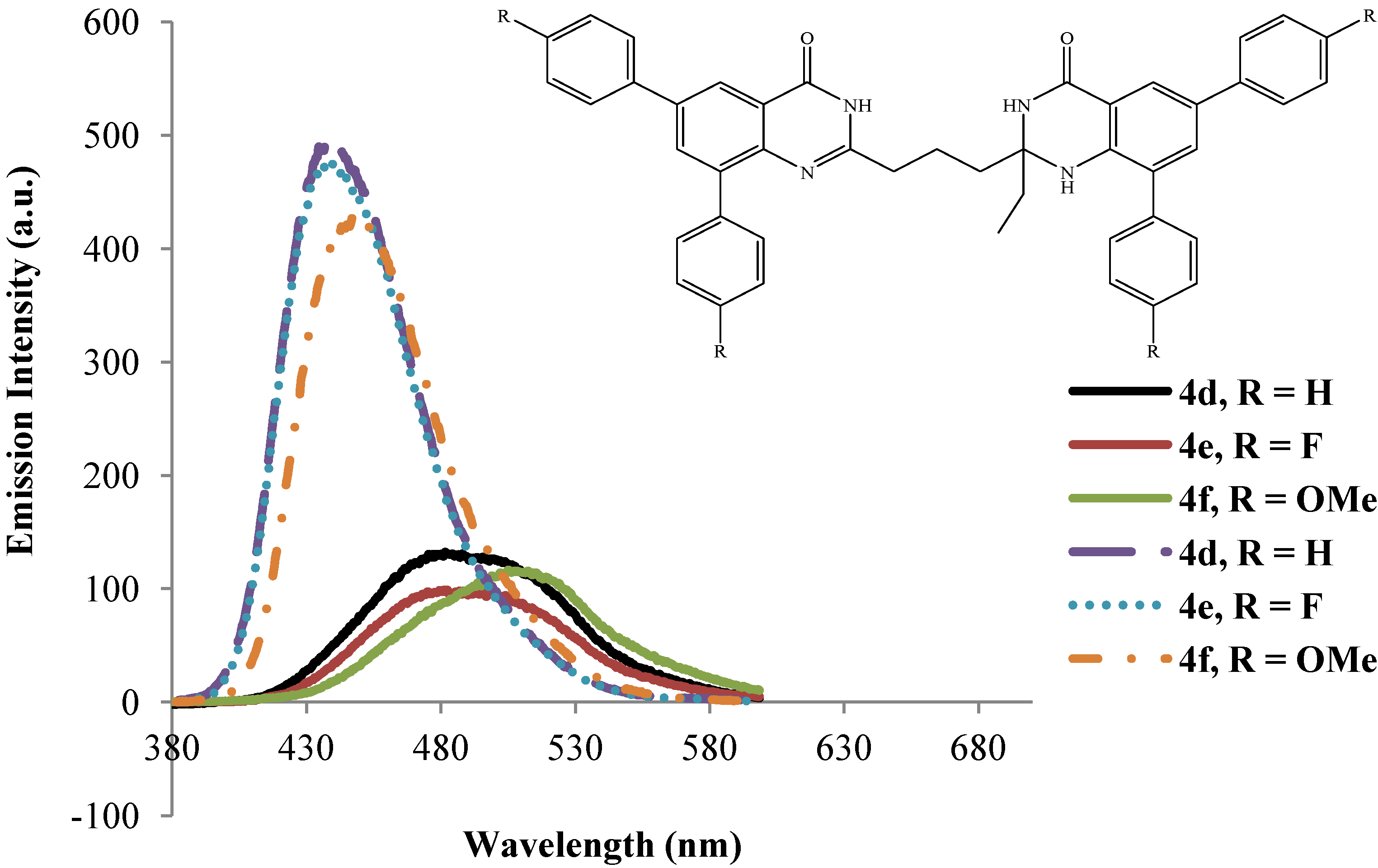

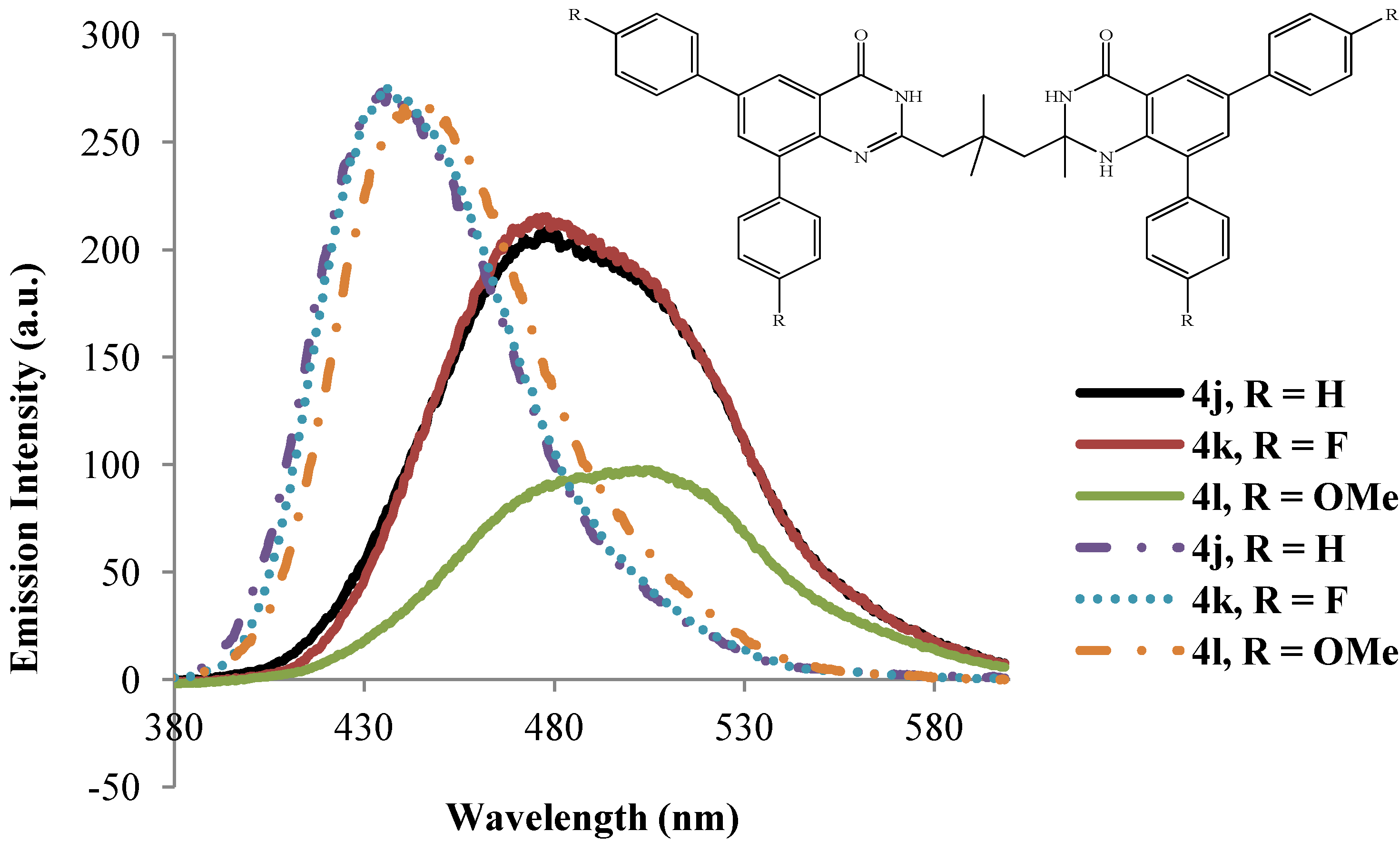
| 4 | λmax (nm) | (ε) × 104 | λmax (nm) | (ε) × 104 | λem (nm) | λem (nm) | (a) Quantum | (a) Quantum | Stokes Shift | Stokes Shift |
|---|---|---|---|---|---|---|---|---|---|---|
| DMSO | Mol−1cm−1 | AcOH | Mol−1cm−1 | DMSO | AcOH | Yields (Φ) × 10−3 | yields (Φ) × 10−3 | |||
| DMSO | AcOH | DMSO | AcOH | |||||||
| 4a | 263.2 | 4.2 | 255.1 | 5.5 | 438.0 | 479.0 | 7.5 | 2.6 | 174.8 | 223.9 |
| 302.5 | 4.2 | 292.3 | 3.7 | 438.0 | 479.0 | 7.5 | 3.8 | 135.5 | 186.7 | |
| 4b | 263.2 | 3.9 | 255.4 | 4.7 | 439.0 | 478.5 | 7.8 | 2.0 | 175.8 | 223.1 |
| 300.1 | 3.6 | 283.6 | 3.4 | 439.0 | 478.5 | 8.3 | 2.7 | 138.9 | 194.9 | |
| 4c | 291.4 | 5.3 | 258.1 | 4.4 | 448.0 | 505.5 | 5.5 | 2.0 | 156.6 | 247.4 |
| 288.1 | 5.2 | 505.5 | 1.7 | 217.4 | ||||||
| 4d | 262.0 | 4.3 | 257.8 | 3.9 | 439.0 | 481.5 | 8.2 | 2.4 | 177.0 | 223.7 |
| 303.4 | 3.9 | 293.8 | 2.6 | 439.0 | 481.5 | 9.0 | 3.6 | 135.6 | 187.7 | |
| 4e | 261.7 | 3.6 | 257.5 | 4.6 | 439.0 | 484.5 | 9.1 | 1.5 | 177.3 | 227.0 |
| 302.2 | 3.6 | 291.4 | 3.3 | 439.0 | 484.5 | 9.3 | 2.1 | 136.8 | 193.1 | |
| 4f | 291.4 | 4.5 | 259.0 | 5.2 | 447.5 | 505.0 | 6.8 | 1.6 | 156.1 | 246.0 |
| 286.3 | 5.8 | 505.0 | 1.4 | 218.7 | ||||||
| 4g | 263.2 | 3.4 | 257.5 | 4.6 | 437.5 | 475.5 | 8.0 | 2.8 | 174.3 | 218.0 |
| 302.5 | 3.2 | 292.3 | 3.2 | 437.5 | 475.5 | 8.4 | 4.1 | 135.0 | 183.2 | |
| 4h | 263.5 | 3.3 | 257.8 | 4.5 | 437.0 | 476.5 | 7.7 | 2.4 | 173.5 | 218.7 |
| 300.4 | 3.2 | 288.4 | 3.3 | 437.0 | 476.5 | 7.8 | 3.3 | 136.6 | 188.1 | |
| 4i | 290.5 | 4.8 | 257.2 | 4.7 | 443.0 | 504.0 | 5.4 | 1.6 | 152.5 | 246.8 |
| 285.1 | 5.2 | 504.0 | 1.5 | 218.9 | ||||||
| 4j | 262.0 | 3.0 | 254.8 | 3.7 | 434.5 | 477.5 | 6.4 | 4.0 | 172.5 | 222.7 |
| 304.0 | 3.0 | 295.3 | 2.7 | 434.5 | 477.5 | 6.4 | 5.5 | 130.5 | 182.2 | |
| 4k | 262.6 | 3.7 | 254.8 | 5.2 | 436.0 | 477.0 | 5.3 | 2.9 | 173.4 | 222.2 |
| 300.1 | 3.7 | 288.1 | 3.7 | 436.0 | 477.0 | 5.3 | 4.2 | 135.9 | 188.9 | |
| 4l | 293.3 | 4.6 | 259.6 | 4.2 | 447.5 | 502.0 | 4.2 | 1.7 | 154.2 | 242.4 |
| 288.7 | 4.8 | 502.0 | 1.5 | 213.3 |
2.4. Quantum Chemical Calculations
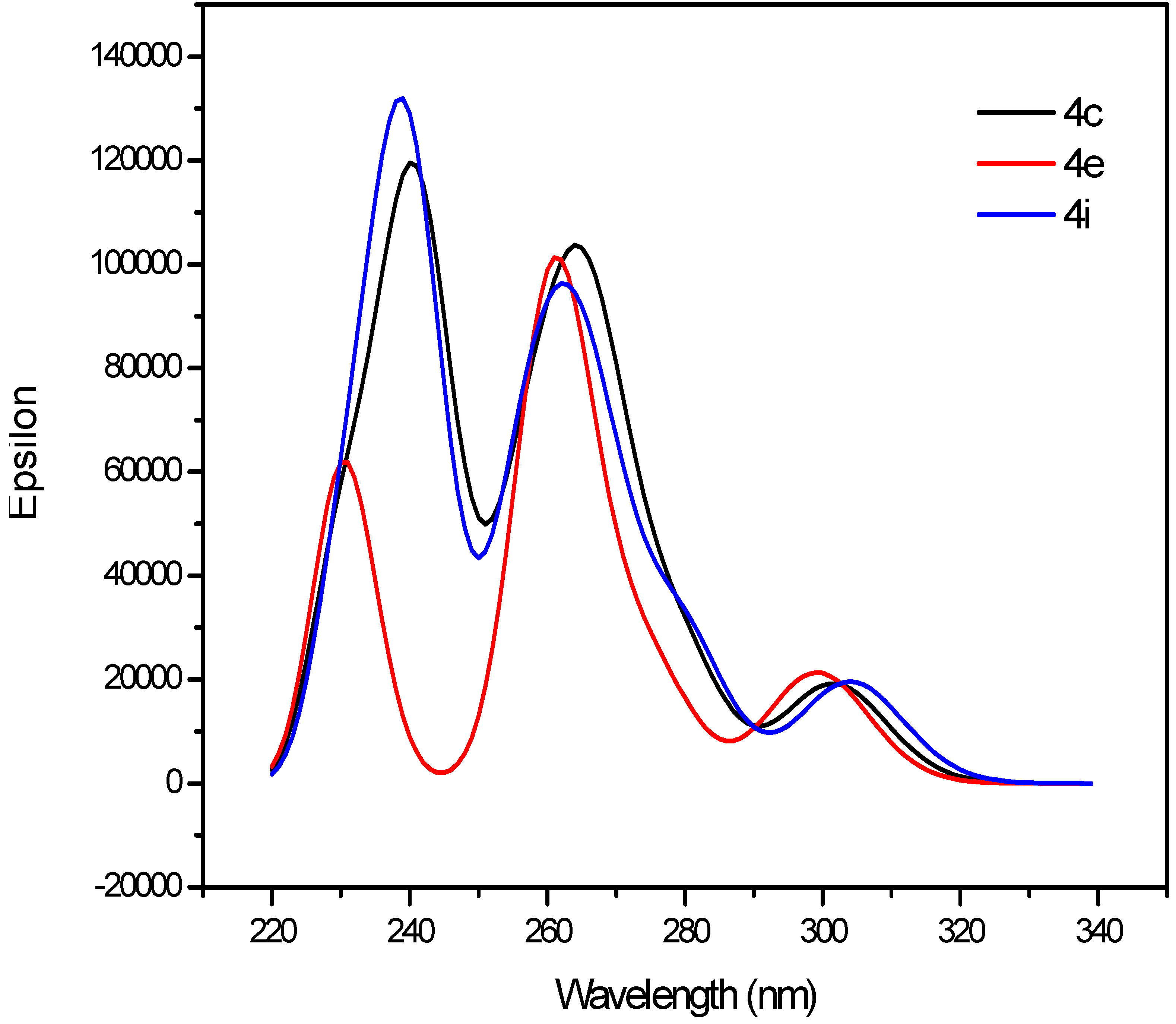
| Compound | Dground (Debye) | Dexcited (Debye) | λmax (nm) |
|---|---|---|---|
| 4a | 7.66 | 8.24 | 260, 299 |
| 4b | 7.45 | 8.37 | 261, 300 |
| 4c | 5.96 | 5.44 | 266, 301 |
| 4d | 7.70 | 8.09 | 261, 299 |
| 4e | 8.19 | 8.33 | 262, 299 |
| 4f | 6.35 | 7.41 | 266, 301 |
| 4g | 5.71 | 6.62 | 262, 301 |
| 4h | 9.84 | 9.92 | 261, 300 |
| 4i | 8.99 | 9.02 | 260, 304 |
| 4j | 5.62 | 6.07 | 262, 299 |
| 4k | 6.45 | 6.47 | 262, 298 |
| 4l | 5.81 | 5.94 | 260, 297 |
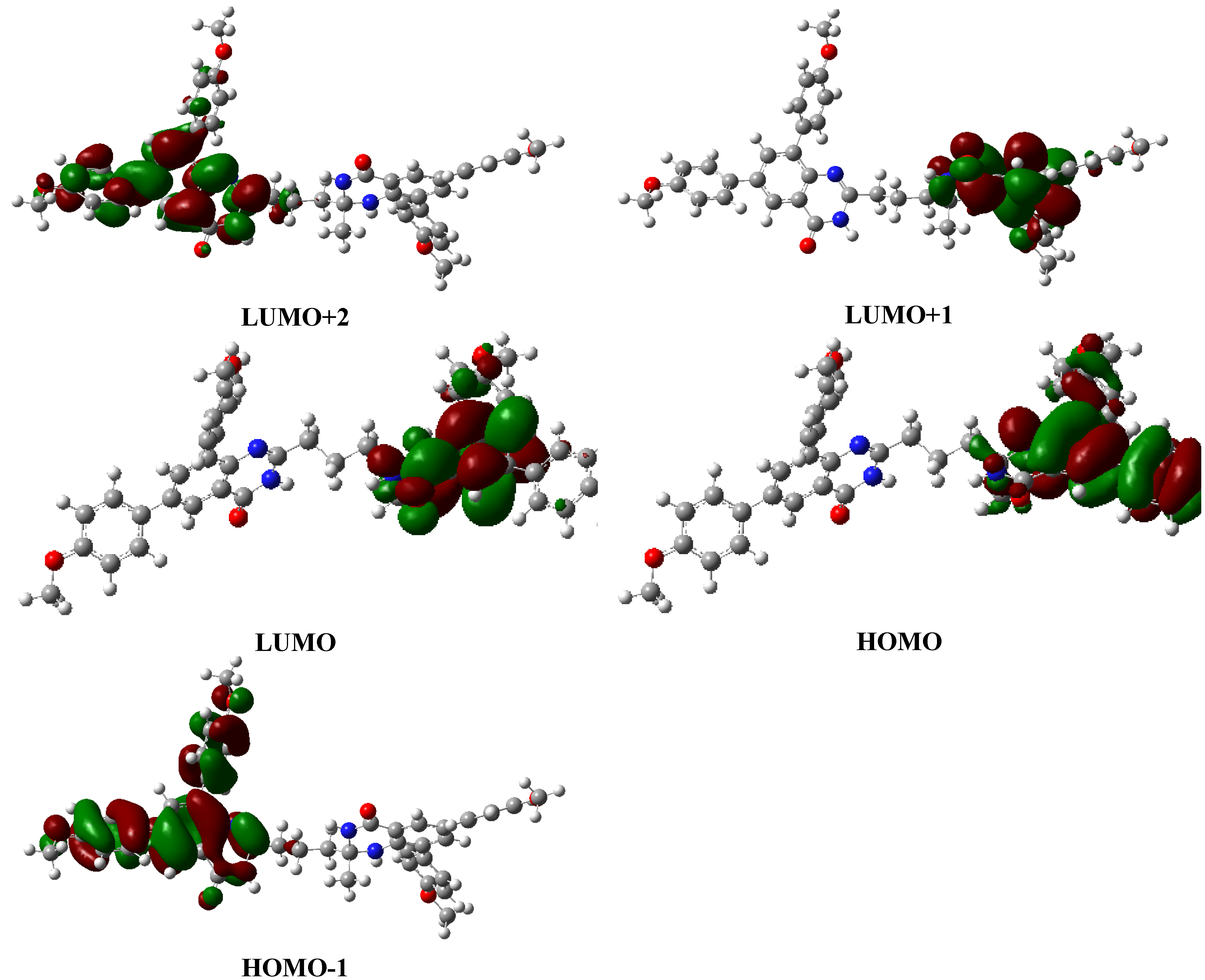
3. Experimental
3.1. General Information
3.2. Synthesis of 3,5-Dibromobenzamide (1)
3.3. Typical Procedure for the Preparation of the 2-(3-(2-Alkyl-6,8-dibromo-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl)-propyl)-6,8-dibromoquinazolin-4(3H)-ones 3a–d
3.4. Typical Procedure for the Suzuki Cross-Coupling of 4a–l
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Egushi, S. Quinazoline analogues and related chemistry. Top. Heterocycl. Chem. 2006, 6, 113–156. [Google Scholar]
- Neil, G.L.; Li, L.H.; Burskirk, H.H.; Moxley, T.E. Antitumor effects of the antispermatogenic agent, 2,3-dihydro-2-(1-naphthyl)-4(1H)-quinazolinone (NSC-145669). Cancer Chemother. 1972, 56, 163–173. [Google Scholar]
- Xia, Y.; Yang, Y.Z.; Hour, M.J.; Kuo, S.C.; Xia, P.; Bastow, K.F.; Nakanishi, Y.; Nampoothiri, P.; Hackl, T.; Hamel, E.; et al. Antitumor agents. Part 204: Synthesis and biological evaluation of substituted 2-aryl quinazolinones. Bioorg. Med. Chem. Lett. 2001, 11, 1193–1196. [Google Scholar]
- Abdel Gawad, N.M.A.; Georgey, H.H.; Youssef, R.M.; El-Sayed, N.A. Synthesis and antitumor activity of some 2,3-disubstituted quinazolin-4(3H)-ones and 4,6-disubstituted-1,2,3,4-tetrahydroquinazolin-2H-ones. Eur. J. Med. Chem. 2010, 45, 6058–6067. [Google Scholar]
- Hour, M.-J.; Huang, L.-J.; Kuo, S.-C.; Bastow, K.; Nakanishi, Y.; Hamel, E.; Lee, K.-H. 6-Alkylamino- and 2,3-dihydro-3ꞌ-methoxy-2-phenyl-4-quinazolinones and related compounds: Their synthesis, cytotoxicity, and inhibition of tubulin polymerization. J. Med. Chem. 2000, 43, 4479–448. [Google Scholar]
- Sim, Y.-L.; Omer, N.; Khan, M.N.; Pratt, L.M. Ultraviolet–visible study on acid-base equilibria of 2-substituted-4(3H)-quinazolinones. Tetrahedron 2013, 69, 2524–2533. [Google Scholar]
- Bakalova, S.M.; Santos, A.G.; Timcheva, I.; Kaneti, J.; Filipova, I.L.; Dobrikov, G.M.; Dimitrov, V.D. Electronic absorption and emission spectra and computational studies of some 2-aryl, 2-styryl, and 2-(4-aryl)butadienyl quinazolin-4-ones. J. Mol. Struct.-Theochem 2004, 710, 229–234. [Google Scholar] [CrossRef]
- Eshimberov, A.; Kristallovich, E.; Abullaev, N.; Tulyaganov, T.; Shakhidoyatov, K.M. AM1/CI, CNDO/S and ZINDO/S computations of absorption bands and their intensities in the UV spectra of some 4(3H)-quinazolinones. Spectrochim. Acta A 2006, 65, 299–307. [Google Scholar]
- Zerner, M.C. Semiempirical molecular orbital methods. In Reviews in Computational Chemistry; Lipkowitz, K.B., Boyd, D.B., Eds.; VCH Publishing: New York, NY, USA, 1991; Volume 2, p. 313. [Google Scholar]
- Baghbanzadeh, M.; Salehi, P.; Dabiri, M.; Kozehgary, G. Water-accelerated synthesis of novel bis-2,3-dihydroquinazolin-4(1H)-one derivatives. Synthesis 2006, 344–348. [Google Scholar]
- Reddy, G.M.; Reddy, P.S.N. Antibacterial acivity of some N,N′-linked bisazaheterocycles, benzimidazoline and quinoxaline derivatives. Rasayan J. Chem. 2008, 1, 179–184. [Google Scholar]
- Reddy, P.S.N.; Mittapelli, V.; Reddy, V.D. Antibacterial, antifungal and antifeedant activity of quinazolinonyl-β-lactams/quinazolinones and bis(quinazolinonyl-β-lactams). Rasayan J. Chem. 2010, 3, 635–640. [Google Scholar]
- Liu, Y.; Lu, L.; Zhou, Y.-J.; Wang, X.-S. Green synthesis of bis-quinazolinone derivatives catalyzed by iodine in ionic liquids. Res. Chem. Intermediat. 2013. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, M.-M.; Jiang, H.; Wang, X.-S. Structurally diversified products from the reactions of 2-aminobenzamides with 1,3-cyclohexanediones catalyzed by iodine. Tetrahedron Lett. 2013, 54, 757–760. [Google Scholar]
- Mphahlele, M.J.; Paumo, H.K.; El-Nahas, A.M.; El-Hendawy, M.M. Synthesis and photophysical property studies of the 2,6,8-triaryl-4-(phenylethynyl)quinazolines. Molecules 2014, 19, 795–818. [Google Scholar]
- Maluleka, M.M.; T.A. Khoza, T.A. Suzuki Cross-coupling of the 2-aryl-6,8-dibromo-2,3-dihydroquinazolin-4(1H)-ones and transformation of the resulting 2,6,8-triaryl-2,3-dihydroquinazolin-4(1H)-ones. Bull. Chem. Soc. Ethiop. 2014, 28, 81–90. [Google Scholar]
- Garcia, Y.; Schoenebeck, F.; Legault, C.Y.; Merlic, C.A.; Houk, K.N. Theoretical bond dissociation energies of halo-heterocycles: Trends and relationships to regioselectivity in palladium-catalyzed cross-coupling reactions. J. Am. Chem. Soc. 2009, 131, 6632–6639. [Google Scholar]
- Kappaun, S.; Sovic, T.; Stelzer, F.; Pogantsch, A.; Zojer, E.; Slugovc, C. Molecular fluorescent pH-probes based on 8-hydroxyquinoline. Org. Biomol. Chem. 2006, 4, 1503–1511. [Google Scholar]
- Amatore, C.; Jutand, A. Mechanistic and kinetic studies of palladium catalytic systems. J. Organomet. Chem. 1999, 576, 254–278. [Google Scholar]
- She, W. Palladium catalyzed coupling of aryl chlorides with arylboronic acids. Tetrahedron Lett. 1997, 38, 5575–5578. [Google Scholar]
- Haman, B.C.; Hartwig, J.F. Sterically hindered chelating alkyl phosphines provide large rate accelerations in palladium-catalyzed amination of aryl iodides, bromides, and chlorides, and the first amination of aryl tosylates. J. Am. Chem. Soc. 1998, 120, 7369–7370. [Google Scholar]
- Barnard, C. Palladium-catalysed C–C coupling: Then and now. Platinum Met. Rev. 2008, 52, 38–45. [Google Scholar]
- Wang, P.; Wu, S. A study on the spectroscopy and photophysical behaviour of chalcone derivatives. J. Photochem. Photobiol. A 1994, 77, 127–131. [Google Scholar]
- Raju, B.B.; Varadarajan, T.S. Spectroscopic studies of 7-diethylamino-3-styryl coumarins. J. Photochem. Photobiol. A 1995, 85, 263–267. [Google Scholar]
- Buhaceanu, R.; Lungu, N.C.; Forna, N.C.; Asaftei, I.V.; Chirita, P.; Birsa, M.L. The influence of bromine atoms on optical properties of some 1,3-dithiolium derivatives. Rev. Chim.-Bucharest 2013, 64, 960–964. [Google Scholar]
- Aaron, J.J.; Tine, A.; Gaye, M.D.; Parkanyi, C.; Boniface, C.; Bieze, T.W.N. Effects of solvent on the electronic absorption and fluorescence spectra of quinazolines, and determination of their ground and excited singlet-state dipole moments. Spectrochim. Acta A 1991, 47, 419–430. [Google Scholar]
- Harriman, A.; Rockett, B.W. Comparative study of spin-orbital coupling for halogenated ethylbenzenes by a study of their fluorescence. J. Chem. Soc. Perkin Trans. 2 1974, 217–219. [Google Scholar]
- Homocianu, M.; Airinei, A.; Dorohoi, D.O. Solvent effects on the electronic absorption and fluorescence spectra. J. Adv. Res. Phys. 2011, 2, 011105. [Google Scholar]
- Diaz, A.N. Absorption and emission spectroscopy and photochemistry of 1,10-anthraquinone derivatives: A review. J. Photochem. Photobiol. A 2009, 53, 141–167. [Google Scholar]
- Reichardt, C. Solvents and Solvent Effects in Organic Chemistry, 3rd ed.; Wiley-VCH: Weinheim Germany, 2003. [Google Scholar]
- Tsin, A.T.C.; Pedrozo-Fernandez, H.A.; Gallas, J.M.; Chambers, J.P. The fluorescence quantum yield of vitamin A2. Life Sci. 1988, 43, 1379–1384. [Google Scholar]
- Iikura, H.; Tsuneda, T.; Yanai, T.; Hirao, K. Long-range correction scheme for generalized-gradient-approximation exchange functionals. J. Chem. Phys. 2001, 115, 3540–3544. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comp. Chem. 2003, 24, 669–681. [Google Scholar]
- Wang, S.; Lou, H.; Liu, Y.; Yu, G.; Lu, P.; Zhu, D. Greenish-yellow electroluminescent devices using a novel dihydroquinazolinone derivative as emitting layer. J. Mater. Chem. 2001, 11, 2971–2973. [Google Scholar]
- Sample Availability: Samples of the compounds 1-4 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mmonwa, M.M.; Mphahlele, M.J.; El-Hendawy, M.M.; El-Nahas, A.M.; Koga, N. Synthesis and Photophysical Properties of the 2-(3-(2-Alkyl-6,8-diaryl-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl)propyl)-6,8-diarylquinazolin-4(3H)-ones. Molecules 2014, 19, 9712-9735. https://doi.org/10.3390/molecules19079712
Mmonwa MM, Mphahlele MJ, El-Hendawy MM, El-Nahas AM, Koga N. Synthesis and Photophysical Properties of the 2-(3-(2-Alkyl-6,8-diaryl-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl)propyl)-6,8-diarylquinazolin-4(3H)-ones. Molecules. 2014; 19(7):9712-9735. https://doi.org/10.3390/molecules19079712
Chicago/Turabian StyleMmonwa, Mmakwena M., Malose J. Mphahlele, Morad M. El-Hendawy, Ahmed M. El-Nahas, and Nobuaki Koga. 2014. "Synthesis and Photophysical Properties of the 2-(3-(2-Alkyl-6,8-diaryl-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl)propyl)-6,8-diarylquinazolin-4(3H)-ones" Molecules 19, no. 7: 9712-9735. https://doi.org/10.3390/molecules19079712
APA StyleMmonwa, M. M., Mphahlele, M. J., El-Hendawy, M. M., El-Nahas, A. M., & Koga, N. (2014). Synthesis and Photophysical Properties of the 2-(3-(2-Alkyl-6,8-diaryl-4-oxo-1,2,3,4-tetrahydroquinazolin-2-yl)propyl)-6,8-diarylquinazolin-4(3H)-ones. Molecules, 19(7), 9712-9735. https://doi.org/10.3390/molecules19079712