In Vivo and In Vitro Anti-Tumor Effects of Fungal Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Different Fungi Extracts Exhibit Anti-Tumor Activities in Different Cancer Cells
| S-180 | HepG2 | A549 | HCT-116 | MDA-MB-231 | |
|---|---|---|---|---|---|
| F. pinicola | |||||
| Water extract | 78.9 ± 5.6 * | 96.6 ± 5.8 | 97.0 ± 2.4 | 62.5 ± 8.5 ** | 60.1 ± 7.2 ** |
| EtOH extract | 17.2 ± 3.4 *** | 28.7 ± 7.5 *** | 7.1 ± 3.2 *** | 12.1 ± 2.9 *** | 34.1 ± 5.1 *** |
| G. sinense | |||||
| Water extract | 47.7 ± 3.5 ** | 94.6 ± 3.5 | 96.9 ± 2.3 | 72.1 ± 5.7 * | 61.3 ± 6.9 ** |
| EtOH extract | 31.2 ± 10.2 *** | 41.2 ± 10.2 ** | 80.3 ± 7.6 * | 14.7 ± 2.1 *** | 28.5 ± 4.8 *** |
| F. officinalis | |||||
| Water extract | 64.0 ± 4.9 ** | 98.9 ± 0.9 | 97.9 ± 1.4 | 20.6 ± 6.2 *** | 60.8 ± 8.8 ** |
| EtOH extract | 34.1 ± 9.1 ** | 31.1 ± 5.1 ** | 62.0 ± 4.4 * | 15.7 ± 4.0 *** | 28.8 ± 1.1 *** |
| P. melanopus | |||||
| Water extract | 61.7 ± 8.1 ** | 99.9 ± 0.1 | 97.7 ± 0.9 | 48.4 ± 4.6 ** | 73.6 ± 4.9 * |
| EtOH extract | 33.3 ± 7.7 *** | 31.5 ± 2.7 ** | 82.5 ± 7.5 * | 16.0 ± 3.3 *** | 28.7 ± 5.8 *** |
| T. camphoratus | |||||
| Water extract | 98.2 ± 5.6 | 44.8 ± 5.6** | 94.6 ± 2.5 | 72.5 ± 6.7 * | 51.1 ± 4.2 ** |
| EtOH extract | 48.5 ± 3.2 ** | 36.2 ± 6.2 ** | 9.8 ± 2.4 *** | 12.9 ± 1.9 *** | 29.1 ± 3.6 *** |
2.2. Ethanol Extract of F. pinicola Significantly Decreases S-180 Cell Viability

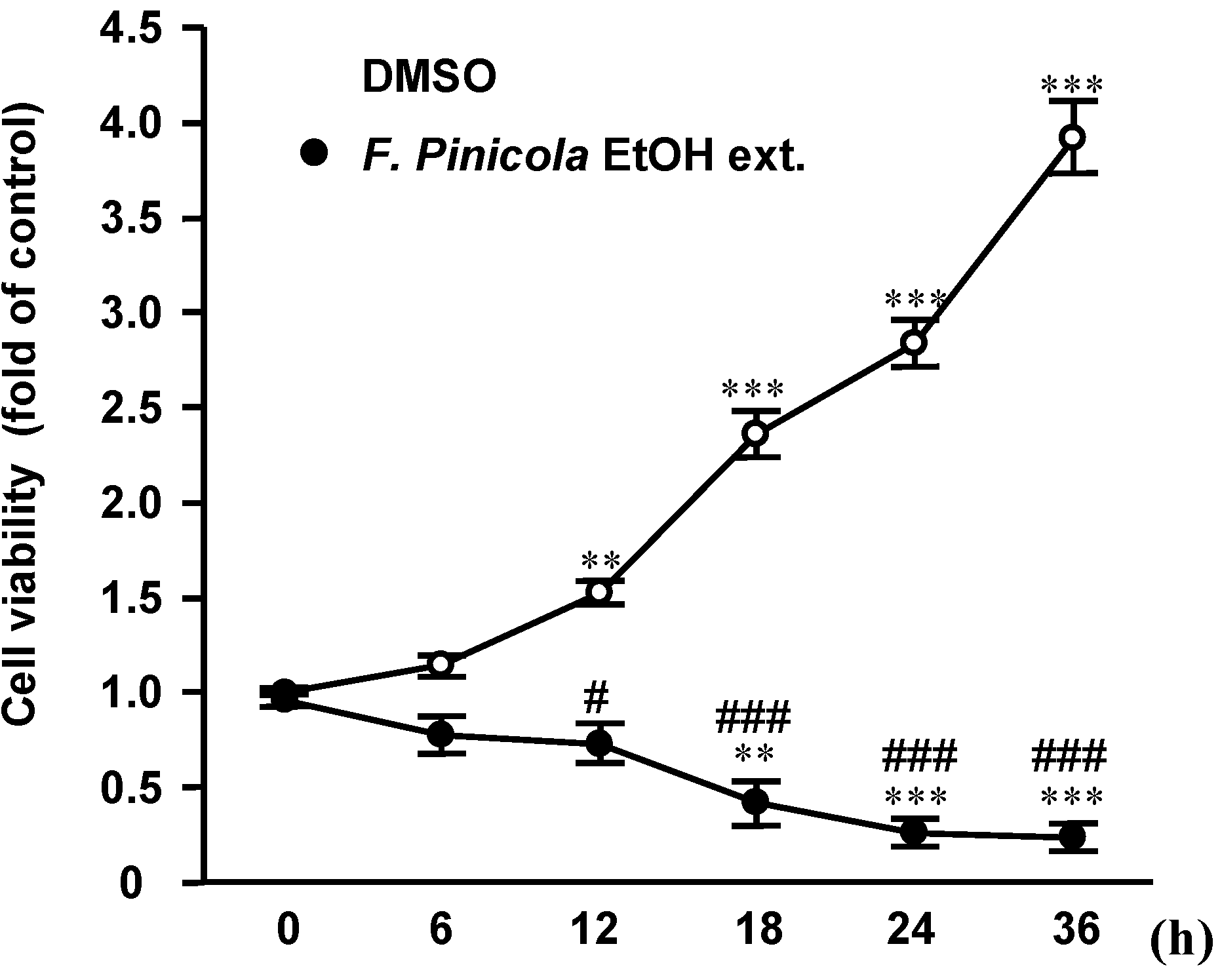
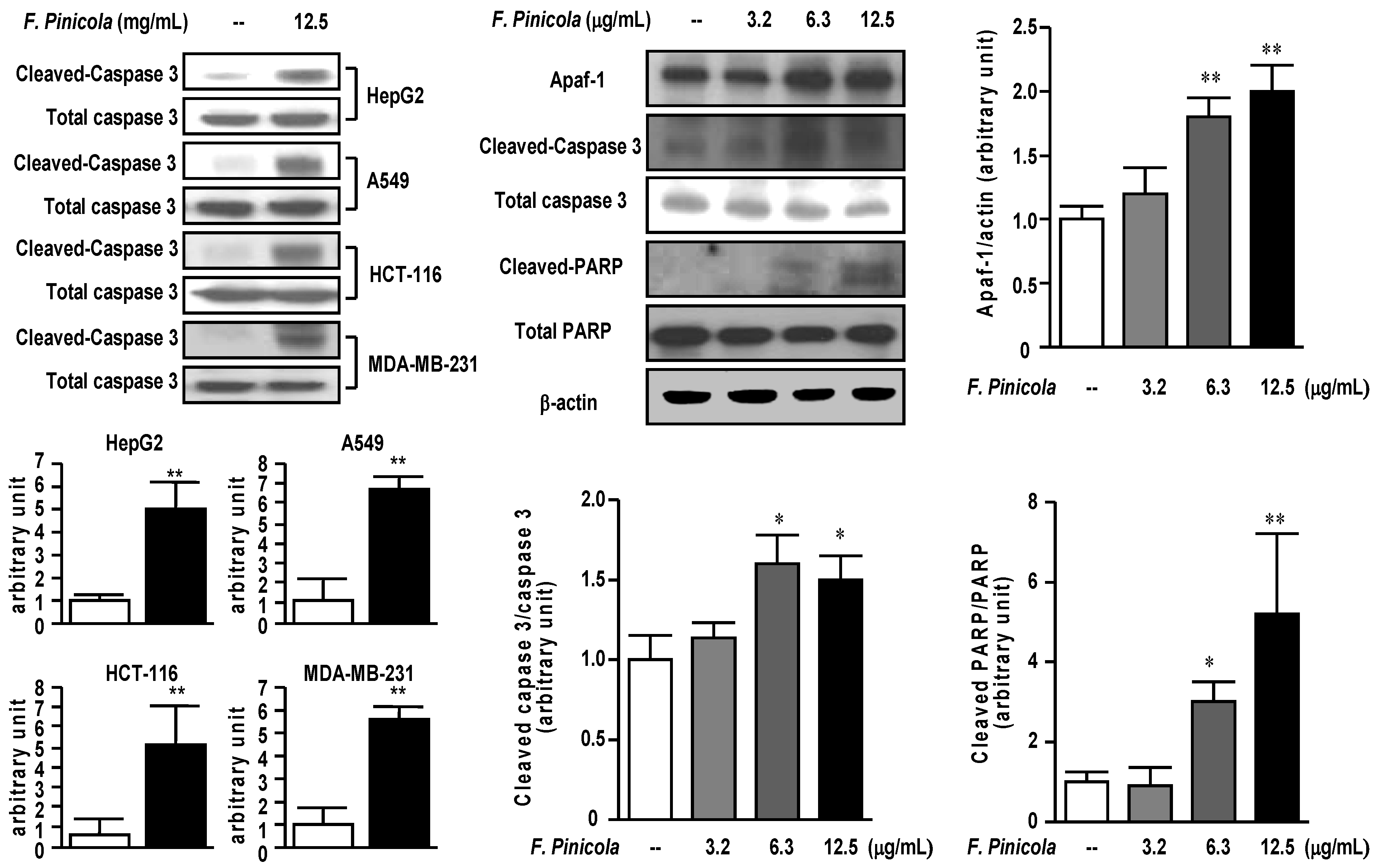
2.3. Ethanol Extract of F. pinicola Induces Cell Apoptosis in Cancer Cells
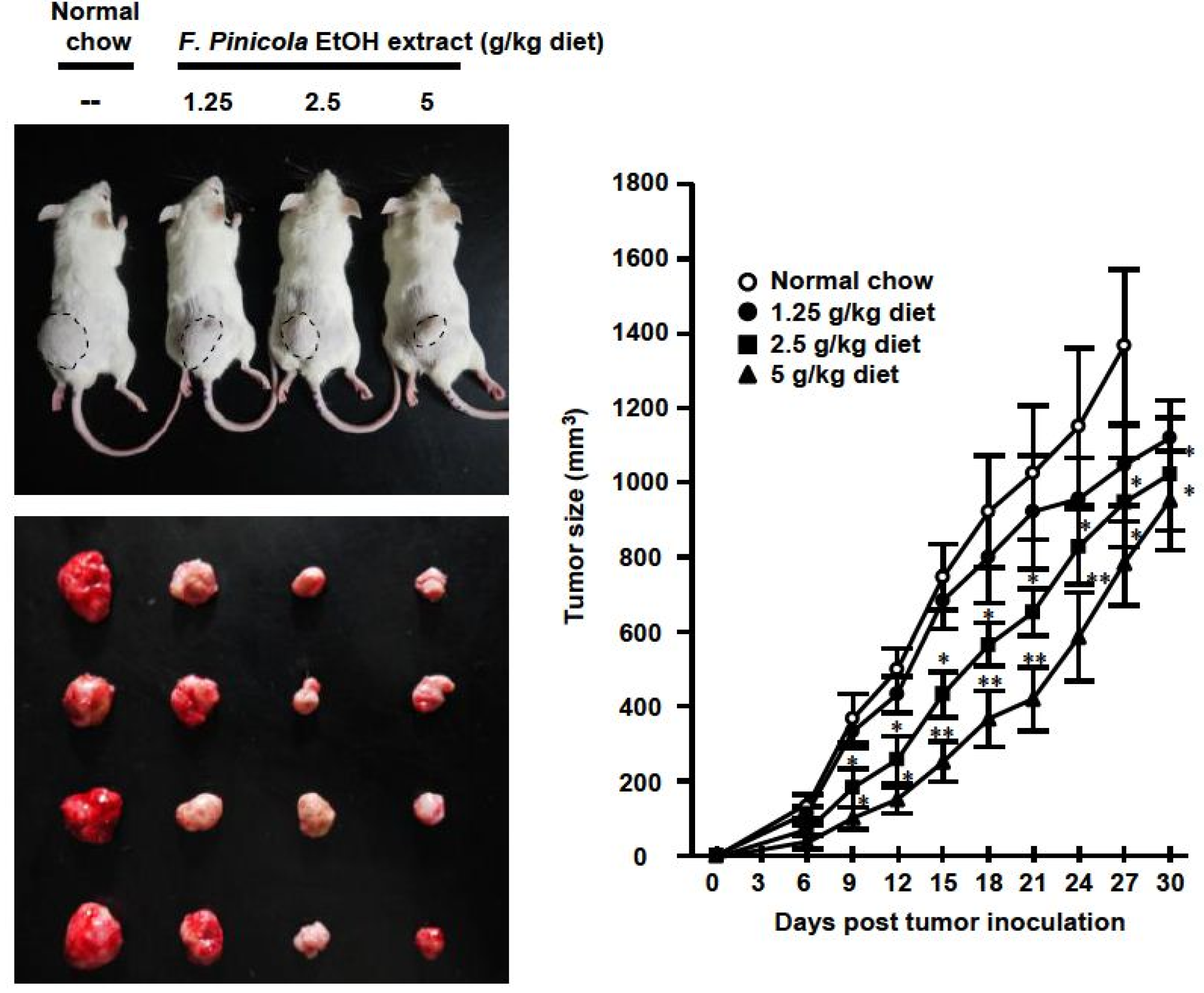
2.4. Anti-Tumor Activity of F. pinicola Ethanol Extract In Vivo
2.5. F. pinicola Ethanol Extract Synergistically Enhances Anti-Tumor Activity of Cisplatin in S-180 Mouse Sarcoma Cells
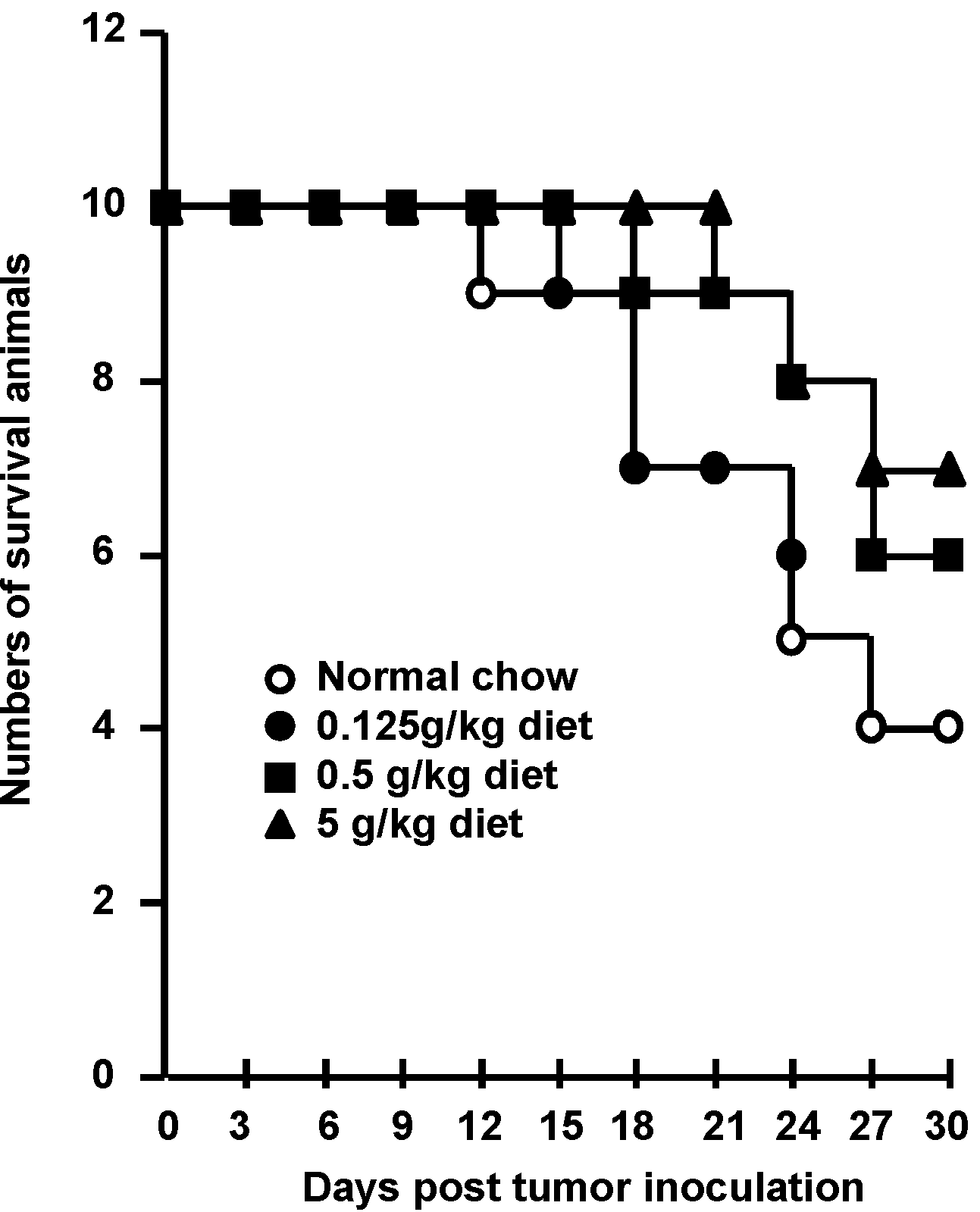

3. Experimental
3.1. Plant Material and Extraction
3.2. Cell Cultures
3.3. MTT Assay
3.4. Western Blotting
3.5. S-180 Bearing Animal Model
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zheng, R.; Jie, S.; Hanchuan, D.; Moucheng, W. Characterization and immunomodulating activities of polysaccharide from Lentinus edodes. Int. Immunopharmacol. 2005, 5, 811–820. [Google Scholar] [CrossRef]
- Yuen, J.W.; Gohel, M.D. Anticancer effects of Ganoderma lucidum: A review of scientific evidence. Nutr. Cancer 2005, 53, 11–17. [Google Scholar] [CrossRef]
- Kim, H.J.; Chang, W.K.; Kim, M.K.; Lee, S.S.; Choi, B.Y. Dietary factors and gastric cancer in Korea: A case-control study. Int. J. Cancer 2002, 97, 531–535. [Google Scholar] [CrossRef]
- Hara, M.; Hanaoka, T.; Kobayashi, M.; Otani, T.; Adachi, H.Y.; Montani, A.; Natsukawa, S.; Shaura, K.; Koizumi, Y.; Kasuga, Y.; et al. Cruciferous vegetables, mushrooms, and gastrointestinal cancer risks in a multicenter, hospital-based case-control study in Japan. Nutr. Cancer 2003, 46, 138–147. [Google Scholar] [CrossRef]
- Zhang, M.; Huang, J.; Xie, X.; Holman, C.D. Dietary intakes of mushrooms and green tea combine to reduce the risk of breast cancer in Chinese women. Int. J. Cancer 2009, 124, 1404–1408. [Google Scholar] [CrossRef]
- Rosecke, J.; Pietsch, M.; Konig, W.A. Volatile constituents of wood-rotting basidiomycetes. Phytochemistry 2000, 54, 747–750. [Google Scholar] [CrossRef]
- Keller, A.C.; Maillard, M.P.; Hostettmann, K. Antimicrobial steroids from the fungus Fomitopsis pinicola. Phytochemistry 1996, 41, 1041–1046. [Google Scholar] [CrossRef]
- Zjawiony, J.K. Biologically active compounds from Aphyllophorales (polypore) fungi. J. Nat. Prod. 2004, 67, 300–310. [Google Scholar] [CrossRef]
- Lee, S.I.; Kim, J.S.; Oh, S.H.; Park, K.Y.; Lee, H.G.; Kim, S.D. Antihyperglycemic effect of Fomitopsis pinicola extracts in streptozotocin-induced diabetic rats. J. Med. Food 2008, 11, 518–524. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Inoue, M.; Matsumoto, Y.; Sakakibara, C.; Miyataka, H.; Matsumoto, H.; Arihara, S. Lanostane triterpenoids and triterpene glycosides from the fruit body of Fomitopsis pinicola and their inhibitory activity against COX-1 and COX-2. J. Nat. Prod. 2005, 68, 69–73. [Google Scholar] [CrossRef]
- Usui, T.; Hosokawa, S.; Mizuno, T.; Suzuki, T.; Meguro, H. Investigation of the heterogeneity of heterogalactan from the fruit bodies of Fomitopsis pinicola, by employing concanavalin A-Sepharose affinity chromatography. J. Biochem. 1981, 89, 1029–1037. [Google Scholar]
- Franceschi, S.; Wild, C.P. Meeting the global demands of epidemiologic transition—The indispensable role of cancer prevention. Mol. Oncol. 2013, 7, 1–13. [Google Scholar] [CrossRef]
- Liu, Y.W.; Gao, J.L.; Guan, J.; Qian, Z.M.; Feng, K.; Li, S.P. Evaluation of antiproliferative activities and action mechanisms of extracts from two species of Ganoderma on tumor cell lines. J. Agric. Food Chem. 2009, 57, 3087–3093. [Google Scholar] [CrossRef]
- Bishayee, A.; Ahmed, S.; Brankov, N.; Perloff, M. Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front. Biosci. 2011, 16, 980–996. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002, 60, 258–274. [Google Scholar] [CrossRef]
- Borchers, A.T.; Krishnamurthy, A.; Keen, C.L.; Meyers, F.J.; Gershwin, M.E. The immunobiology of mushrooms. Exp. Biol. Med. 2008, 233, 259–276. [Google Scholar] [CrossRef]
- Mattila, P.; Suonpaa, K.; Piironen, V. Functional properties of edible mushrooms. Nutrition 2000, 16, 694–696. [Google Scholar] [CrossRef]
- Zaidman, B.Z.; Yassin, M.; Mahajna, J.; Wasser, S.P. Medicinal mushroom modulators of molecular targets as cancer therapeutics. Appl. Microbiol. Biotechnol. 2005, 67, 453–468. [Google Scholar] [CrossRef]
- Petrova, A.; Popov, S.; Gjosheva, M.; Bankova, V. A new triterpenic alcohol from Fomitopsis pinicola. Nat. Prod. Res. 2007, 21, 401–405. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Castro, F.O.; Alves, A.P.; Pessoa, C.; Moraes, M.O.; Silveira, E.R.; Lima, M.A.; Elmiro, F.J.; Costa-Lotufo, L.V. In vivo growth-inhibition of Sarcoma 180 by piplartine and piperine, two alkaloid amides from Piper. Braz. J. Med. Biol. Res. 2006, 39, 801–807. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the water or ethanol extracts of Fomitopsis pinicola (F. pinicola), Ganoderma sinense, Fomitopsis officinalis, Polyporus melanopus, and Taiwanofungus camphorates are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, H.-T.; Lu, F.-H.; Su, Y.-C.; Ou, H.-Y.; Hung, H.-C.; Wu, J.-S.; Yang, Y.-C.; Chang, C.-J. In Vivo and In Vitro Anti-Tumor Effects of Fungal Extracts. Molecules 2014, 19, 2546-2556. https://doi.org/10.3390/molecules19022546
Wu H-T, Lu F-H, Su Y-C, Ou H-Y, Hung H-C, Wu J-S, Yang Y-C, Chang C-J. In Vivo and In Vitro Anti-Tumor Effects of Fungal Extracts. Molecules. 2014; 19(2):2546-2556. https://doi.org/10.3390/molecules19022546
Chicago/Turabian StyleWu, Hung-Tsung, Feng-Hwa Lu, Yu-Chu Su, Horng-Yih Ou, Hao-Chang Hung, Jin-Shang Wu, Yi-Ching Yang, and Chih-Jen Chang. 2014. "In Vivo and In Vitro Anti-Tumor Effects of Fungal Extracts" Molecules 19, no. 2: 2546-2556. https://doi.org/10.3390/molecules19022546
APA StyleWu, H.-T., Lu, F.-H., Su, Y.-C., Ou, H.-Y., Hung, H.-C., Wu, J.-S., Yang, Y.-C., & Chang, C.-J. (2014). In Vivo and In Vitro Anti-Tumor Effects of Fungal Extracts. Molecules, 19(2), 2546-2556. https://doi.org/10.3390/molecules19022546




