Abstract
This work deals with the formation of supramolecular complexes between ascorbic acid (AA), the guest, and β-cyclodextrin (β-CD), the host, that was first potentiodynamically immobilized on the surface of a carbon paste electrode (CPE) throughout the formation of a β-CD-based conducting polymer (poly-β-CD). With the bare CPE and the β-CD-modified CPE, an electrochemical study was performed to understand the effect of such surface modification on the electrochemical response of the AA. From this study it was shown that on the modified-CPE, the AA was surface-immobilized through formation of an inclusion complex with β-CD, which provoked the adsorption of AA in such a way that this stage became the limiting step for the electrochemical oxidation of AA. Moreover, from the analysis of the experimental voltammetric plots recorded during AA oxidation on the CPE/poly-β-CD electrode surfaces, the Gibbs’ standard free energy of the inclusion complex formed by the oxidation product of AA and β-CD has been determined for the first time, ∆G0inclus = −36.4 kJ/mol.
1. Introduction
Ascorbic acid, AA, also termed vitamin C (Figure 1a), is quite an important biocomponent widely present in living organisms [1] as anti-oxidation promoter. However, such a wide scale presence in diverse biological media can also be seen as the origin of interference problems, particularly when analytical research studies aim to effect quantitative determinations of other biologically relevant analytes, which happen to be present in much smaller concentrations, but that are nonetheless specifically important, such as the catecholamines (CAs). The latter produce, as a result of chemical or electrochemical interactions, an oxidation signal overlapping that of the AA, in particular when undertaking quantitative determinations of either and both are present in the same solution [2,3,4,5,6,7].
In chemometric terms, it is not just this last problem that needs to be contended with, but also the acute disparity of concentrations in biological samples and the actual signal separation of both analytes that has given rise to quite a significant interest in characterizing the electrochemical behavior of AA in different systems. For instance, during the quantitative determination of CAs using electrochemical techniques AA is considered an interfering analyte [8,9,10,11,12,13]. Therefore, diverse electrode surface modifying agents have been proposed in order to overcome this problem. In the studies carried out to form supramolecular complexes [14,15,16] with CAs in the presence of the AA [2,3,4,5,6,7,17], it was important to also study the supramolecular interactions that the electrode surface-modifying agents may induce in the AA, specifically when membranes are formed. Some of these studies have been done with β-cyclodextrin, β-CD, which comprises 7 α-D-glucopyranose units [18,19] (Figure 1b). Its interaction with organic, inorganic and biological molecules has seemed possible because the molecular structure of the β-CD resembles a truncated cone basket, where the inner surface of the cavity displays a hydrophobic character, and is capable of hosting some of the said molecules, provided they have the polarities and sizes adequate to fit and form guest-host-type complexes. On consideration of the aforementioned results, this work studies the influence of surface–modifying agents like β-CD in the form of its polymer poly-β-CD [20,21 on a carbon paste electrode, CPE, [22,23] used to analyze the supramolecular interactions with the AA. It is important to mention that some complex polymers that include β-CD in their chemical structure have been reported for the controlled release of drugs [24,25,26,27].
2. Results and Discussion
2.1. Electrochemical Behavior of AA over a CPE
Figure 2 shows typical experimental cyclic voltammograms, CVs, recorded in the system CPE/0.1 M NaCl, at pH 3.0, and 1 mM AA, within the −200 to 1200 mV potential window. When the potential scan started in the anodic direction an oxidation process can be observed through the peak at Epa = 574 mV at 100 mV s−1 scan rate. Subsequently, reverting the scan direction there were no reduction peaks displayed, therefore, the electrochemical process conducted is considered irreversible. When plotting the jpa as a function of v1/2 (see the inset in Figure 2), a linear trend was obtained, thus indicating that diffusion is the rate controlling step for the oxidation process under study [28].
Figure 1.
Molecular structures of: (a) Ascorbic acid, (5R)-[(1S)-1,2-dihydroxyethyl]-3,4-dihydroxyfuran-2(5H)-one, and (b) β-cyclodextrin, C42H70O35 [27].
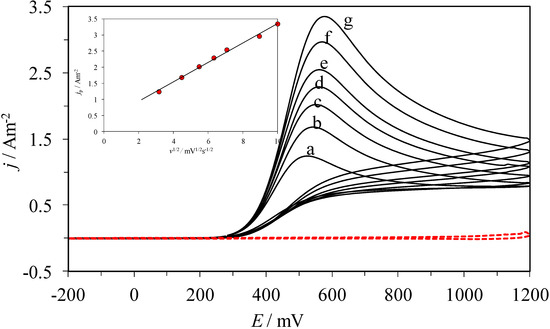
Figure 2.
Family of cyclic voltammograms recorded in the system CPE/0.1 M NaCl, 1 mM AA at pH 3.0, at the different scan rates, ν, indicated: (a) 10, (b) 20, (c) 30, (d) 40, (e) 50, (f) 80, (g) 100, mV s−1. The inset shows the variation of the oxidation peak’s current density, jp, as a function of ν1/2, where the dots (˙) correspond to the experimental data and the line to the linear fitting. The broken line corresponds to the cyclic voltammogram recorded in the same system but in the absence of AA.
2.2. Synthesis of the β-CD Polymer, Poly-β-CD
Figure 3a shows the electrochemical response recorded during the electropolymerization of β-CD over the CPE through application of 20 consecutive CV cycles. It can be clearly noted that as the cycle number increased, the current density also increased, which portrays the typical behavior of the process of electrochemical formation of a conducting polymer [29,30,31]. Moreover, as an example of the morphology of the poly-β-CD electrodeposited, Figure 3b,c show an AFM and a SEM image, respectively, of the indium tin oxide (ITO) electrode surface after the potentiodynamic deposition of poly-β-CD. It is important to note that both rod- and sphere-like poly-β-CD deposition can be recognized from the AFM and SEM images, which are related to a progressive nucleation mechanisms involving differently aged nuclei; the larger ones with spherical shapes seem to be older than the smaller rod-like deposits which grew at later stages. From concentration differences, before and after β-CD polymerization, the evaluation of the amount of β-CD molecules that were deposited was obtained. The [β-CD] was evaluated from a polarimetry calibration plot, of the specific rotation evaluated at wavelength of 545 nm, [α]546, as a function of [β-CD]. This way, it was found that 4.52 × 1018 β-CD molecules were used to form the poly-β-CD.
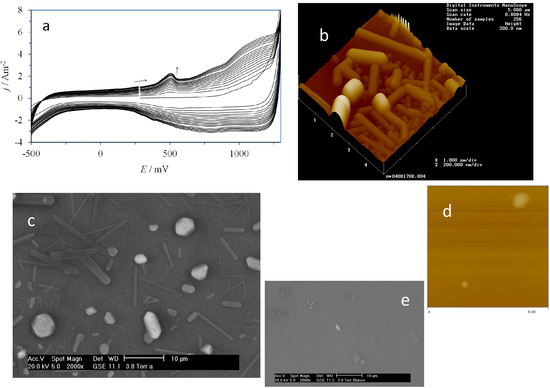
Figure 3.
(a) Experimental CV plots recorded during formation of the β-CD polymer by applying 20 consecutive cycles on the surface of the CPE in a solution of 0.01 M β-CD and 1 M HClO4; (b) AFM and (c) SEM images taken on the surface of an indium tin oxide (ITO) electrode after the electrodeposition of poly-β-CD; The images of the bare surface of the ITO electrode are shown in (d) using AFM and (e) using SEM.
2.3. Electrochemical Behavior of AA over a CPE/Poly-β-CD Electrode
Figure 4 shows the family of CVs recorded in the system CPE/poly-β-CD/1 mM AA, 0.1 M NaCl at pH 3.0, at different potential scan rates. Just like in the previous case, only one oxidation peak is observed, which corroborates that the process is still irreversible, although the oxidation peak is located at about 200 mV, a comparatively smaller potential. When plotting the jpa as a function of v, (see the inset in Figure 4), a linear trend is obtained in the 10 to 100 mV s−1 potential range, which indicates that the process is now adsorption-controlled [28].
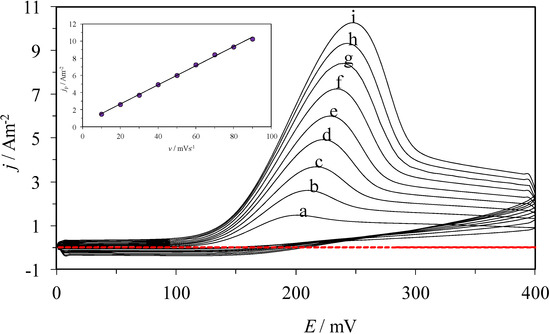
Figure 4.
(a) CVs obtained from the system CPE/poly-β-CD/1 mM AA, 0.1 M NaCl, at pH 3.0, at different potential scan rates, as indicated: (a) 10, (b) 20, (c) 30, (d) 40, (e) 50, (f) 60, (g) 70, (h) 80, (i) 90, mV s−1. The inset shows the variation of the peak’s current density, jp, as a function of ν, the circles correspond to experimental data (○) of the anodic peak, while the line corresponds to the linear fitting. The broken line corresponds to the cyclic voltammogram recorded in the same system but in the absence of AA.
The AA adsorption on the CPE/poly-β-CD can be explained by the formation of an inclusion complex between the AA and the β-CD molecules that constitute the poly-β-CD polymer, see Scheme 1. Notwithstanding that Scheme 1 is a representation of the adsorption process of AA on the surfaces of the poly-β-CD modified CPE, it is based on our previous study [32] regarding a quantum chemical study of the stability of the complex formed by the neutral form of AA, that predominate at pH 3.0, where the pKa value of AA is 4.17 [33], and β-CD. In this study [32] we found that two different structures are energetically favored for this inclusion complex namely that formed by the interaction through the functional hydroxyl groups of the lactone in AA and the primary hydroxyl groups of the β-CD or the β-CD-AA complex formation through the alcohol group of AA, as is represented in Scheme 1. Furthermore, it should be added that the value of the thermodynamic constant of this inclusion complex formation has been reported by our group from both spectrophotometric and electrochemical calculations [18].
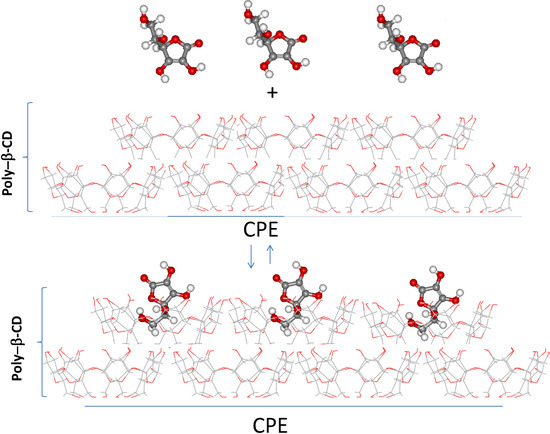
Scheme 1.
Surface inclusion complex formation between AA and β-CD immobilized on the surface of a CPE.
Considering that the species adsorbed are electroactive [21,32,34,35], Equation (1) has been proposed to describe the i-E experimental plots [34,35]:
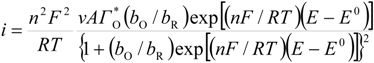 where A is the electrode surface area, n is the number of electrons transferred during the heterogeneous reaction, v is the potential scan rate, R, T and F are the universal gas constant, absolute temperature and Faraday constant, respectively. E0 is the formal potential, Γо* is the surface coverage, bO and bR are related with the Gibbs’ standard free energy of the surface inclusion complex formation, ∆G0inclusion, see Scheme 1, of the AA’s oxidized (O) and reduced (R) species, respectively.
where A is the electrode surface area, n is the number of electrons transferred during the heterogeneous reaction, v is the potential scan rate, R, T and F are the universal gas constant, absolute temperature and Faraday constant, respectively. E0 is the formal potential, Γо* is the surface coverage, bO and bR are related with the Gibbs’ standard free energy of the surface inclusion complex formation, ∆G0inclusion, see Scheme 1, of the AA’s oxidized (O) and reduced (R) species, respectively.
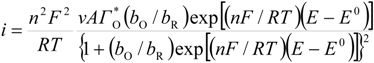
The peaks’ potentials and the currents are given as follows:
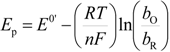
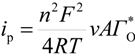
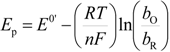
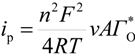
Substituting Equations (2) and (3) in (1) Equation (4) results in:
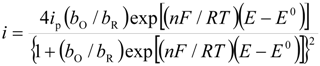
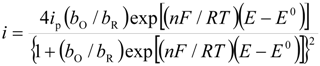
Parameterizing Equation (4) Equation (5) results in:
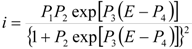 where:
where:
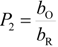
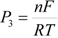
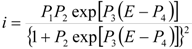
P1 = 4ip
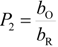
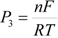
P4 = E0
Performing a non-linear fitting procedure to Equation (1), allows one to obtain theoretical CVs that can be compared to the experimental ones of the system CPE/poly-β-CD/1 mM AA, 0.1 M NaCl, at pH 3.0 and at 10 mV s−1 (Figure 5), where the adsorption model observed, derived from Equation (1), adequately describes the results from the experimental electrochemical oxidation taking place on the poly-β-CD-modified CPE. It is important to note that the fitting was quite good, regardless of the scan rate used. From this analysis, the values of the best fit parameters P1 to P4 were obtained. In particular, from the value of the parameter P2 obtained at 50 mV s−1 (1.7) and the thermodynamic constant value obtained through electrochemical techniques, reported in [18], for the inclusion complex between the reduced form of AA and β-CD, (lnKincl. = 8.52), the ∆G0inclus. of the oxidation product of AA was obtained as −36.4 kJ/mol. To the best of the authors’ knowledge, this is the first time that the Gibbs’ standard free energy of formation for this inclusion complex has been determined.
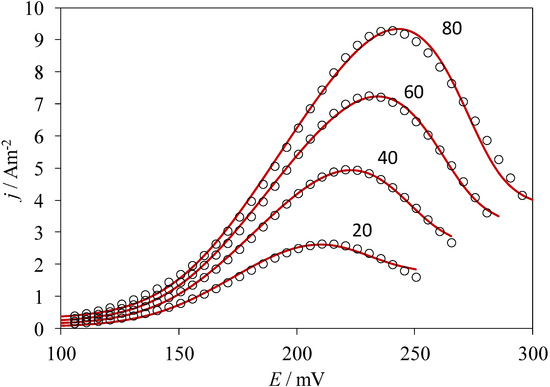
Figure 5.
Comparison of the experimental CVs from the system CPE/poly-β-CD/1 mM AA, 0.1 M NaCl, at pH 3.0 (points) and theoretical plots (lines) generated through linear non-linear fitting of Equation (1), at different potential scan rates as indicated, in mV s−1.
In order to corroborate further that the AA molecules were indeed adsorbed on the surfaces of the poly-β-CD-modified CPE, this electrode was immersed in an aqueous solution containing AA for a few minutes, after which the electrode was withdrawn and immediately placed in another aqueous solution containing solely NaCl to perform a CV experiment. Figure 6 depicts two experimental CVs recorded in the system CPE/poly-β-CD/0.1 M NaCl, at pH 3.0, where it is possible to note that when the CPE/poly-β-CD electrode was previously immersed in the AA solution an oxidation peak becomes apparent due to AA oxidation, otherwise there were none.
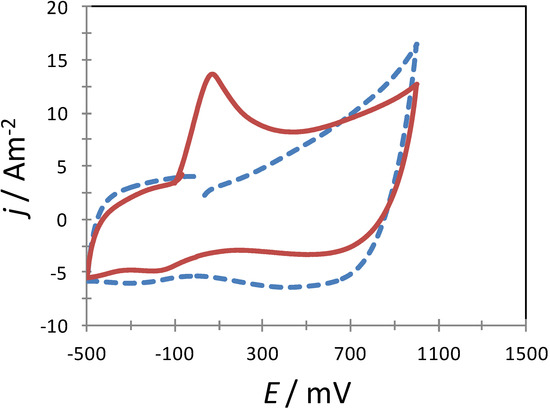
Figure 6.
Experimental CVs recorded in the system CPE/poly-β-CD/0.1 M NaCl, at pH 3.0 (points) at 120 mV s−1 scan rate. In one case (solid line) the CPE/poly-β-CD was previously immersed in 1 mM AA, 0.1 M NaCl, at pH 3.0 for 5 min. whereas in the other case (broken line) the electrode was not exposed to the AA solution.
Figure 7a shows a comparison of CV plots recorded during AA oxidation using the electrodes considered in this work. It is possible to note that both the current density and the anodic peaks’ potential vary drastically as a function of the electrode used.
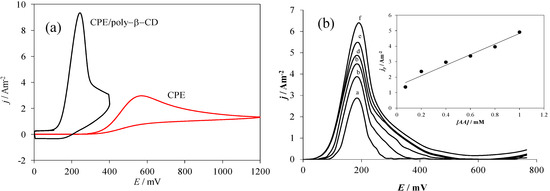
Figure 7.
(a) Comparison of experimental CV plots recorded in the system electrode/0.1 mM AA, 0.1 M NaCl at pH 3.0, for different electrodes as indicated in the Figure, in both cases at 80 mV s−1 potential scan rate. (b) Family of Differential Pulse Voltammetry plots recorded in the system CPE/poly-β-CD/0.1 M NaCl, at pH 3.0 and different AA concentration: (a) 0.07, (b) 0.1. (c) 0.3, (d) 0.6, (e) 0.8 and (f) 1 mM, at a scan rate of 20 mVs−1, the inset show the calibration plot for AA quantification.
When modified electrodes were used, the respective peak potential, Ep,m, moves towards less positive potential values. This change makes the difference between the peaks’ potentials of the bare carbon paste electrode, Ep,b, and Ep,m, ∆Ep, 324 mV, see Table 1. Moreover, the ratio between the peak’s current density recorded with the modified electrodes, jp,m and that obtained with the bare CPE, jp,b is greater than 3. The change in the AA oxidation potential towards less positive values can be attributed to the formation of the surface inclusion complex, that apart from provoking its strong adsorption it may give rise to an increase of the heterogeneous reaction rate constant as was observed in the case of the interaction of dopamine (DA) with the same modified electrode [35]. Moreover, using a similar treatment to the one described in the present work, Palomar-Pardavé et al. [35], showed that the CPE/poly-β-CD/electrode can be successfully used as working electrode during the electrochemical determination of DA, in the presence of ascorbic acid at pH 3.0. Notwithstanding, in this work we shown that due to the negative value found for the ∆G0inclus., this electrode can also be adequately use for the electrochemical quantification of AA, see Figure 7b, with a sensitivity of 3.41 Am−2 mM−1 and a detection limit of 0.22 µM.

Table 1.
Variation of the voltammetric parameters for AA oxidation as a function of the electrode used.
| Electrode | Ep/mV | jp/Am−2 | ∆Ep = Ep,b − Ep,m/mV | jp,m/jp,b |
|---|---|---|---|---|
| CPE | 564 | 2.96 | 0 | 1 |
| CPE/poly-β-CD | 240 | 9.19 | 324 | 3.14 |
3. Experimental
3.1. Reagents and Chemicals
All solutions were made from Merck (Mexico D.F., Mexico) analytical grade reagents and deionized water type 1 with 18.2 MΩcm resistivity, free from organic matter, obtained from a U.S. Filter PURE-LAB Plus UV (EU, San Diego, CA, USA). The pH was adjusted to pH 3.0 with HCl (37%). The resulting solutions were degassed with nitrogen and freshly prepared prior to each determination. They were also protected from the incidence of light even during the performance of the experiments.
3.2. Instrumentation
The electrochemical experiments were carried out with the aid of a potentiostat-galvanostat Autolab PGSTAT 30 (Utrecht, The Netherlands) in conjunction with a typical three-electrode electrochemical cell, with the carbon paste electrode, CPE, as working electrode. The CPE was prepared as described by Ramirez-Silva et al. [22,23], by mixing 1 µM, 99.9% graphite powder, from Johnson Matthey (Devens, MA, USA), with nujol. The exposed surface area of the CPE to the electrolyte solution was 0.25 cm2. A Pt wire was used as counterelectrode and a saturated Ag/AgCl electrode as reference, to which all potentials herein reported should be referred to unless otherwise stated. All reported measurements were recorded at 25 °C.
3.3. CPE Modified with Poly-β-CD
A 10 mM β-CD solution was prepared in 1 M HClO4 which was used to polymerize it over the CPE, as described by Roa-Morales et al. [20] and Corona-Avendaño et al. [21], by means of CV during different number of cycles. The poly-β-CD electrodeposited on an indium tin oxide (ITO) electrode was characterized ex situ by atomic force microscopy (AFM) applying the tapping method, with a Digital SPM Multimode instruments, Nanoscope IIIA (Santa Barbara, CA, USA) and by scanning electrode microscopy (SEM) using a JEOL JXA-8200 microscope (JEOL, Peabody, MA, USA).
4. Conclusions
This work presented the influence of the β-CD polymer, poly-β-CD, on the electrochemical response of AA. It was shown that the poly-β-CD drastically influenced the AA oxidation process. On the bare CPE the AA electrochemical oxidation was mass transfer-controlled while in the system CPE/poly-β-CD the process becomes adsorption-controlled, due to formation of an inclusion complex, on the electrode surface between β-CD and AA. Furthermore, the said complex catalyzed the AA oxidation reaction.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/19/5/5952/s1.
Acknowledgements
Manuel Palomar-Pardavé likes to thank ICyTDF for project ICyTDF/327/2011 “Síntesis, ensamblaje y caracterización de nanopartículas mono y multi-metálicas, para catalizadores en la generación de hidrógeno y oxidación de moléculas orgánicas en celdas de combustible directo y en sensores electroquímicos de neurotransmisores”. Silvia Corona-Avendaño and María Teresa Ramírez-Silva thank CONACyT for projects 80305 and 82932 of which they are responsible, respectively. Also, Silvia Corona-Avendaño, Mario Romero-Romo, Manuel Palomar-Pardavé, Georgina Alarcón-Angeles and María Teresa Ramírez-Silva thank the “Sistema Nacional de Investigadores” for the distinction of their membership and the stipend received. Manuel Palomar-Pardavé, Silvia Corona-Avendaño and Mario Romero-Romo thankfully acknowledge the support of the Departamento de Materiales at UAM-A through the research projects from the Área Ingeniería de Materiales and to Jesus Morales from Área de Física Atómica Molecular y Aplicada for the AFM facilities, Elizabeth Garfias, Área Ingeniería de Materiales for helping us with the AFM characterization and Perla Morales-Gil, “Instituto Mexicano del Petroleo” for helping us with the SEM characterization.
Author Contributions
María Teresa Ramírez-Silva; Manuel Palomar-Pardavé and Mario Romero-Romo participated in the conception and design of the study. Silvia Corona-Avendaño and Georgina Alarcón-Angeles carried out the electrochemical experiments. María Teresa Ramírez-Silva; Manuel Palomar-Pardavé; Mario Romero-Romo; Silvia Corona-Avendaño and Georgina Alarcón-Angeles participated in the analysis and discussion of results and the developed of the theoretical model used.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shaidarova, L.G.; Gedmina, A.V.; Chelnokova, I.A.; Budnikov, G.K. Electrocatalytic oxidation and flow-injection determination of ascorbic acid at a graphite electrode modified with a polyaniline film containing electrodeposited palladium. J. Anal. Chem. 2006, 61, 601–608. [Google Scholar] [CrossRef]
- Alarcón-Ángeles, G.; Corona-Avendaño, S.; Palomar-Pardavé, M.; Rojas-Hernández, A.; Romero-Romo, M.; Ramírez-Silva, M.T. Selective electrochemical determination of dopamine in the presence of ascorbic acid using sodium dodecyl sulfate micelles as masking agent. Electrochim. Acta 2008, 53, 3013–3020. [Google Scholar] [CrossRef]
- Corona-Avendaño, S.; Alarcón-Angeles, G.; Romero-Romo, M.; Cuán, A.; Ramírez-Silva, M.T.; Hernández-Martínez, L.; Palomar-Pardavé, M. Influence of CTAB on the electrochemical behavior of dopamine and on its analytic determination in the presence of ascorbic acid. J. Appl. Electrochem. 2010, 40, 463–474. [Google Scholar] [CrossRef]
- Corona-Avendaño, S.; Alarcón-Angeles, G.; Romero-Romo, M.; Merkoçi, A.; Rojas-Hernández, A.; Ramírez-Silva, M.T.; Palomar-Pardavé, M. Supramolecular systems construction for the selective quantitative determination of dopamine in the presence of ascorbic acid. Electrochem. Soc. Trans. 2011, 36, 385–392. [Google Scholar]
- Alarcón-Ángeles, G.; Pérez-López, B.; Palomar-Pardavé, M.; Ramírez-Silva, M.T.; Alegret, S.; Merkoçi, A. Enhanced host–guest electrochemical recognition of dopamine using cyclodextrin in the presence of carbon nanotubes. Carbon 2008, 46, 898–906. [Google Scholar] [CrossRef]
- Colín-Orozco, E.; Ramírez-Silva, M.T.; Corona-Avendaño, S.; Romero-Romo, M.; Palomar-Pardavé, M. Electrochemical quantification of dopamine in the presence of ascorbic acid and uric acid using a simple carbon paste electrode modified with SDS micelles at pH 7. Electrochim. Acta 2012, 85, 307–313. [Google Scholar] [CrossRef]
- Fragoso, A.; Almirall, E.; Cao, R.; Echegoyen, L.; González-Jonte, R. A supramolecular approach to the selective detection of dopamine in the presence of ascorbate. Chem. Commun. 2004, 19, 2230–2231. [Google Scholar]
- Wang, C.; Yuan, R.; Chai, Y.; Chen, S.; Zhang, Y.; Hu, F.; Zhang, M. Non-covalent iron(III)-porphyrin functionalized multi-walled carbon nanotubes for the simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite. Electrochim. Acta 2012, 62, 109–115. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, Z.; Lang, Q. Fabrication of poly(orthanilic acid)–multiwalled carbon nanotubes composite film-modified glassy carbon electrode and its use for the simultaneous determination of uric acid and dopamine in the presence of ascorbic acid. J. Solid State Electrochem. 2011, 15, 801–809. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Yang, R.; Xia, M.; Qu, L. Simultaneous voltammetric detection of dopamine and uric acid in the presence of high concentration of ascorbic acid using multi-walled carbon nanotubes with methylene blue composite film-modified electrode. J. Solid State Electrochem. 2011, 15, 1909–1918. [Google Scholar] [CrossRef]
- Kalimuthu, P.; John, S.A. Electropolymerized film of functionalized thiadiazole on glassy carbon electrode for the simultaneous determination of ascorbic acid, dopamine and uric acid. Bioelectrochemistry 2009, 77, 13–18. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Taei, M.; Khayamian, T.; Arabzadeh, A. Highly selective determination of ascorbic acid, dopamine, and uric acid by differential pulse voltammetry using poly(sulfonazo III) modified glassy carbon electrode. Sens. Actuators B 2010, 147, 213–221. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Taei, M.; Khayamian, T. A differential pulse voltammetric method for simultaneous determination of ascorbic acid, dopamine, and uric acid using poly(3-(5-chloro-2-hydroxyphenylazo)-4,5-dihydroxynaphthalene-2,7-disulfonic acid) film modified glassy carbon electrode. J. Electroanal. Chem. 2009, 633, 212–220. [Google Scholar] [CrossRef]
- Lehn, J.M. Supramolecular chemistry. Science 1993, 260, 1762–1763. [Google Scholar]
- Lehn, J.M. Supramolecular Chemistry; Wiley-VCH: New York, NY, USA, 1995; pp. 1–29. [Google Scholar]
- Kaifer, A.E.; Gomez-Kaifer, M. Supramolecular Electrochemistry; Wiley-VCH: Weinheim, Germany; New York, NY, USA; Chichester, UK; Brisbane, Australia; Singapore, Singapore; Toronto, ON, Canada, 1999; pp. 89–125. [Google Scholar]
- Wang, J.; Li, M.; Shi, Z.; Li, N.; Gu, Z. Electrocatalytic oxidation of norepinephrine at a glassy carbon electrode modified with single wall carbon nanotubes. Electroanalysis 2002, 14, 225–230. [Google Scholar] [CrossRef]
- Palomar-Pardavé, M.; Alarcón-Angeles, G.; Ramírez-Silva, M.T.; Romero-Romo, M.; Rojas-Hernández, A.; Corona-Avendaño, S. Electrochemical and spectrophotometric determination of the formation constants of the ascorbic acid-β-cyclodextrin and dopamine-β-cyclodextrin inclusion complexes. J. Incl. Phenom. Macrocycl. Chem. 2011, 69, 92–99. [Google Scholar]
- Ikeda, H.; Nakamura, M.; Ise, N.; Oguma, N.; Nakamura, A.; Ikeda, T.; Toda, F.; Ueno, A. Fluorescent cyclodextrins for molecule sensing: Fluorescent properties, NMR characterization, and inclusion phenomena of n-dansylleucine-modified cyclodextrins. J. Am. Chem. Soc. 1996, 118, 10980–10998. [Google Scholar] [CrossRef]
- Roa-Morales, G.; Ramirez-Silva, T.; Galicia, L. Carbon paste electrodes electrochemically modified with cyclodextrins. J. Solid State Electrochem. 2003, 7, 355–360. [Google Scholar]
- Corona-Avendaño, S.; Ramírez-Silva, M.T.; Romero-Romo, M.; Rojas-Hernández, A.; Palomar-Pardavé, M. Influence of the HClO4 concentration on the β-CD electropolimerization over a carbon paste electrode and on dopamine’s electrochemical response. Electrochim. Acta 2013, 89, 854–860. [Google Scholar] [CrossRef]
- Ramírez, M.T.; Palomar, M.E.; González, I.; Rojas-Hernández, A. Carbon paste electrodes with electrolytic binder: Influence of the preparation method. Electroanalysis 1995, 7, 184–188. [Google Scholar] [CrossRef]
- Martínez, R.; Ramírez, M.T.; González, I. Voltammetric characterization of carbon paste electrodes with a nonconducting binder. Part I: Evidence of the influence of electroactive species dissolution into the paste on the voltammetric response. Electroanalysis 1998, 10, 336–342. [Google Scholar] [CrossRef]
- Xin, J.; Guo, Z.; Chen, X.; Jiang, W.; Li, J.; Li, M. Study of branched cationic β-cyclodextrin polymer/indomethacin complex and its release profile from alginate hydrogel. Int. J. Pharm. 2010, 386, 221–228. [Google Scholar] [CrossRef]
- Wu, W.; Liu, J.; Cao, S.; Tan, H.; Li, J.; Xu, F.; Zhang, X. Drug release behaviors of a pH sensitive semi-interpenetrating polymer network hydrogel composed of poly(vinyl alcohol) and star poly[2-(dimethylamino)ethylmethacrylate]. Int. J. Pharm. 2011, 416, 104–109. [Google Scholar] [CrossRef]
- He, Q.; Wu, W.; Xiu, K.; Zhang, Q.; Xu, F.; Li, J. Controlled drug release system based on cyclodextrin-conjugated poly(lactic acid)-β-poly(ethylene glycol) micelles. Int. J. Pharm. 2013, 443, 110–119. [Google Scholar] [CrossRef]
- Li, S.; Purdy, W.C. Cyclodextrins and their applications in analytical chemistry. Chem. Rev. 1992, 92, 1457–1470. [Google Scholar] [CrossRef]
- Gosser, D.K. Cyclic Voltametry Simulation and Analysis of Reaction Mechanisms; Wiley-VCH: Weinheim, Germany; New York, NY, USA; Chichester, UK; Brisbane, Australia; Singapore, Singapore; Toronto, ON, Canada, 1993; pp. 27–97. [Google Scholar]
- Cobos-Murcia, J.A.; Galicia, L.; Rojas-Hernández, A.; Ramírez-Silva, M.T.; Álvarez-Bustamante, R.; Romero-Romo, M.; Rosquete-Pina, G.; Palomar-Pardave, M. Electrochemical polymerisation of 5-amino-1,10-phenanthroline onto different substrates. Experimental and theoretical study. Polymer 2005, 46, 9053–9063. [Google Scholar] [CrossRef]
- Garfias-García, E.; Romero-Romo, M.; Ramírez-Silva, M.T.; Morales, J.; Palomar-Pardavé, M. Mechanism and kinetics of the electrochemical formation of polypyrrole under forced convection conditions. J. Electroanal. Chem. 2008, 613, 67–79. [Google Scholar] [CrossRef]
- De Licona-Sánchez, T.J.; Álvarez-Romero, G.A.; Mendoza-Huizar, L.H.; Galán-Vidal, C.A.; Palomar-Pardavé, M.; Romero-Romo, M.; Herrera-Hernández, H.; Uruchurtu, J.; Juárez-García, J.M. Nucleation and growth kinetics of electrodeposited sulfate-doped polypyrrole: Determination of the diffusion coefficient of SO42− in the polymeric membrane. J. Phys. Chem. B 2010, 114, 9737–9743. [Google Scholar] [CrossRef]
- Cuán, A.; Velasco, A.; Palomar-Pardavé, M.E.; Ramírez-Silva, M.T.; Romero-Romo, M.; Cortés-Romero, C.M.; Corona-Avendaño, S. Quantumchemical calculations of the structural stability of β-cyclodextrin/dopamine and β-cyclodextrin/ascorbic acid systems. Electrochem. Soc. Trans. 2013, 47, 53–67. [Google Scholar]
- Yi, S.Y.; Chan, H.Y.; Cho, H.H.; Park, Y.C.; Lee, S.H.; Bae, Z.U. Resolution of dopamine and ascorbic acid using nickel(II) complex polymer-modified electrodes. J. Electroanal. Chem. 2007, 602, 217–225. [Google Scholar] [CrossRef]
- Corona-Avendaño, S.; Alarcón-Ángeles, G.; Ramírez-Silva, M.T.; Rosquete-Pina, G.; Romero-Romo, M.; Palomar-Pardavé, M. On the electrochemistry of dopamine in aqueous solution. Part I: The role of [SDS] on the voltammetric behavior of dopamine on a carbon paste electrode. J. Electroanal. Chem. 2007, 609, 17–26. [Google Scholar] [CrossRef]
- Palomar-Pardavé, M.; Corona-Avendaño, S.; Romero-Romo, M.; Alarcón-Ángeles, G.; Merkoçi, A.; Ramírez-Silva, M.T. Supramolecular interaction of dopamine with β-cyclodextrin: An experimental and theoretical electrochemical study. J. Electroanal. Chem. 2014, 717–718, 103–109. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).