Abstract
A novel method for the synthesis of bile acid derivatives has been developed using “click chemistry”. Intermolecular 1,3-dipolar cycloaddition of the propargyl ester of bile acids and azide groups of 1,3,5-tris(azidomethyl)benzene gave a new quasi-podands with 1,2,3-triazole rings. The structures of the products were confirmed by spectral (1H-NMR, 13C-NMR, and FT-IR) analysis, mass spectrometry and PM5 semiempirical methods. Estimation of the pharmacotherapeutic potential has been accomplished for synthesized compounds on the basis of Prediction of Activity Spectra for Substances (PASS).
1. Introduction
Bile acids were isolated from the bile of mammals in 1828 by L. Gmelin. They are produced from cholesterol in the liver and are stored in the gallbladder [1,2,3,4,5,6], from where contraction with feeding releases bile acids into the intestine. The terminal carboxylic acid group at C(17) in the side chain can be conjugated with taurine or glycine. The specific structure of bile acids with a large rigid and curved skeleton, chirality, the orientation of their chemically different polar hydroxy groups (3α; 3α,7α and 3α,7α,12α) toward the center of a concave face, as well as their amphiphilic properties make bile acids and their derivatives very interesting starting materials for the synthesis of macrocyclic molecular dimers, molecular tweezers or cholaphanes [7,8,9,10].
In recent times, much attention was given to the synthesis of molecular pockets, molecular umbrellas and quasi-podands from bile acids [11,12,13,14,15,16,17]. The molecular pockets and umbrellas are composed of two or more facial amphiphiles that are connected to a central, chaining and labile scaffold. On the other hand quasi-podands have a rigid benzyl platform [11,12,13,14,15,16,17]. The potential applications of these compounds include use as delivery vehicles for biological molecules, as molecular containers as well as hydrogelators. Bile acid dimers can be used for the synthesis of macrocyclic compounds as artificial receptors [18,19,20,21,22]. Furthermore some derivatives of bile acids are very good organogelators [23,24,25].
Synthetic or natural podands have acyclic structures where polyether chains are linked to the same binding centre, which can be different heteroatoms, e.g., nitrogen, phosphorus and sulphur. In view of their specific properties, these compounds are so-called open-chain simple analogues of crown ethers and cryptands [26]. Like the above compounds, podand hosts are generally able to form stable complexes with monovalent cations [27]. Synthetic podands have advantages over biological ones, in terms of facile synthesis and molecular structure versatility, therefore the synthesis of compounds containing a steroid skeleton is very interesting and useful. In our previous work, we reported the synthesis and physicochemical properties of new bile acid esters of 1,3,5-tris(bromomethyl)benzene or 1,2,4,5-tetrakis(bromomethyl)benzene and 3α-acetoxy-5β-cholanic acid, 3α,12α-diacetoxy-5β-cholanic acid and 3α,7α,12α-triacetoxy-5β-cholanic acid [17]. To get new quasi-podands we decided to modify the structure of bile acids by introduction of additional 1,2,3-triazole rings using “click chemistry” methods.
“Click chemistry” is a relatively new and very attractive trend in modern organic synthesis. It includes a broad spectrum of carbon–heteroatom bond forming reactions that fulfil specified requirements such as high efficiency and selectivity, simple reaction conditions and easy product isolation [28]. Moreover the products of “click chemistry” are stable in various solvents, including water [28,29]. A very important and effective method is the copper(I)-catalyzed 1,3-dipolar cycloaddition (the Huisgen reaction) between azides and terminal alkynes which is regarded as an important example of the “click” reaction. This is a very convenient and simple method of 1,2,3-triazole synthesis. Regioselective cycloaddition reactions lead to the formation of various substituted triazoles. Depending on the catalyst used 1,4-disubstituted triazoles, 1,5-disubstituted triazoles or a mixture of both are formed (Scheme 1) [30,31].
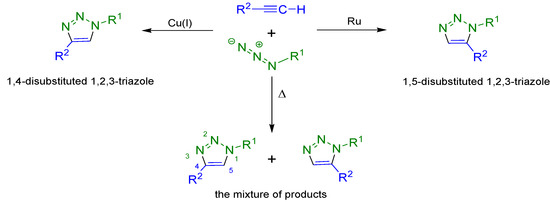
Scheme 1.
The possible “click” reaction pathways occurring between terminal alkynes and azides.
Compounds of this type are very resistant to the hydrolysis, oxidation and reduction conditions of metabolic degradation. Moreover, they can participate in the formation of hydrogen bonds which are crucial interactions in biological systems. In particular, 1,4-disubstitued 1,2,3-triazoles show the ability to participate in hydrogen bonds and dipole interactions [32]. The Cu(I)-catalyzed “click” reaction is thus an extremely useful method to obtain new 1,2,3-triazole derivatives of bile acids [33,34,35,36,37,38,39].
2. Results and Discussion
This work reports the synthesis and physicochemical properties of new quasi-podands linked with 1,2,3-triazole rings from propargyl esters of bile acid derivatives and 1,3,5-tris(azidomethyl)benzene. The propargyl esters of bile acids and 1,3,5-tris(azidomethyl)benzene were prepared according to the literature procedures (Scheme 2) [40,41,42].
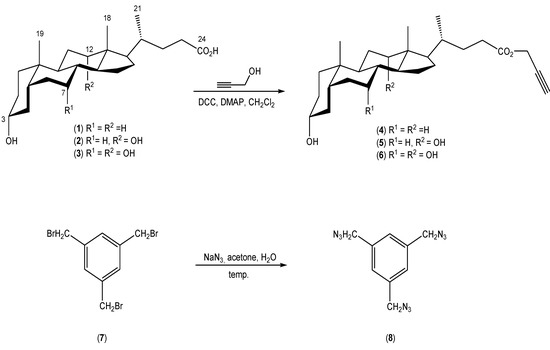
Scheme 2.
Synthesis of propargyl esters of bile acids 4–6 and 1,3,5-tris(azidomethyl)-benzene (8).
The syntheses of compounds 9–13 are shown in Scheme 3. We have obtained trisubstituted products 11–13 and we have also isolated and characterized two disubstituted products 9–10. These conjugates, in contrast to molecular pockets or umbrellas have a rigid benzyl platform. This allows the easier creation of conformers with an appropriate geometry. The aromatic ring is flat, so it can easily interact with the surface of biopolymers, for example. It performs the function of a specific anchor. Ramírez-López et al. have described this type of connection obtained from hormones such as estrone and estradiol and the corresponding azide. These compounds demonstrate differently gelling properties [43,44].
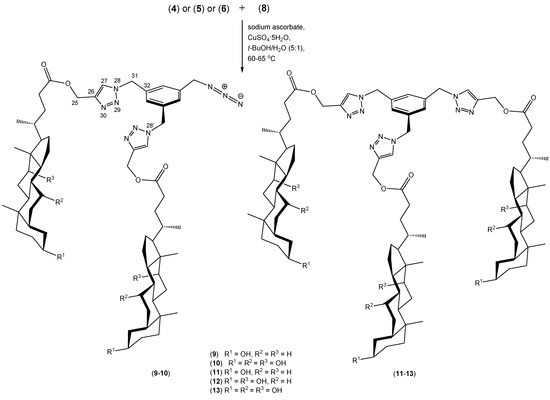
Scheme 3.
Synthesis of quasi podands of bile acids derivatives 9–13 linked by 1,2,3-triazole rings.
The potential pharmacological activity of synthesized compounds has been determined on the basis of computer-aided drug discovery approaches with the Prediction of Activity Spectra for Substances (PASSs) program. It is based on a robust analysis of structure–activity relationships in a heterogeneous training set currently including about 60,000 biologically active compounds from different chemical series with about 4,500 types of biological activity. Since only the structural formula of the chemical compound is necessary to obtain a PASS prediction, this approach can be used as the earliest stage of an investigation. There are many examples of the successful use of the PASS approach leading to new pharmacological agents [45,46,47,48,49].
Additionally, analyses of the biological prediction activity spectra for the new esters prepared herein are good examples of in silico studies of chemical compounds. The biological activity spectra were predicted with PASS for only two synthesized compounds (9 and 10). We also selected the types of activity that were predicted for a potential compound with the highest probability (focal activities) (Table 1). According to these data the most frequently predicted types of biological activity are: cholesterol antagonist, hypolipemic and inhibitors of acylcarnitine hydrolase, glyceryl-ether monooxygenase, alkenylglycerophosphocholine hydrolase or alkylacetylglycerophosphatase. We could not determine the potential biological properties of the compounds 11–13 because their molecular weight was over 1.200 g/mol.

Table 1.
Probability ‘to be Active’ (PA) values for the predicted biological activity of compounds 9 and 10.
| Focal Predicted Activity (PA > 0.70) | Compound | |
|---|---|---|
| 9 | 10 | |
| Acylcarnitine hydrolase inhibitor | 0.79 | 0.90 |
| Glyceryl-ether monooxygenase inhibitor | 0.72 | 0.84 |
| Alkenylglycerophosphocholine hydrolase inhibitor | 0.82 | 0.82 |
| Biliary tract disorders treatment | 0.73 | 0.81 |
| Hypolipemic | – | 0.77 |
| Alkylacetylglycerophosphatase inhibitor | 0.76 | 0.76 |
| Cholesterol antagonist | 0.79 | 0.72 |
The structures of all synthesized compounds were determined from their 1H- and 13C-NMR, FT-IR and ESI-MS spectra. Moreover, PM5 calculations were performed on all compounds [50,51,52]. The 1H- NMR spectra of compounds 9–10 and 11–13 show characteristic multiplets in the 3.68–3.15 ppm range assigned to the C3β–H protons of the steroid skeleton, and two hydrogen singlets ranging from 0.66–0.56 and 0.93–0.81, and characteristic doublets at 0.93–0.88 ppm assigned to CH3–18, CH3–19, and CH3–21, respectively. In the spectra of compounds 10, 12 and 13 characteristic broad singlets in the 3.96–3.78 ppm range due to the C12β–H protons and singlets in the 3.82–3.61 ppm range for the C7β–H protons (10 and 13) were observed. The 1H-NMR spectra of 9 and 10 showed a signal at 4.35 ppm for the protons of the –CH2–N3 group. It is a diagnostic signal, which is not observed in the spectra of 11–13. The signals for two methylene protons of the CO–CH2–triazole ring and Ph–CH2–triazole ring groups occurred as singlets in the 5.21–5.10 ppm and 5.57–5.48 ppm range, respectively (Figure 1).
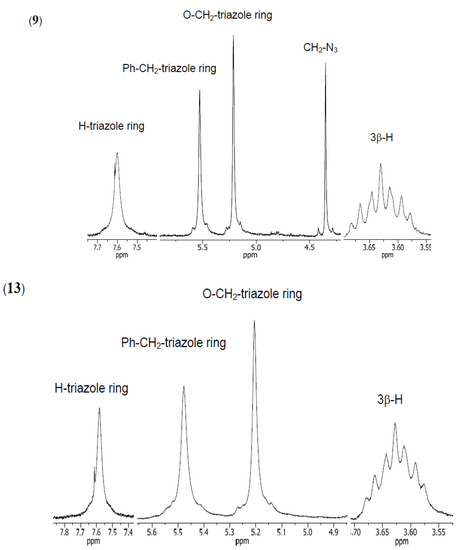
Figure 1.
1H-NMR spectra in the region (7.8–3.55 ppm) of the most characteristic signals of compounds 9 and 13.
Moreover, the 1H-NMR spectra of compounds 9 and 10 showed characteristic, diagnostic singlets at 7.62–7.61 ppm assigned to the two protons of the triazole rings. In the spectra of compounds 11–13 were three triazole ring protons in the 8.17–7.58 ppm range were seen. In the 1H-NMR spectra of compounds 9–13 the most characteristic signals were observed for the aromatic protons of the 1,3,5-trisubstituted benzene. These signals appeared as singlets at 7.21–7.19 and 7.26–7.16 ppm for 9–10 and 11–13, respectively.
The 13C-NMR spectra of compounds 9–13 show characteristic signals at 12.4–12.0 ppm, 23.4–22.8 ppm, and 18.3–17.5 ppm, which are assigned to CH3–18, CH3–19, and CH3–21, respectively. The carbon atoms of the CO2–CH2–triazole ring unit resonate in the range of 173.9–173.6 ppm (CO2 ) and 65.7–63.1 ppm (CH2). In the 13C-NMR spectra of compounds 9 and 10 the signals due to CH2 in N3-CH2-Ph group appear in the 53.89–53.88 ppm range. The spectra of compounds 11–13 show signals associated with CH2 atoms in triazole ring-CH2-Ph. The carbon atoms in the triazole ring are located at 143.72–142.20 ppm and 124.94–123.81 ppm, and are assigned to C(26)=C(27)–N(28), respectively.
The most characteristic feature of the FT-IR spectra (film) of all synthesized compounds are bands at 3,441–3,358 cm−1 assigned to the ν(O-H) stretching vibrations of the O(3)-H, O(7)-H and O(12)-H groups. A very weak hydrogen bond between the OH groups of the steroid skeleton is evidenced by the relatively narrow bands and low intensity. Moreover, two strong characteristic bands in the 1,739–1,732 cm−1 and 1,260–1,225 cm−1 region are present, which are assigned to the ν(C=O) and ν(C-O), respectively. For the compounds 9 and 10 are also observed a very strong band at 2,101–2,100 cm−1 associated with the presence of ν(N=N+=N−) groups (Figure 2).
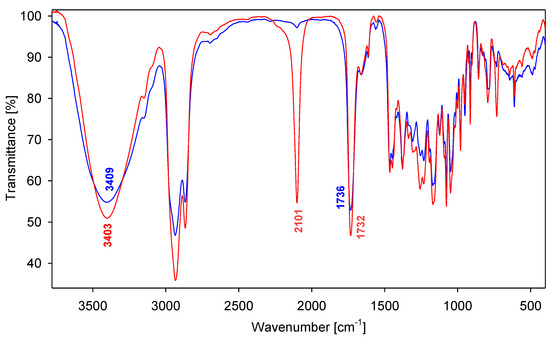
Figure 2.
FT-IR spectra of 10 (red) and 13 (blue) in the 3,700–400 cm−1 region.
The ESI-MS spectra were recorded in methanol. In all cases, the molecular ion [M]+ is present, which is associated with the presence in positive ion mode (ES+) as well as negative ion mode (ES−) of a ion with proton, alkali metals or halides. In Figure 3 we present the ESI-MS spectrum of conjugate 10. In the spectrum of this conjugate, the ion peaks are observed at m/z 1,137.47 (35%) [M+H]+, m/z 1,159.45 (70%) [M+Na]+ and m/z 1,175.56 (100%) [M+K]+. For this compound in the ESI-MS spectrum in negative ion mode molecular ion is present at m/z 1,170.67 (100%) [M+Cl]−.
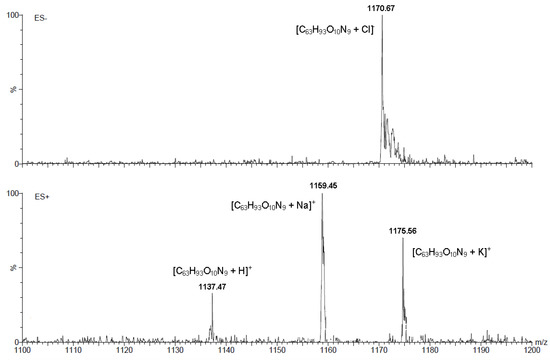
Figure 3.
ESI-MS spectrum of conjugate 10.
PM5 semiempirical calculations were performed using the WinMopac 2003 program. The final heat of formation (HOF), distances of N(28)-N(28') atoms and bond angle of N(28)-C(31)-C(32) of compounds 9–13 is presented in Table 2.

Table 2.
Heat of formation (HOF) [kcal/mol], distances of N(28)-N(28') atoms [Å] and bond angle [°] of N(28)-C(31)-C(32) of compounds (9–13).
| Compound | Heat of Formation [kcal/mol] | Distance [Å] of N(28)-N(28') | Bond Angle [°] of N(28)-C(31)-C(32) |
|---|---|---|---|
| 9 | −217.30 | 5.49 | 110.8 |
| 10 | −382.73 | 5.49 | 110.8 |
| 11 | −440.71 | 5.75 | 110.8 |
| 12 | −568.23 | 5.73 | 111.7 |
| 13 | −694.62 | 5.46 | 110.8 |
Representative compounds 10 and 13 are shown in Figure 4. The lowest HOF value is observed for cholic acid derivative 13, where an increasing number of hydroxyl groups facilitate the formation of intramolecular hydrogen bonds. Monosubstituted derivatives of bile acids linked to 1,2,3-triazole rings are not formed because the heat of formation is very high (approx. −66 kcal/mol).
In the all quasi-podands π–π stacking sandwich type interactions between two triazole rings were observed. The calculated interplanar separation is about 5.5 Å. These distances are greater about 1.7 Å in a comparison to the classical π–π stacking interactions because the triazole ring is attached to a rigid aromatic ring which imposes an increasing distance. This is confirmed by the size of the angle between N(28)-C(31)-C(32) atoms (Table 2). Moreover this spatial arrangement of bile acids and 1,2,3-triazole rings can facilitate the formation of stable host-guest complexes.
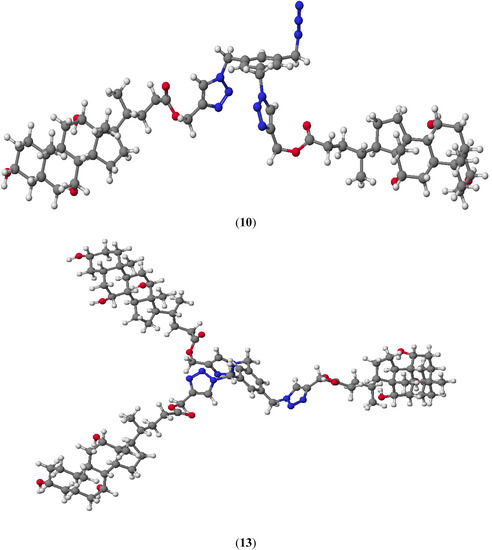
Figure 4.
Molecular models of representative compounds 10 and 13 calculated by the PM5 method.
3. Experimental
3.1. General
The NMR spectra were measured with a Varian Mercury 300 MHz NMR spectrometer (Oxford, UK), operating at 300.07 and 75.4614 MHz for 1H and 13C, respectively. Typical conditions for the proton spectra were: pulse width 32°, acquisition time 5 s, FT size 32 K and digital resolution 0.3 Hz per point, and for the carbon spectra pulse width 60°, FT size 60 K and digital resolution 0.6 Hz per point, the number of scans varied from 1200 to 10,000 per spectrum. The 13C and 1H chemical shifts were measured in CDCl3 or DMSO-d6 (compound 13) relative to an internal standard of TMS. Infrared spectra were recorded in the KBr pellets using a FT-IR Bruker IFS 66 spectrometer (Karlsruhe, Germany). The ESI (electron spray ionization) mass spectra were recorded on a Waters/Micromass (Manchester, UK) ZQ mass spectrometer equipped with a Harvard Apparatus (Saint Laurent, QC, Canada), syringe pump. The sample solutions were prepared in methanol at the concentration of approximately 10−5 M. The standard ESI-MS mass spectra were recorded at the cone voltage 30 V.
Synthesis: General Procedure for the Synthesis of Compounds 9 and 11
1,3,5-Tris(azidomethyl)benzene (43 mg, 0.177 mmol) was dissolved in mixture of t-butanol and water (10 mL, 5:1), propargyl lithocholate (200 mg, 0.482 mmol) was added, the mixture heated at 60–65 °C for 15 min. To the homogenous solution CuSO4·5H2O (3 mg, 3 mol%) and sodium ascorbate (9 mg, 20 mol%) in water (0.3 mL) were added. The mixture was heated to 60–65 °C for 2 h. The mixture was extracted with chloroform (10 mL), washed with brine (15 mL), and dried over anhydrous MgSO4. Evaporation of the solvent and purification of the residue over silica gel (CHCl3/EtOAc, 50:1) gave 83.6 mg of product 9 and 133.8 mg of product 11.
1-Azidomethylene-3,5-di[4-methylenelitocholate-1-methylene-(1,2,3-triazol-1-yl)]benzene (9): Oil (34%). 1H-NMR: δH 7.61 (s, 2H, triazole ring), 7.20 (s, 3H, Ar–H), 5.53 (s, 4H, Ph–CH2–triazole ring), 5.21 (s, 4H, O–CH2–triazole ring), 4.35 (s, 2H, CH2–N3), 3.68–3.58 (m, 2H, 3β–H), 0.91(s, 6H, CH3–19), 0.88 (d, J = 6.0 Hz, 6H, CH3–21), 0.62 (s, 6H, CH3–18). 13C-NMR (CDCl3, TMS, ppm): δC 174.15, 143.53, 137.94, 136.21, 127.85, 124.08, 71.85, 57.24, 56.47, 55.87, 53.89, 53.71, 42.70, 42.06, 40.40, 40.37, 36.40, 35.81, 35.31, 34.54, 31.04, 30.82, 30.50, 28.19, 27.16, 26.40, 26.21, 24.18, 23.36, 20.80, 18.24, 12.01. FT-IR (film) νmax: 3,441, 2,101, 1,735, 1,260, 1,163, 784. ESI-MS (m/z): 1,073.46 [C63H93O6N9+H]+, 1,095.44 [C63H93O6N9+Na]+, 1,106.67 [C63H93O6N9+Cl]−.
1-Azidomethylene-3,5-di[4-methylenecholate-1-methylene-(1,2,3-triazol-1-yl)]benzene (10): Oil (21%). 1H-NMR δH 7.62 (s, 2H, triazole ring), 7.21 and 7.19 (two s, 3H, Ar–H), 5.54 (s, 4H, Ph–CH2–triazole ring), 5.19 (s, 4H, O–CH2–triazole ring), 4.35 (s, 2H, CH2–N3), 3.93 (s, 2H, 12β–H), 3.82 (s, 2H, 7β–H), 3.47–3.34 (m, 2H, 3β–H), 0.93 (d, J = 6.0 Hz, 6H, CH3–21), 0.87 (s, 6H, CH3–19), 0.64 (s, 6H, CH3–18). 13C-NMR (CDCl3, TMS, ppm): δC 174.23, 143.60, 137.77, 136.36, 127.81, 127.32, 123.95, 72.97, 71.84, 68.38, 57.45, 53.88, 53.48, 46.71, 46.37, 41.63, 41.45, 39.49, 39.44, 35.29, 34.72, 34.61, 31.02, 30.75, 30.28, 29.67, 28.13, 27.55, 26.35, 23.20, 22.45, 17.24, 12.40, 0.99. FT-IR (film) νmax: 3,403, 2,100, 1,732, 1,258, 1,169, 732. ESI-MS (m/z): 1,137.46 [C63H93O10N9+H]+, 1,159.46 [C63H93O10N9+Na]+, 1,175.59 [C63H93O10N9+K]+, 1,170.68 [C63H93O10N9+Cl]−.
1,3,5-Tris[4-methylenelitocholate-1-methylene-(1,2,3-triazol-1-yl)]benzene (11): Oil (54%). 1H-NMR: δH 7.58 (two s, 3H, triazole ring), 7.20 and 7.16 (s, 3H, Ar–H), 5.48 (s, 6H, Ph–CH2–triazole ring), 5.21 (s, 6H, O–CH2–triazole ring), 3.66–3.59 (m, 3H, 3β–H), 0.91(s, 9H, CH3–19), 0.88 (d, J = 6.1 Hz, 9H, CH3–21), 0.62 (s, 9H, CH3–18).13C-NMR (CDCl3, TMS, ppm): δC 174.13, 143.71, 136.76, 127.68, 123.91, 71.82, 57.35, 56.48, 55.89, 53.34, 42.71, 42.06, 40.15, 40.40, 36.42, 35.81, 35.31, 35.30, 34.55, 31.05, 30.84, 30.52, 28.19, 27.16, 26.40, 24.18, 23.36, 20.81, 18.25, 12.01. FT-IR (film) νmax: 3,358, 1,732, 1,225, 1,163, 757. ESI-MS (m/z): 1,487.05 [C90H135O9N9+H]+, 1,509.04 [C90H135O9N9+Na]+, 1,525.08 [C90H135O9N9+K]+, 1,521.01 [C90H135O9N9+Cl]−.
1,3,5-Tris[4-methylenedeoxycholate-1-methylene-(1,2,3-triazol-1-yl)]benzene (12): Oil (43%). 1H-NMR: δH 8.02 (s, 3H, triazole ring), 7.17 (s, 3H, Ar–H), 5.49 (s, 6H, Ph–CH2–triazole ring), 5.19 (s, 6H, O–CH2–triazole ring), 3.96 (s, 3H,12β–H), 3.61-3.59 (m, 3H, 3β–H), 0.92 (d, J = 6.1 Hz, 9H, CH3–21), 0.91 (s, 9H, CH3–19), 0.66 (s, 9H, CH3–18). 13C-NMR (CDCl3, TMS, ppm): δC 174.08, 143.72, 136.77, 127.73, 123.81, 73.05, 71.70, 57.42, 53.32, 48.24, 47.10, 46.46, 42.04, 35.97, 35.23, 35.10, 34.08, 33.58, 31.42, 31.07, 30.76, 30.45, 28.64, 27.50, 27.09, 26.12, 23.64, 23.11, 17.24, 12.67. FT-IR (film) νmax: 3,434, 1,739, 1,255, 1,167, 758. ESI-MS (m/z): 1,535.04 [C90H135O12N9+H]+, 1,557.02 [C90H135O12N9+Na]+, 1,568.98 [C90H135O12N9+Cl]−, 1,612.96 [C90H135O12N9+Br]−.
1,3,5-Tris[4-methylenecholate-1-methylene-(1,2,3-triazol-1-yl)]benzene (13): oil (55%). 1H-NMR: δH 8.17 (s, 3H, triazole ring), 7.26 (s, 3H, Ar–H), 5.57 (s, 6H, Ph–CH2–triazole ring), 5.10 (s, 6H, O–CH2–triazole ring), 4.33 (d, 3H, J = 3 Hz, 7α–OH), 4.11 (d, 3H, J = 3 Hz, 3α–OH), 4.01 (d, 3H, J = 3 Hz, 12α–OH), 3.78 (s, 3H, 12β–H), 3.61 (s, 3H, 7β–H), 3.21–3.15 (s, 3H, 3β–H), 0.90 (d, 9H, J = 6.1 Hz, CH3–21), 0.81(s, 9H, CH3–19), 0.56 (s, 9H, CH3–18). 13C-NMR (DMSOd6, TMS, ppm): δC 173.11, 142.20, 137.12, 127.54, 124.94, 72.86, 70.99, 70.44, 66.25, 56.95, 52.30, 46.09, 45.76, 41.52, 41.37, 35.32, 35.02, 34.88, 34.40, 30.65, 30.58, 30.41, 28.52, 28.51, 27.28, 26.21, 22.82, 22.64, 16.88, 12.29. FT-IR (film) νmax: 3,409, 1,736, 1,255, 1,162, 759. ESI-MS (m/z): 1,584.07 [C90H135O15N9+H]+, 1,606.03 [C90H135O15N9+Na]+, 1,620.96 [C90H135O15N9+K]+, 1,616.98 [C90H135O15N9+Cl]−, 1,660.95 [C90H135O15N9+Br]−.
4. Conclusions
In conclusion, five new bile acid esters 9–13 were prepared from propargyl esters of lithocholic, deoxycholic and cholic acid and 1,3,5-tris(azidomethyl)benzene t-butanol/water mixture in the presence of sodium ascorbate and CuSO4·5H2O at 65 °C. These new compounds were characterized by spectroscopic and molecular structure methods. These bile acid esters may find applications in molecular recognition, supramolecular chemistry, and in pharmacology.
Acknowledgments
This work was supported by the funds from Adam Mickiewicz University, Faculty of Chemistry.
Author Contributions
The listed authors contributed to this work as described in the following. Tomasz Pospieszny carried out of the synthetic work, interpretation of results and prepared the manuscript. He performed semiempirical calculations and Prediction of Activity Spectra for Substances (PASS). Hanna Koenig was purifying compounds. Iwona Kowalczyk and Bogumił Brycki contributed with valuable discussions and scientific input. All authors approved the final version.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nicolaou, K.C.; Montagnon, T. Steroids and the Pill. In Molecules that Changed the World; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 79–90. [Google Scholar]
- Dewick, P.M. Steroids. In Medicinal Natural Products A Biosynthetic Approach, 3rd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 275–277. [Google Scholar]
- Lack, L.; Donity, F.O.; Walker, T.; Singletary, G.D. Synthesis of conjugated bile acids by means of a peptide coupling reagent. J. Lipid Res. 1973, 14, 367–370. [Google Scholar]
- Tserng, K.-Y.; Hachey, D.L.; Klein, P.D. An improved procedure for the synthesis of glycine and taurine conjugates of bile acids. J. Lipid Res. 1977, 18, 404–407. [Google Scholar]
- Batta, A.K.; Salen, G.; Shefer, S. Substrate specificity of cholylglycine hydrolase for the hydrolysis of bile acid conjugates. J. Biol. Chem. 1984, 259, 15035–15039. [Google Scholar]
- Huijghebaert, S.M.; Hofmann, A.F. Influence of the amino acid moiety on deconjugation of bile acid amidates by cholylglycine hydrolase or human fecal cultures. J. Lipid Res. 1986, 27, 742–752. [Google Scholar]
- Yuexian, L.; Dias, J.R. Dimeric and oligomeric steroids. Chem. Rev. 1997, 97, 283–304. [Google Scholar]
- Davis, A.P. Cholaphanes, et al; steroids as structural components in molecular engineering. Chem. Soc. Rev. 1993, 22, 243–253. [Google Scholar] [CrossRef]
- Willimann, P.; Marti, T.; Fürer, A.; Diederich, F. Steroids in molecular recognition. Chem. Rev. 1997, 97, 1567–1608. [Google Scholar] [CrossRef]
- Tamminen, J.; Kolehmainen, E. Bile acids as building blocks of supramolecular hosts. Molecules 2001, 6, 21–46. [Google Scholar] [CrossRef]
- Maitra, U.; Mukhopadhyay, S.; Sarkar, A.; Rao, P.; Indi, S.S. Hydrophobic pockets in a nonpolymeric aqueous gel: Observation of such a gelation process by color change. Angew. Chem. Int. Ed. 2001, 40, 2281–2283. [Google Scholar] [CrossRef]
- Luo, J.; Chen, Y.; Zhu, X.X. Invertible amphiphilic molecular pockets made of cholic acid. Langmuir 2009, 25, 10913–10917. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, J.; Zhu, X.X.; Junk, M.J.N.; Hinderberger, D. Molecular pockets derived from cholic acid as chemosensors for metal ions. Langmuir 2010, 26, 2958–2962. [Google Scholar]
- Zhang, J.; Junk, M.J.N.; Luo, J.; Hinderberger, D.; Zhu, X.X. 1,2,3-Triazole-containing molecular pockets derived from cholic acid: The influence of structure on host−guest coordination properties. Langmuir 2010, 26, 13415–13421. [Google Scholar] [CrossRef]
- Janout, V; Jing, B.W.; Regen, S.L. Molecular umbrella-assisted transport of an oligonucleotide across cholesterol-rich phospholipid bilayers. J. Am. Chem. Soc. 2005, 127, 15862–15870. [Google Scholar] [CrossRef]
- Janout, V; Jing, B.W.; Regen, S.L. Bioconjugate-based molecular umbrellas. Bioconjugate Chem. 2009, 20, 183–192. [Google Scholar] [CrossRef]
- Pospieszny, T.; Koenig, H.; Brycki, B. Synthesis and spectroscopic studies of new quasi podands from bile acid derivatives. Tetrahedron Lett. 2013, 54, 4700–4704. [Google Scholar] [CrossRef]
- Gao, H.; Dias, J.R. Synthesis and characterization of dimeric bile acid ester derivatives. J. Prakt. Chem. 1997, 339, 187–190. [Google Scholar] [CrossRef]
- Li, Y.X.; Dias, J.R. Synthesis of α- and β-dimers of lithocholic acid esters. Org. Prep. Proced. Int. 1996, 28, 201–207. [Google Scholar]
- Hsieh, H.P.; Muller, J.G.; Burrows, C.J. Structural effects in novel steroidal polyamine-DNA binding. J. Am. Chem. Soc. 1994, 116, 12077–12078. [Google Scholar] [CrossRef]
- Guthrie, J.P.; Cullimore, P.A.; McDonald, R.S.; O’Leary, S. Large hydrophobic interactions with clearly defined geometry. A dimeric steroid with catalytic properties. Can. J. Chem. 1982, 60, 747–764. [Google Scholar] [CrossRef]
- Paryzek, Z.; Joachimiak, R.; Piasecka, M.; Pospieszny, T. A new approach to steroid dimers and macrocycles by the reaction of 3-chlorocarbonyl derivatives of bile acids with O,O-, N,N-, and S,S-dinucleophile. Tetrahedron Lett. 2012, 46, 6212–6215. [Google Scholar]
- Willemen, H.M.; Vermonden, T.; Marcelis, A.T.M.; Sudhölter, E.J.R. N-Cholyl amino acid alkyl esters−a novel class of organogelators. Eur. J. Org. Chem. 2001, 12, 2329–2335. [Google Scholar]
- Willemen, H.M.; Vermonden, T.; Marcelis, A.T.M.; Sudhölter, E.J.R. Alkyl derivatives of cholic acid as organogelators: One-component and two-component gels. Langmuir 2002, 18, 7102–7106. [Google Scholar] [CrossRef]
- Valkonen, A.; Lahtinen, M.; Virtanen, E.; Kaikkonen, S.; Kolehmainen, E. Bile acid amidoalcohols: Simple organogelators. Biosens. Bioelectron. 2004, 20, 1233–1241. [Google Scholar] [CrossRef]
- Steed, J.W.; Atwood, J.L. Supramolecular Chemistry, 2nd ed.; John Wiley & Sons, Ltd.: London, UK, 2009; pp. 118–120, 278–281. [Google Scholar]
- Maia, A.; Landini, D.; Leska, B.; Schroeder, G. Silicon polypodands: Powerful metal cation complexing agents and solid–liquid phase-transfer catalysts of new generation. Tetrahedron Lett. 2003, 44, 4149–4151. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Narayan, S.; Muldoon, J.; Finn, M.G.; Fokin, V.V.; Kolb, H.C.; Sharpless, K.B. “On water”: Unique reactivity of organic compounds in aqueous suspension. Angew. Chem. Int. Ed. 2005, 44, 3275–3279. [Google Scholar] [CrossRef]
- Tornøe, Ch. W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-Triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Xue, P.; Sun, H.H.Y.; Williams, I.D.; Sharpless, K.B.; Fokin, V.V.; Jia, G. Ruthenium-catalyzed cycloaddition of alkynes and organic azides. J. Am. Chem. Soc. 2005, 127, 15998–15999. [Google Scholar]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click-Chemie: Diverse chemische funktionalität mit einer handvoll guter reaktionen. Angew. Chem. 2001, 113, 2056–2075. [Google Scholar] [CrossRef]
- Tron, G.C.; Pirali, T.; Billington, R.A.; Canonico, P.L.; Sorba, G.; Genazzani, A.A. Click chemistry reactions in medicinal chemistry: Applications of the 1,3-dipolar cycloaddition between azides and alkynes. Med. Res. Rev. 2008, 28, 278–308. [Google Scholar] [CrossRef]
- Latyshev, G.V.; Baranov, M.S.; Kazantsev, A.V.; Averin, A.D.; Lukashev, N.V.; Beletskaya, I.P. Copper-catalyzed [1,3]-dipolar cycloaddition for the synthesis of macrocycles containing acyclic, aromatic and steroidal moieties. Synthesis 2009, 2605–2615. [Google Scholar]
- Pore, V.S.; Aher, N.G.; Kumar, M.; Shukla, P.K. Design and synthesis of fluconazole/bile acid conjugate using click reaction. Tetrahedron 2006, 62, 11178–11186. [Google Scholar] [CrossRef]
- Kumar, A.; Pandey, P.S. Anion Recognition by 1,2,3-triazolium receptors: Application of click chemistry in anion recognition. Org. Lett. 2008, 10, 165–168. [Google Scholar] [CrossRef]
- Kumar, A.; Chhatra, R.K.; Pandey, P.S. Synthesis of click bile acid polymers and their application in stabilization of silver nanoparticles showing iodide sensing property. Org. Lett. 2010, 12, 24–27. [Google Scholar] [CrossRef]
- Vatmurge, N.S.; Hazra, B.G.; Pore, V.S.; Shirazi, F.; Deshpande, M.V.; Kadreppa, S.; Chattopadhyay, S.; Gonnade, R.G. Synthesis and biological evaluation ofbile aciddimers linked with 1,2,3-triazoleandbis-β-lactam. Org. Biomol. Chem. 2008, 6, 3823–3830. [Google Scholar] [CrossRef]
- Pospieszny, T.; Małecka, I.; Paryzek, Z. Synthesis and spectroscopic studies of new bile acid derivatives linked by a 1,2,3-triazole ring. Tetrahedron Lett. 2012, 53, 301–305. [Google Scholar] [CrossRef]
- Garrett, T.M.; McMurry, T.J.; Hosseini, M.W.; Reyes, Z.E.; Hahn, F.E.; Raymond, K.N. Ferric ion sequestering agents. 22. Synthesis and characterization of macrobicyclic iron(III) sequestering agents. J. Am. Chem. Soc. 1991, 113, 2965–2977. [Google Scholar] [CrossRef]
- Aher, N.G.; Pore, V.S.; Patil, S.P. Design, synthesis, and micellar properties of bile acid dimers and oligomers linked with a 1,2,3-triazole ring. Tetrahedron 2007, 63, 12927–12934. [Google Scholar] [CrossRef]
- Maue, M.; Bernitzki, K.; Eilermann, M.; Schrader, T. Bifunctional bisamphiphilic transmembrane building blocks for artificial – signal transduction. Synthesis 2008, 14, 2247–2256. [Google Scholar]
- Ramírez-López, P.; de la Torre, M.C.; Montenegro, H.E.; Asenjo, M.; Sierra, M.A. A straightforward synthesis of tetrameric estrone-based macrocycles. Org. Lett. 2008, 10, 3555–3558. [Google Scholar] [CrossRef]
- Ramírez-López, P.; de la Torre, M.C.; Asenjo, M.; Ramírez-Castellanos, J.; González-Calbet, J.M.; Rodrígez-Gimeno, A.; Ramírez de Arellano, C.; Sierra, M.A. A new family of “clicked” estradiol-based low-molecular-weight gelatores having highly symmetry-dependent gelation ability. Chem. Commun. 2011, 47, 10281–10283. [Google Scholar] [CrossRef]
- Pharma Expert Predictive Services Version 2.0 © 2011–2013. Available online: http://www.pharmaexpert.ru/PASSOnline/ (accessed on 1 November 2013).
- Poroikov, V.V.; Filimonov, D.A.; Borodina, Y.V.; Lagunin, A.A.; Kos, A. Robustness of biological activity spectra predicting by computer program PASS for noncongeneric sets of chemical compounds. J. Chem. Inf. Comput. Sci. 2000, 40, 1349–1355. [Google Scholar] [CrossRef]
- Poroikov, V.V.; Filimonov, D.A. How to acquire new biological activities in old compounds by computer prediction. J. Comput. Aided Mol. Des. 2002, 16, 819–824. [Google Scholar] [CrossRef]
- Poroikov, V.V.; Filimonov, D.A. Predictive Toxicology; Helma, C., Ed.; Taylor and Francis: Boca Raton, FL, USA, 2005; pp. 459–478. [Google Scholar]
- Stepanchikova, A.V.; Lagunin, A.A.; Filimonov, D.A.; Poroikov, V.V. Prediction of biological activity spectra for substances: Evaluation on the diverse sets of drug-like structures. Curr. Med. Chem. 2003, 10, 225–233. [Google Scholar] [CrossRef]
- Fujistsu. CAChe 5.04 UserGuide, Fujitsu: Chiba, Japan, 2003.
- Stewart, J.J.P. Optimization of parameters for semiempirical methods. III Extension of PM3 to Be, Mg, Zn, Ga, Ge, As, Se, Cd, In, Sn, Sb, Te, Hg, Tl, Pb, and Bi. J. Comput. Chem. 1991, 12, 320–341. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods I. Method. J. Comput. Chem. 1989, 10, 209–220. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 9–13 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).