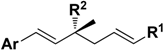
Synthesis of ∆3-2-Hydroxybakuchiol Analogues and Their Growth Inhibitory Activity against Rat UMR106 Cells
Abstract
:1. Introduction
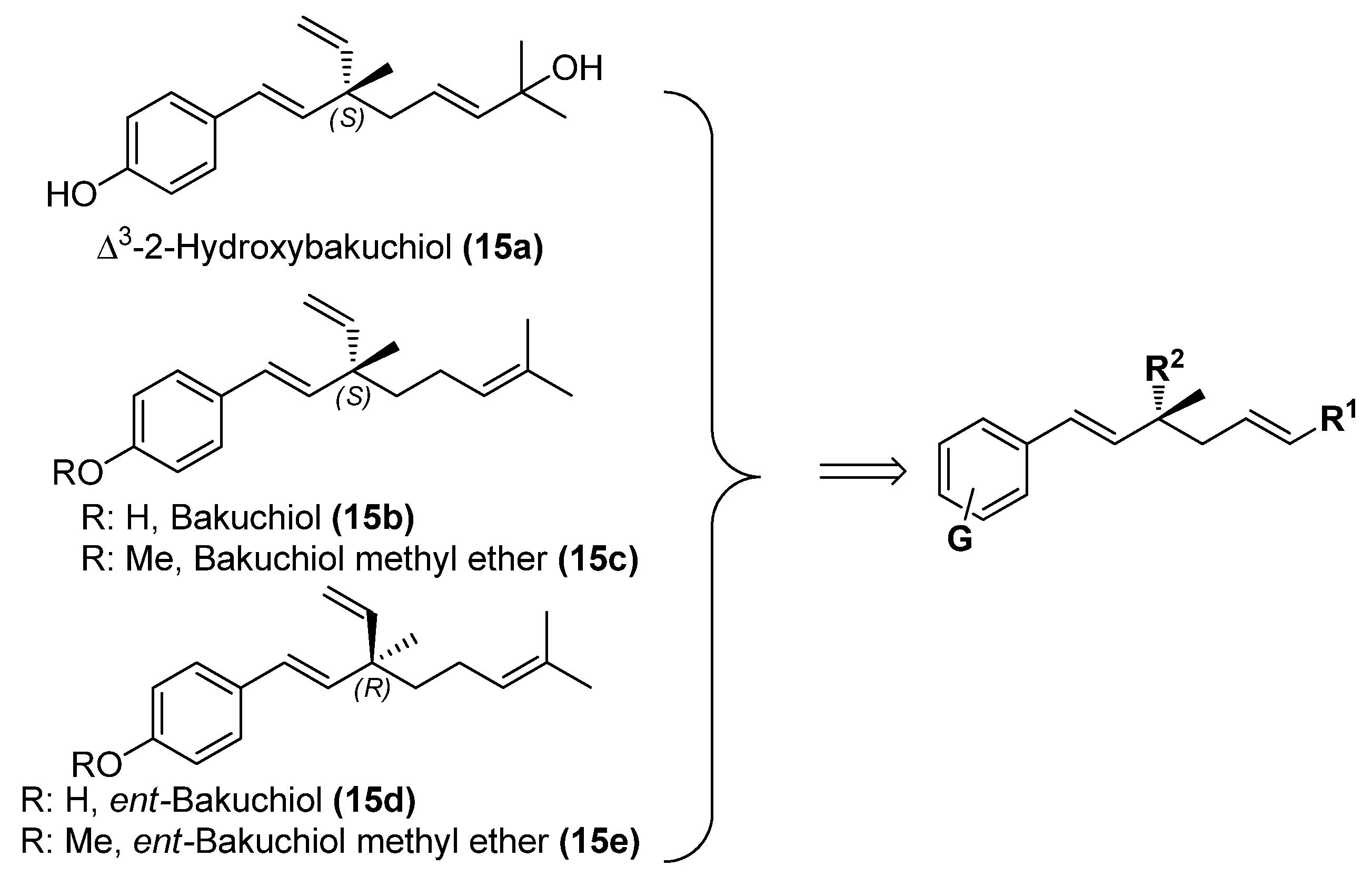
2. Results and Discussion
2.1. Chemistry
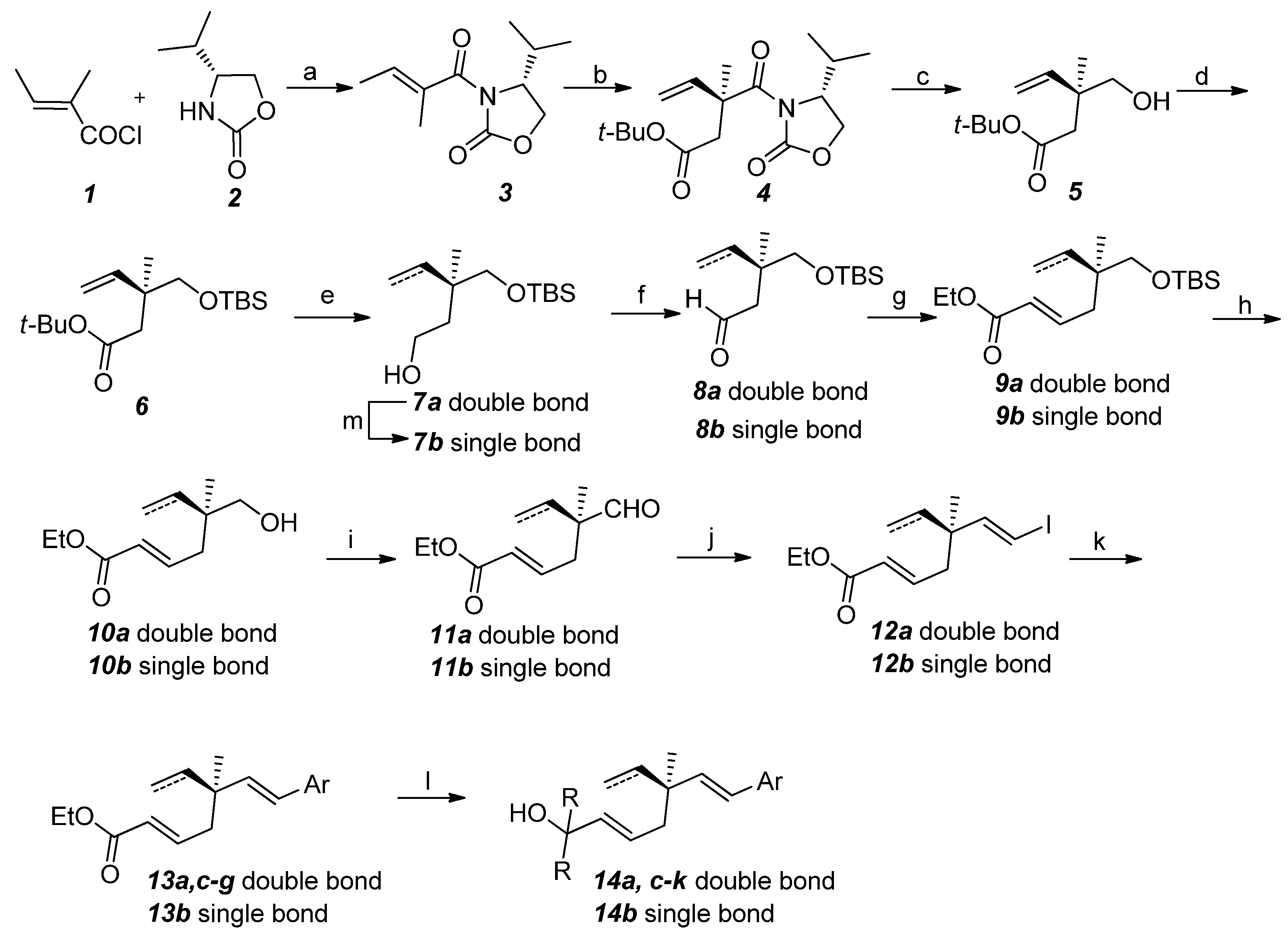
2.2. Activity against Rat UMR106 Cell
| Entry | Comp. | Ar | R1 | R2 | IC50 (µM) | |
|---|---|---|---|---|---|---|
| 1 | 13a | 4-MeO-C6H4 | CO2Et | CH=CH2 | 27 | |
| 2 | 13c | C6H5 | CO2Et | CH=CH2 | 330 | |
| 3 | 13d | 4-Me-C6H4 | CO2Et | CH=CH2 | 263 | |
| 4 | 13e | 2-C10H7 | CO2Et | CH=CH2 | >500 | |
| 5 | 13f | 4-CF3-C6H4 | CO2Et | CH=CH2 | 242 | |
| 6 | 13g | 3,4-OCH2O-C6H4 | CO2Et | CH=CH2 | 511 | |
| 7 | 14a | 4-MeO-C6H4 | Me2CH(OH) | CH=CH2 | 66 | |
| 8 | 14c | C6H5 | Me2CH(OH) | CH=CH2 | 107 | |
| 9 | 14d | 4-Me-C6H4 | Me2CH(OH) | CH=CH2 | 87 | |
| 10 | 14e | 2-C10H7 | Me2CH(OH) | CH=CH2 | 128 | |
| 11 | 14f | 4-CF3-C6H4 | Me2CH(OH) | CH=CH2 | 46 | |
| 12 | 14g | 3,4-OCH2O-C6H4 | Me2CH(OH) | CH=CH2 | 115 | |
| 13 | 14h | 3-HO-C6H4 | Me2CH(OH) | CH=CH2 | 123 | |
| 14 | 14i | 4-HO-C6H4 | Et2CH(OH) | CH=CH2 | 57 | |
| 15 | 14j | 4-HO-C6H4 | n-Bu2CH(OH) | CH=CH2 | 17 | |
| 16 | 14b | 4-HO-C6H4 | Me2CH(OH) | CH2CH3 | 71 | |
| 17 | bakuchiol methyl ether (15c) |  | 60 | |||
| 18 | bakuchiol (15b) | 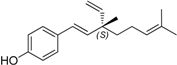 | 62 | |||
| 19 | ent-bakuchiol methyl ether (15e) |  | 161 | |||
| 20 | ent-bakuchiol (15d) |  | 33 | |||
| 21 | ∆3-2-hydroxybakuchiol (15a) |  | 69 | |||
3. Experimental
3.1. General Information
3.2. Synthetic Procedures for the New Compounds
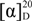 +4.8 (c 0.65, CHCl3). 1H-NMR (CDCl3) δ 3.64 (t, J = 5.6 Hz, 2H), 3.41 (brs, 1H), 3.39 (d, J = 10.0 Hz, 1H), 3.34 (d, J = 10.0 Hz, 1H), 1.64–1.48 (m, 2H), 1.39–1.20 (m, 2H), 0.91 (s, 9H), 0.85–0.78 (m, 6H), 0.08 (s, 6H). 13C-NMR (CDCl3) δ 70.6, 58.8, 41.2, 37.4, 29.6, 25.8, 21.7, 18.2, 7.7, −5.6. ESI-MS: 247.1 [M+H], HRMS (ESI): Calcd. for C13H30O2Si [M+H]: 247.2088, found: 247.2091.
+4.8 (c 0.65, CHCl3). 1H-NMR (CDCl3) δ 3.64 (t, J = 5.6 Hz, 2H), 3.41 (brs, 1H), 3.39 (d, J = 10.0 Hz, 1H), 3.34 (d, J = 10.0 Hz, 1H), 1.64–1.48 (m, 2H), 1.39–1.20 (m, 2H), 0.91 (s, 9H), 0.85–0.78 (m, 6H), 0.08 (s, 6H). 13C-NMR (CDCl3) δ 70.6, 58.8, 41.2, 37.4, 29.6, 25.8, 21.7, 18.2, 7.7, −5.6. ESI-MS: 247.1 [M+H], HRMS (ESI): Calcd. for C13H30O2Si [M+H]: 247.2088, found: 247.2091.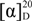 −2.6 (c 0.50, CHCl3). 1H-NMR (CDCl3) δ 9.84 (t, J = 3.1 Hz, 1H), 3.42 (d, J = 9.7 Hz, 1H), 3.35 (d, J = 9.7 Hz, 1H), 2.32–2.21 (m, 2H), 1.46–1.37 (m, 2H), 0.96 (s, 3H), 0.92–0.83 (m, 12H), 0.03 (s, 6H). 13C-NMR (CDCl3) δ 203.8, 69.6, 51.0, 42.2, 37.8, 29.4, 25.8, 21.3, 7.9, −5.6. ESI-MS: 283.7 [M+K].
−2.6 (c 0.50, CHCl3). 1H-NMR (CDCl3) δ 9.84 (t, J = 3.1 Hz, 1H), 3.42 (d, J = 9.7 Hz, 1H), 3.35 (d, J = 9.7 Hz, 1H), 2.32–2.21 (m, 2H), 1.46–1.37 (m, 2H), 0.96 (s, 3H), 0.92–0.83 (m, 12H), 0.03 (s, 6H). 13C-NMR (CDCl3) δ 203.8, 69.6, 51.0, 42.2, 37.8, 29.4, 25.8, 21.3, 7.9, −5.6. ESI-MS: 283.7 [M+K]. 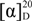 −11.5 (c 0.60, CHCl3). 1H-NMR (CDCl3) δ 6.97 (dt, J = 15.7, 7.9 Hz, 1H), 5.81 (d, J = 15.7 Hz, 1H), 4.18 (q, J = 7.1 Hz, 2H), 3.28 (d, J = 9.7 Hz, 1H), 3.23 (d, J = 9.7 Hz, 1H), 2.14 (d, J = 7.9 Hz, 2H), 1.28 (t, J = 7.1 Hz, 3H), 0.89 (s, 9H), 0.85–0.76 (m, 6H), 0.02 (s, 6H). 13C-NMR (CDCl3) δ 166.6, 146.8, 123.2, 68.6, 60.1, 39.4, 38.8, 29.0, 3.82, 21.1, 18.2, 14.3, 7.8, −5.6. ESI-MS: 315.2 [M+H], HRMS(ESI): Calcd. for C17H35O3Si [M+H]: 315.2350, found: 315.2356.
−11.5 (c 0.60, CHCl3). 1H-NMR (CDCl3) δ 6.97 (dt, J = 15.7, 7.9 Hz, 1H), 5.81 (d, J = 15.7 Hz, 1H), 4.18 (q, J = 7.1 Hz, 2H), 3.28 (d, J = 9.7 Hz, 1H), 3.23 (d, J = 9.7 Hz, 1H), 2.14 (d, J = 7.9 Hz, 2H), 1.28 (t, J = 7.1 Hz, 3H), 0.89 (s, 9H), 0.85–0.76 (m, 6H), 0.02 (s, 6H). 13C-NMR (CDCl3) δ 166.6, 146.8, 123.2, 68.6, 60.1, 39.4, 38.8, 29.0, 3.82, 21.1, 18.2, 14.3, 7.8, −5.6. ESI-MS: 315.2 [M+H], HRMS(ESI): Calcd. for C17H35O3Si [M+H]: 315.2350, found: 315.2356.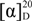 −14.7 (c 0.60, CHCl3). 1H-NMR (CDCl3) δ 7.10–6.88 (m, 1H), 5.86 (ddd, J = 15.5, 3.0, 1.7 Hz, 1H), 4.19 (q, J = 7.1 Hz, 2H), 3.40 (d, J = 10.8 Hz, 1H), 3.36 (d, J = 10.8 Hz, 1H), 2.18 (dd, J = 7.9, 1.3 Hz, 2H), 1.37–1.25 (m, 5H), 0.91–0.81 (m, 6H). 13C-NMR (CDCl3) δ 166.4, 146.0, 123.6, 68.9, 60.2, 39.1, 38.6, 28.8, 21.1, 14.33, 7.8. ESI-MS: 201.1 [M+H], HRMS(ESI): Calcd. for C11H21O3 [M+H]: 201.1485, found: 201.1489.
−14.7 (c 0.60, CHCl3). 1H-NMR (CDCl3) δ 7.10–6.88 (m, 1H), 5.86 (ddd, J = 15.5, 3.0, 1.7 Hz, 1H), 4.19 (q, J = 7.1 Hz, 2H), 3.40 (d, J = 10.8 Hz, 1H), 3.36 (d, J = 10.8 Hz, 1H), 2.18 (dd, J = 7.9, 1.3 Hz, 2H), 1.37–1.25 (m, 5H), 0.91–0.81 (m, 6H). 13C-NMR (CDCl3) δ 166.4, 146.0, 123.6, 68.9, 60.2, 39.1, 38.6, 28.8, 21.1, 14.33, 7.8. ESI-MS: 201.1 [M+H], HRMS(ESI): Calcd. for C11H21O3 [M+H]: 201.1485, found: 201.1489.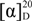 −21.6 (c 0.45, CHCl3) 1H-NMR (CDCl3) δ 7.04–6.89 (m, 1H), 5.86 (ddd, J = 15.5, 3.0, 1.7 Hz, 1H), 4.19 (q, J = 7.1 Hz, 2H), 3.40 (d, J = 10.8 Hz, 1H), 3.36 (d, J = 10.8 Hz, 1H), 2.18 (dd, J = 7.9, 1.3 Hz, 2H), 1.44–1.22 (m, 5H), 0.92–0.79 (m, 6H). 13C-NMR (CDCl3) δ 205.3, 166.0, 143.6, 124.6, 60.4, 49.3, 36.7, 27.9, 18.6, 14.2, 8.3. ESI-MS: 237.1, [M+K] HRMS(ESI): Calcd. for C11H19O3 [M+H]: 199.1329, found: 199.1331.
−21.6 (c 0.45, CHCl3) 1H-NMR (CDCl3) δ 7.04–6.89 (m, 1H), 5.86 (ddd, J = 15.5, 3.0, 1.7 Hz, 1H), 4.19 (q, J = 7.1 Hz, 2H), 3.40 (d, J = 10.8 Hz, 1H), 3.36 (d, J = 10.8 Hz, 1H), 2.18 (dd, J = 7.9, 1.3 Hz, 2H), 1.44–1.22 (m, 5H), 0.92–0.79 (m, 6H). 13C-NMR (CDCl3) δ 205.3, 166.0, 143.6, 124.6, 60.4, 49.3, 36.7, 27.9, 18.6, 14.2, 8.3. ESI-MS: 237.1, [M+K] HRMS(ESI): Calcd. for C11H19O3 [M+H]: 199.1329, found: 199.1331.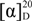 −39.2 (c 0.65, CHCl3) 1H-NMR (CDCl3) δ 6.96–6.75 (m, 1H), 6.44 (d, J = 14.7 Hz, 1H), 5.97 (d, J = 14.7 Hz, 1H), 5.82 (d, J = 15.5 Hz, 1H), 4.19 (q, J = 7.1 Hz, 2H), 2.26–2.13 (m, 2H), 1.43–1.24 (m, 5H), 0.98 (s, 3H), 0.82 (t, J = 7.1 Hz, 3H). 13C-NMR (CDCl3) δ 166.2, 153.2, 144.8, 124.0, 74.3, 60.2, 44.3, 42.6, 32.6, 22.1, 14.2, 8.4. ESI-MS: 323.0 [M+H], HRMS(ESI): Calcd. for C12H20IO2 [M+H]: 323.0502, found: 323.0511.
−39.2 (c 0.65, CHCl3) 1H-NMR (CDCl3) δ 6.96–6.75 (m, 1H), 6.44 (d, J = 14.7 Hz, 1H), 5.97 (d, J = 14.7 Hz, 1H), 5.82 (d, J = 15.5 Hz, 1H), 4.19 (q, J = 7.1 Hz, 2H), 2.26–2.13 (m, 2H), 1.43–1.24 (m, 5H), 0.98 (s, 3H), 0.82 (t, J = 7.1 Hz, 3H). 13C-NMR (CDCl3) δ 166.2, 153.2, 144.8, 124.0, 74.3, 60.2, 44.3, 42.6, 32.6, 22.1, 14.2, 8.4. ESI-MS: 323.0 [M+H], HRMS(ESI): Calcd. for C12H20IO2 [M+H]: 323.0502, found: 323.0511.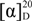 −40.4 (c 0.50, CHCl3). 1H-NMR (CDCl3) δ 7.77–7.66 (m, 4H), 7.46–7.31 (m, 6H), 7.10 (d, J = 8.4 Hz, 2H), 6.91 (dt, J = 15.4, 7.6 Hz, 1H), 6.70 (d, J = 8.4 Hz, 2H), 6.16 (d, J = 16.3 Hz, 1H), 5.90 (d, J = 16.3 Hz, 1H), 5.81 (d, J = 15.5 Hz, 1H), 4.16 (q, J = 7.1 Hz, 2H), 2.31–2.18 (m, 2H), 1.49–1.33 (m, 2H), 1.26 (t, J = 7.1 Hz, 3H), 1.09 (s, 9H), 1.03 (s, 3H), 0.80 (t, J = 7.4 Hz, 3H). 13C-NMR (CDCl3) δ 166.4, 154.8, 146.2, 135.7, 135.5, 132.9, 130.6, 129.9, 127.8, 127.2, 126.9, 123.4, 119.6, 60.1, 43.7, 39.6, 33.5, 26.5, 19.4, 14.3, 8.5. ESI-MS: 527.2 [M+H].
−40.4 (c 0.50, CHCl3). 1H-NMR (CDCl3) δ 7.77–7.66 (m, 4H), 7.46–7.31 (m, 6H), 7.10 (d, J = 8.4 Hz, 2H), 6.91 (dt, J = 15.4, 7.6 Hz, 1H), 6.70 (d, J = 8.4 Hz, 2H), 6.16 (d, J = 16.3 Hz, 1H), 5.90 (d, J = 16.3 Hz, 1H), 5.81 (d, J = 15.5 Hz, 1H), 4.16 (q, J = 7.1 Hz, 2H), 2.31–2.18 (m, 2H), 1.49–1.33 (m, 2H), 1.26 (t, J = 7.1 Hz, 3H), 1.09 (s, 9H), 1.03 (s, 3H), 0.80 (t, J = 7.4 Hz, 3H). 13C-NMR (CDCl3) δ 166.4, 154.8, 146.2, 135.7, 135.5, 132.9, 130.6, 129.9, 127.8, 127.2, 126.9, 123.4, 119.6, 60.1, 43.7, 39.6, 33.5, 26.5, 19.4, 14.3, 8.5. ESI-MS: 527.2 [M+H].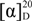 −3.1 (c 0.55, CHCl3). 1H-NMR (CDCl3) δ 7.39–7.17 (m, 5H), 6.93 (dt, J = 15.4, 7.6 Hz, 1H), 6.36 (d, J = 16.2 Hz, 1H), 6.20 (d, J = 16.2 Hz, 1H), 5.93–5.82 (m, 2H), 5.11 (d, J = 10.7 Hz, 1H), 5.06 (d, J = 17.5 Hz, 1H), 4.19 (q, J = 7.1 Hz, 2H), 2.41 (d, J = 7.6 Hz, 2H), 1.28 (t, J = 7.1 Hz, 3H), 1.23 (s, 3H). 13C-NMR (CDCl3) δ 166.3, 145.3, 144.4, 137.3, 136.4, 128.5, 128.1, 127.2, 126.2, 123.9, 113.1, 60.2, 43.8, 42.62, 23.7, 14.2. ESI-MS: 271.2 [M+H], 293.1 [M+Na], HRMS (ESI): Calcd. for C18H22O2Na [M+H]: 293.1512, found: 293.1518.
−3.1 (c 0.55, CHCl3). 1H-NMR (CDCl3) δ 7.39–7.17 (m, 5H), 6.93 (dt, J = 15.4, 7.6 Hz, 1H), 6.36 (d, J = 16.2 Hz, 1H), 6.20 (d, J = 16.2 Hz, 1H), 5.93–5.82 (m, 2H), 5.11 (d, J = 10.7 Hz, 1H), 5.06 (d, J = 17.5 Hz, 1H), 4.19 (q, J = 7.1 Hz, 2H), 2.41 (d, J = 7.6 Hz, 2H), 1.28 (t, J = 7.1 Hz, 3H), 1.23 (s, 3H). 13C-NMR (CDCl3) δ 166.3, 145.3, 144.4, 137.3, 136.4, 128.5, 128.1, 127.2, 126.2, 123.9, 113.1, 60.2, 43.8, 42.62, 23.7, 14.2. ESI-MS: 271.2 [M+H], 293.1 [M+Na], HRMS (ESI): Calcd. for C18H22O2Na [M+H]: 293.1512, found: 293.1518.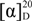 +3.3 (c 0.55, CHCl3). 1H-NMR (CDCl3) δ 7.25 (d, J = 7.7 Hz, 2H), 7.11 (d, J = 7.7 Hz, 2H), 6.92 (dt, J = 15.4, 7.6 Hz, 1H), 6.32 (d, J = 16.2 Hz, 1H), 6.14 (d, J = 16.2 Hz, 1H), 5.94–5.77 (m, 2H), 5.12–5.01 (m, 2H), 4.17 (q, 7.2 Hz, 2H), 2.40 (d, J = 7.4 Hz, 2H), 2.33 (s, 3H), 1.28 (t, J = 7.2 Hz, 3H), 1.21 (s, 3H). 13C-NMR (CDCl3) δ 166.3, 145.5, 144.6, 137.0, 135.4, 134.5, 129.2, 127.9, 126.1, 123.8, 113.0, 60.2, 43.8, 42.6, 23.7, 21.1, 14.2. ESI-MS: 307.1 [M+Na], HRMS(ESI): Calcd. for C19H25O2 [M+H]: 285.1849, found: 285.1855.
+3.3 (c 0.55, CHCl3). 1H-NMR (CDCl3) δ 7.25 (d, J = 7.7 Hz, 2H), 7.11 (d, J = 7.7 Hz, 2H), 6.92 (dt, J = 15.4, 7.6 Hz, 1H), 6.32 (d, J = 16.2 Hz, 1H), 6.14 (d, J = 16.2 Hz, 1H), 5.94–5.77 (m, 2H), 5.12–5.01 (m, 2H), 4.17 (q, 7.2 Hz, 2H), 2.40 (d, J = 7.4 Hz, 2H), 2.33 (s, 3H), 1.28 (t, J = 7.2 Hz, 3H), 1.21 (s, 3H). 13C-NMR (CDCl3) δ 166.3, 145.5, 144.6, 137.0, 135.4, 134.5, 129.2, 127.9, 126.1, 123.8, 113.0, 60.2, 43.8, 42.6, 23.7, 21.1, 14.2. ESI-MS: 307.1 [M+Na], HRMS(ESI): Calcd. for C19H25O2 [M+H]: 285.1849, found: 285.1855.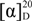 −4.4 (c 0.50, CHCl3). 1H-NMR (CDCl3) δ 7.84–7.74 (m, 3H), 7.71 (s, 1H), 7.59 (d, J = 8.6 Hz, 1H), 7.50–7.37 (m, 2H), 6.96 (dt, J = 15.4, 7.5 Hz, 1H), 6.52 (d, J = 16.2 Hz, 1H), 6.33 (d, J = 16.2 Hz, 1H), 6.00–5.82 (m, 2H), 5.17–5.05 (m, 2H), 4.18 (q, 7.1 Hz, 2H), 2.46 (d, J = 7.5 Hz, 2H), 1.30–1.23 (m, 6H). ESI-MS: 343.3 [M+Na], HRMS (ESI): Calcd. for C22H25O2 [M+H]: 321.1849, found: 321.1857.
−4.4 (c 0.50, CHCl3). 1H-NMR (CDCl3) δ 7.84–7.74 (m, 3H), 7.71 (s, 1H), 7.59 (d, J = 8.6 Hz, 1H), 7.50–7.37 (m, 2H), 6.96 (dt, J = 15.4, 7.5 Hz, 1H), 6.52 (d, J = 16.2 Hz, 1H), 6.33 (d, J = 16.2 Hz, 1H), 6.00–5.82 (m, 2H), 5.17–5.05 (m, 2H), 4.18 (q, 7.1 Hz, 2H), 2.46 (d, J = 7.5 Hz, 2H), 1.30–1.23 (m, 6H). ESI-MS: 343.3 [M+Na], HRMS (ESI): Calcd. for C22H25O2 [M+H]: 321.1849, found: 321.1857.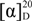 +9.8 (c 0.50, CHCl3). 1H-NMR (CDCl3) δ 7.55 (d, J = 8.2 Hz, 2H), 7.45 (d, J = 8.2 Hz, 2H), 6.98–6.84 (m, 1H), 6.39 (d, J = 16.2 Hz, 1H), 6.29 (d, J = 16.2 Hz, 1H), 5.96–5.82 (m, 2H), 5.20–5.11 (m, 1H), 5.08 (dd, J = 17.4, 0.8 Hz, 1H), 4.18 (q, J = 7.1 Hz, 2H), 2.43 (dd, J = 7.6, 1.3 Hz, 2H), 1.28 (t, J = 7.1 Hz, 3H), 1.24 (s, 3H). 13C-NMR (CDCl3) δ 166.2 (C4), 144.9 (C7), 143.9 (C11), 140.8 (C15), 139.1 (C14), 128.9 (C18), 127.0 (C10), 126.3 (C16), 125.5 (C17), 124.1 (C5), 113.6 (13), 60.3 (C2), 43.6 (C8), 42.8 (C9), 23.5 (C12), 14.2 (C1).ESI-MS: 361.2 [M+Na], HRMS(ESI): Calcd. for C19H22F3O2 [M+H]: 339.1566, found: 339.1575.
+9.8 (c 0.50, CHCl3). 1H-NMR (CDCl3) δ 7.55 (d, J = 8.2 Hz, 2H), 7.45 (d, J = 8.2 Hz, 2H), 6.98–6.84 (m, 1H), 6.39 (d, J = 16.2 Hz, 1H), 6.29 (d, J = 16.2 Hz, 1H), 5.96–5.82 (m, 2H), 5.20–5.11 (m, 1H), 5.08 (dd, J = 17.4, 0.8 Hz, 1H), 4.18 (q, J = 7.1 Hz, 2H), 2.43 (dd, J = 7.6, 1.3 Hz, 2H), 1.28 (t, J = 7.1 Hz, 3H), 1.24 (s, 3H). 13C-NMR (CDCl3) δ 166.2 (C4), 144.9 (C7), 143.9 (C11), 140.8 (C15), 139.1 (C14), 128.9 (C18), 127.0 (C10), 126.3 (C16), 125.5 (C17), 124.1 (C5), 113.6 (13), 60.3 (C2), 43.6 (C8), 42.8 (C9), 23.5 (C12), 14.2 (C1).ESI-MS: 361.2 [M+Na], HRMS(ESI): Calcd. for C19H22F3O2 [M+H]: 339.1566, found: 339.1575.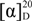 −15.0 (c 0.50, CHCl3). 1H-NMR (CDCl3) δ 6.97–6.86 (m, 2H, C(7)H, C(20)H), 6.81–6.71 (m, 2H, C(16)H, C(17)H), 6.26 (d, J = 16.2 Hz, 1H, C(14)H), 6.02 (d, J = 16.2 Hz, 1H, C(10)H), 5.94 (s, 2H, C(22)H), 5.92–5.81 (m, 2H, C(5)H, C(11)H), 5.10 (dd, J = 10.7, 0.7 Hz, 1H, C(13)H), 5.05 (d, J = 17.5 Hz, 1H, C(13)H), 4.18 (q, J = 7.1 Hz, 2H, C(2)H2), 2.42–2.36 (m, 2H, C(8)H), 1.28 (t, J = 7.1 Hz, 3H, C(1)H), 1.20 (s, 3H, C(12)H3). 13C-NMR (CDCl3) δ 166.3 (C(4)), 147.9 (C(19)), 146.9 (C(18)), 145.4 (C(7)), 144.5 (C(11)), 134.7 (C(14)), 131.8 (C(15)), 127.6 (C(10)), 123.8 (C(5)), 120.7, 113.0 (C(13)), 108.2, 105.5, 101.0 (C(16), C(17), C(20)), 60.2 (C(2)), 43.8 (C(8)), 42.5 (C(9)), 23.7 (C(12)), 14.2 (C(1)). ESI-MS: 315.2[M+H], 337.2 [M+Na], HRMS(ESI): Calcd. for C19H23O4 [M+H]: 315.1591, found: 315.1602.
−15.0 (c 0.50, CHCl3). 1H-NMR (CDCl3) δ 6.97–6.86 (m, 2H, C(7)H, C(20)H), 6.81–6.71 (m, 2H, C(16)H, C(17)H), 6.26 (d, J = 16.2 Hz, 1H, C(14)H), 6.02 (d, J = 16.2 Hz, 1H, C(10)H), 5.94 (s, 2H, C(22)H), 5.92–5.81 (m, 2H, C(5)H, C(11)H), 5.10 (dd, J = 10.7, 0.7 Hz, 1H, C(13)H), 5.05 (d, J = 17.5 Hz, 1H, C(13)H), 4.18 (q, J = 7.1 Hz, 2H, C(2)H2), 2.42–2.36 (m, 2H, C(8)H), 1.28 (t, J = 7.1 Hz, 3H, C(1)H), 1.20 (s, 3H, C(12)H3). 13C-NMR (CDCl3) δ 166.3 (C(4)), 147.9 (C(19)), 146.9 (C(18)), 145.4 (C(7)), 144.5 (C(11)), 134.7 (C(14)), 131.8 (C(15)), 127.6 (C(10)), 123.8 (C(5)), 120.7, 113.0 (C(13)), 108.2, 105.5, 101.0 (C(16), C(17), C(20)), 60.2 (C(2)), 43.8 (C(8)), 42.5 (C(9)), 23.7 (C(12)), 14.2 (C(1)). ESI-MS: 315.2[M+H], 337.2 [M+Na], HRMS(ESI): Calcd. for C19H23O4 [M+H]: 315.1591, found: 315.1602.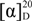 −65.5 (c 0.20, CHCl3). 1H-NMR (CDCl3) δ 7.76–7.67 (m, 4H), 7.46–7.31 (m, 6H), 7.09 (d, J = 8.6 Hz, 2H), 6.69 (d, J = 8.6 Hz, 2H), 6.12 (d, J = 16.3 Hz, 1H), 5.90 (d, J = 16.2 Hz, 1H), 5.64–5.50 (m, 2H), 2.12–1.98 (m, 2H), 1.40–1.32 (m, 2H), 1.27 (s, 6H), 1.09 (s, 9H), 0.98 (s, 3H), 0.79 (t, J = 7.4 Hz, 3H). 13C-NMR (CDCl3) δ 154.6, 140.6, 136.9, 135.5, 132.9, 130.9, 129.8, 127.7, 126.8, 126.5, 123.4, 119.6, 70.7, 43.6, 39.4, 33.3, 29.9, 26.5, 22.9, 19.4, 8.6. ESI-MS: 530.2 [M+NH4].
−65.5 (c 0.20, CHCl3). 1H-NMR (CDCl3) δ 7.76–7.67 (m, 4H), 7.46–7.31 (m, 6H), 7.09 (d, J = 8.6 Hz, 2H), 6.69 (d, J = 8.6 Hz, 2H), 6.12 (d, J = 16.3 Hz, 1H), 5.90 (d, J = 16.2 Hz, 1H), 5.64–5.50 (m, 2H), 2.12–1.98 (m, 2H), 1.40–1.32 (m, 2H), 1.27 (s, 6H), 1.09 (s, 9H), 0.98 (s, 3H), 0.79 (t, J = 7.4 Hz, 3H). 13C-NMR (CDCl3) δ 154.6, 140.6, 136.9, 135.5, 132.9, 130.9, 129.8, 127.7, 126.8, 126.5, 123.4, 119.6, 70.7, 43.6, 39.4, 33.3, 29.9, 26.5, 22.9, 19.4, 8.6. ESI-MS: 530.2 [M+NH4].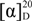 −36.3 (c 0.07, CHCl3). 1H-NMR (CDCl3) δ 7.23 (d, J = 8.6 Hz, 2H), 6.77 (d, J = 8.6 Hz, 2H), 6.18 (d, J = 16.3 Hz, 1H), 5.96 (d, J = 16.3 Hz, 1H), 5.68–5.49 (m, 2H), 2.17–2.03 (m, 2H), 1.46–1.34 (m, 2H), 1.30 (s, 6H), 1.02 (s, 3H), 0.82 (t, J = 7.5 Hz, 3H). 13C-NMR (CDCl3) δ 154.7, 140.5, 136.8, 130.8, 127.2, 126.4, 123.5, 115.3, 70.9, 43.6, 39.5, 33.3, 29.8, 22.8, 8.6. ESI-MS: 530.2 [M+NH4].
−36.3 (c 0.07, CHCl3). 1H-NMR (CDCl3) δ 7.23 (d, J = 8.6 Hz, 2H), 6.77 (d, J = 8.6 Hz, 2H), 6.18 (d, J = 16.3 Hz, 1H), 5.96 (d, J = 16.3 Hz, 1H), 5.68–5.49 (m, 2H), 2.17–2.03 (m, 2H), 1.46–1.34 (m, 2H), 1.30 (s, 6H), 1.02 (s, 3H), 0.82 (t, J = 7.5 Hz, 3H). 13C-NMR (CDCl3) δ 154.7, 140.5, 136.8, 130.8, 127.2, 126.4, 123.5, 115.3, 70.9, 43.6, 39.5, 33.3, 29.8, 22.8, 8.6. ESI-MS: 530.2 [M+NH4].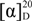 −9.5 (c 0.55, CHCl3). 1H-NMR (CDCl3) δ 7.36 (d, J = 8.0 Hz, 2H), 7.33–7.25 (m, 2H), 7.20 (t, J = 6.6 Hz, 1H), 6.32 (d, J = 16.2 Hz, 1H), 6.20 (d, J = 16.2 Hz, 1H), 5.89 (dd, J = 17.4, 10.7 Hz, 1H), 5.70–5.55 (m, 2H), 5.09–4.98 (m, 2H), 2.23 (d, J = 6.3 Hz, 2H), 1.38 (s, 1H), 1.30 (s, 6H), 1.18 (s, 3H). 13C-NMR (CDCl3) δ 145.3, 141.2, 137.6, 137.4, 128.5, 127.4, 127.0, 126.1, 122.9, 112.3, 70.7, 43.8, 42. 7, 29.9, 23.4. ESI-MS: 274.2 [M+NH4], HRMS (ESI): Calcd. for C18H25O [M+H]: 257.1900, found: 257.1899.
−9.5 (c 0.55, CHCl3). 1H-NMR (CDCl3) δ 7.36 (d, J = 8.0 Hz, 2H), 7.33–7.25 (m, 2H), 7.20 (t, J = 6.6 Hz, 1H), 6.32 (d, J = 16.2 Hz, 1H), 6.20 (d, J = 16.2 Hz, 1H), 5.89 (dd, J = 17.4, 10.7 Hz, 1H), 5.70–5.55 (m, 2H), 5.09–4.98 (m, 2H), 2.23 (d, J = 6.3 Hz, 2H), 1.38 (s, 1H), 1.30 (s, 6H), 1.18 (s, 3H). 13C-NMR (CDCl3) δ 145.3, 141.2, 137.6, 137.4, 128.5, 127.4, 127.0, 126.1, 122.9, 112.3, 70.7, 43.8, 42. 7, 29.9, 23.4. ESI-MS: 274.2 [M+NH4], HRMS (ESI): Calcd. for C18H25O [M+H]: 257.1900, found: 257.1899.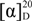 −9.1 (c 0.55, CHCl3). 1H-NMR (CDCl3) δ 7.25 (d, J = 7.3 Hz, 2H), 7.10 (d, J = 7.3 Hz, 2H), 6.28 (d, J = 16.2 Hz, 1H), 6.14 (d, J = 16.2 Hz, 1H), 5.88 (dd, J = 17.4, 10.7 Hz, 1H), 5.70–5.53 (m, 2H), 5.11–4.94 (m, 2H), 2.32 (s, 3H), 2.22 (d, J = 5.9 Hz, 2H), 1.42 (brs, 1H), 1.29 (s, 6H), 1.17 (s, 3H). 13C-NMR (CDCl3) δ 145.4, 141.1, 136.7, 136.4, 134.9, 129.2, 127.3, 126.0, 123.0, 112.2, 70.7, 43.8, 42.6, 29.8, 23.4, 21.1. ESI-MS: 293.2 [M+Na].
−9.1 (c 0.55, CHCl3). 1H-NMR (CDCl3) δ 7.25 (d, J = 7.3 Hz, 2H), 7.10 (d, J = 7.3 Hz, 2H), 6.28 (d, J = 16.2 Hz, 1H), 6.14 (d, J = 16.2 Hz, 1H), 5.88 (dd, J = 17.4, 10.7 Hz, 1H), 5.70–5.53 (m, 2H), 5.11–4.94 (m, 2H), 2.32 (s, 3H), 2.22 (d, J = 5.9 Hz, 2H), 1.42 (brs, 1H), 1.29 (s, 6H), 1.17 (s, 3H). 13C-NMR (CDCl3) δ 145.4, 141.1, 136.7, 136.4, 134.9, 129.2, 127.3, 126.0, 123.0, 112.2, 70.7, 43.8, 42.6, 29.8, 23.4, 21.1. ESI-MS: 293.2 [M+Na].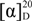 −13.7 (c 0.35, CHCl3). 1H-NMR (CDCl3) δ 7.83–7.74 (m, 3H), 7.70 (s, 1H), 7.59 (d, J = 8.6 Hz, 1H), 7.48–7.38 (m, 2H), 6.49 (d, J = 16.2 Hz, 1H), 6.34 (dd, J = 16.2, 2.0 Hz, 1H), 5.93 (ddd, J = 17.4, 10.7, 2.0 Hz, 1H), 5.72-5.57 (m, 2H), 5.14–5.01 (m, 2H), 2.26 (dd, J = 5.9, 1.7 Hz, 2H), 1.41 (s, 1H), 1.30 (s, 6H), 1.23 (s, 3H). 13C-NMR (CDCl3) δ 145.3, 141.2, 137.9, 135.1, 133.6, 132.7, 128.0, 127.8, 127.6, 126.1, 125.68, 125.5, 123.5, 122.9, 112.4, 70.8, 43.8, 42.8, 29.9, 23.4. ESI-MS: 293.2 [M+Na], HRMS (ESI): Calcd. for C22H26ONa [M+Na]: 329.1876, found: 329.1885.
−13.7 (c 0.35, CHCl3). 1H-NMR (CDCl3) δ 7.83–7.74 (m, 3H), 7.70 (s, 1H), 7.59 (d, J = 8.6 Hz, 1H), 7.48–7.38 (m, 2H), 6.49 (d, J = 16.2 Hz, 1H), 6.34 (dd, J = 16.2, 2.0 Hz, 1H), 5.93 (ddd, J = 17.4, 10.7, 2.0 Hz, 1H), 5.72-5.57 (m, 2H), 5.14–5.01 (m, 2H), 2.26 (dd, J = 5.9, 1.7 Hz, 2H), 1.41 (s, 1H), 1.30 (s, 6H), 1.23 (s, 3H). 13C-NMR (CDCl3) δ 145.3, 141.2, 137.9, 135.1, 133.6, 132.7, 128.0, 127.8, 127.6, 126.1, 125.68, 125.5, 123.5, 122.9, 112.4, 70.8, 43.8, 42.8, 29.9, 23.4. ESI-MS: 293.2 [M+Na], HRMS (ESI): Calcd. for C22H26ONa [M+Na]: 329.1876, found: 329.1885.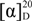 +2.0 (c 0.75, CHCl3). 1H-NMR (CDCl3) δ 7.54 (d, J = 8.2 Hz, 2H), 7.44 (d, J = 8.2 Hz, 2H), 6.35 (d, J = 16.3 Hz, 1H), 6.30 (d, J = 16.3 Hz, 1H), 5.89 (dd, J = 17.5, 10.7 Hz, 1H), 5.70–5.53 (m, 2H), 5.09 (dd, J = 10.7, 1.0 Hz, 1H), 5.03 (dd, J = 17.5, 1.0 Hz, 1H), 2.24 (d, J = 6.6 Hz, 2H), 1.37 (brs, 1H), 1.30 (s, 6H), 1.20 (s, 3H). 13C-NMR (CDCl3) δ 144.7, 141.4, 141.1, 140.2, 126.3, 126.2, 125.4, 125.4, 122.5, 112.8, 70.7, 43.7, 42.9, 29.9, 23.2. ESI-MS: 347.2 [M+Na], HRMS(ESI): Calcd. for C19H24F3O [M+H]: 325.1774, found: 325.1774.
+2.0 (c 0.75, CHCl3). 1H-NMR (CDCl3) δ 7.54 (d, J = 8.2 Hz, 2H), 7.44 (d, J = 8.2 Hz, 2H), 6.35 (d, J = 16.3 Hz, 1H), 6.30 (d, J = 16.3 Hz, 1H), 5.89 (dd, J = 17.5, 10.7 Hz, 1H), 5.70–5.53 (m, 2H), 5.09 (dd, J = 10.7, 1.0 Hz, 1H), 5.03 (dd, J = 17.5, 1.0 Hz, 1H), 2.24 (d, J = 6.6 Hz, 2H), 1.37 (brs, 1H), 1.30 (s, 6H), 1.20 (s, 3H). 13C-NMR (CDCl3) δ 144.7, 141.4, 141.1, 140.2, 126.3, 126.2, 125.4, 125.4, 122.5, 112.8, 70.7, 43.7, 42.9, 29.9, 23.2. ESI-MS: 347.2 [M+Na], HRMS(ESI): Calcd. for C19H24F3O [M+H]: 325.1774, found: 325.1774.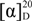 −10.2 (c 0.50, CHCl3). 1H-NMR (CDCl3) δ 6.91 (s, 1H), 6.77 (d, J = 8.0 Hz, 1H), 6.73 (d, J = 8.0 Hz, 1H), 6.22 (d, J = 16.2 Hz, 1H), 6.03 (d, J = 16.2 Hz, 1H), 5.93 (d, J = 1.0 Hz, 2H), 5.87 (dd, J = 17.5, 10.7 Hz, 1H), 5.68–5.52 (m, 2H), 5.05 (d, J = 10.7 Hz, 1H), 5.00 (d, J = 17.5 Hz, 1H), 2.21 (d, J = 6.5 Hz, 2H), 1.42 (brs, 1H), 1.29 (s, 6H), 1.16 (s, 3H). 13C-NMR (CDCl3) δ 147.9, 146.7, 145.3, 141.1, 135.7, 132.2, 127.0, 122. 9, 120.5, 112.3, 108.2, 105.4, 100.9, 70.7, 43.8, 42.6, 29.9, 29.7, 23.4. ESI-MS: 318.4 [M+NH4], HRMS (ESI): Calcd. for C19H24O3Na [M+Na]: 323.1618, found: 323.1639.
−10.2 (c 0.50, CHCl3). 1H-NMR (CDCl3) δ 6.91 (s, 1H), 6.77 (d, J = 8.0 Hz, 1H), 6.73 (d, J = 8.0 Hz, 1H), 6.22 (d, J = 16.2 Hz, 1H), 6.03 (d, J = 16.2 Hz, 1H), 5.93 (d, J = 1.0 Hz, 2H), 5.87 (dd, J = 17.5, 10.7 Hz, 1H), 5.68–5.52 (m, 2H), 5.05 (d, J = 10.7 Hz, 1H), 5.00 (d, J = 17.5 Hz, 1H), 2.21 (d, J = 6.5 Hz, 2H), 1.42 (brs, 1H), 1.29 (s, 6H), 1.16 (s, 3H). 13C-NMR (CDCl3) δ 147.9, 146.7, 145.3, 141.1, 135.7, 132.2, 127.0, 122. 9, 120.5, 112.3, 108.2, 105.4, 100.9, 70.7, 43.8, 42.6, 29.9, 29.7, 23.4. ESI-MS: 318.4 [M+NH4], HRMS (ESI): Calcd. for C19H24O3Na [M+Na]: 323.1618, found: 323.1639.3.3. Activity Tests
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Sun, N.J.; Woo, S.H.; Cassady, J.M.; Snapka, R.M. DNA polymerase and topoisomerase II inhibitors from Psoralea corylifolia. J. Nat. Prod. 1998, 61, 362–366. [Google Scholar] [CrossRef]
- Miura, H.; Nishida, H.; Linuma, M. Effect of Crude Fractions of Psoralea corylifolia Seed Extract on Bone Calcification. Planta Med. 1996, 62, 150–153. [Google Scholar] [CrossRef]
- Yin, S.; Fan, C.-Q.; Yue, J.-M. Cyclobakuchiol C, a new bakuchiol derivative from Psoralea coryllfolia. J. Asian Nat. Prod. Res. 2007, 9, 29–33. [Google Scholar] [CrossRef]
- Matsuda, H.; Sugimoto, S.; Morikawa, T.; Matsuhira, K.; Mizuguchi, E.; Nakamura, S.; Yoshikawa, M. Bioactive constituents from chinese natural medicines. XX. Inhibitors of antigen-induced degranulation in RBL-2H3 cells from the seeds of Psoralea corylifolia. Chem. Pharm. Bull. 2007, 55, 106–110. [Google Scholar] [CrossRef]
- Wu, C.-Z.; Cai, X.F.; Dat, N.T.; Hong, S.S.; Han, A.-R.; Seo, E.-K.; Hwang, B.Y.; Nan, J.-X.; Lee, D.; Lee, J.J. Bisbakuchiols A and B, novel dimeric meroterpenoids from Psoralea corylifolia. Tetrahedron Lett. 2007, 48, 8861–8864. [Google Scholar] [CrossRef]
- Yin, S.; Fan, C.-Q.; Dong, L.; Yue, J.-M. Psoracorylifols A–E, five novel compounds with activity against Helicobacter pylori from seeds of Psoralea corylifolia. Tetrahedron 2006, 62, 2569–2575. [Google Scholar] [CrossRef]
- Backhouse, C.N.; Delporte, C.L.; Negrete, R.E.; Erazo, S.; Zuniga, A.; Pinto, A.; Cassels, B.K. Active constituents isolated from Psoralea glandulosa L. with antiinflammatory and antipyretic activities. J. Ethnopharmacol. 2001, 78, 27–31. [Google Scholar] [CrossRef]
- Majeed, R.; Reddy, M.V.; Chinthakindi, P.K.; Sangwan, P.L.; Hamid, A.; Chashoo, G.; Saxena, A.K.; Koul, S. Bakuchiol derivatives as novel and potent cytotoxic agents: A report. Eur. J. Med. Chem. 2012, 49, 55–67. [Google Scholar] [CrossRef]
- Reddy, M.V.; Thota, N.; Sangwan, P.L.; Malhotra, P.; Ali, F.; Khan, I.A.; Chimni, S.S.; Koul, S. Novel bisstyryl derivatives of bakuchiol: Targeting oral cavity pathogens. Eur. J. Med. Chem. 2010, 45, 3125–3134. [Google Scholar] [CrossRef]
- Chen, H.; Du, X.; Tang, W.; Zhou, Y.; Zuo, J.; Feng, H.; Li, Y. Synthesis and structure–immunosuppressive activity relationships of bakuchiol and its derivatives. Bioorg. Med. Chem. 2008, 16, 2403–2411. [Google Scholar] [CrossRef]
- Chen, Z.; Jin, K.; Gao, L.; Lou, G.; Jin, Y.; Yu, Y.; Lou, Y. Anti-tumor effects of bakuchiol, an analogue of resveratrol, on human lung adenocarcinoma A549 cell line. Eur. J. Pharmcol. 2010, 643, 170–179. [Google Scholar] [CrossRef]
- Yan, D.-M.; Chang, Y.-X.; Wang, Y.-F.; Liu, E.-W.; Li, J.; Kang, L.-Y.; Gao, X.-M. In vivo pharmacokinetics of bakuchiol after oral administration of bakuchiol extraction in rat plasma. J. Ethnopharmacol. 2010, 128, 697–702. [Google Scholar] [CrossRef]
- Choi, S.Y.; Lee, S.; Choi, W.-H.; Lee, Y.; Jo, Y.O.; Ha, T.-Y. Isolation and anti-inflammatory activity of bakuchiol from Ulmus davidiana var. japonica. J. Med. Food 2010, 13, 1019–1023. [Google Scholar] [CrossRef]
- Lim, S.-H.; Ha, T.-Y.; Kim, S.-R.; Ahn, J.; Park, H.J.; Kim, S. Ethanol extract of Psoralea corylifolia L. and its main constituent, bakuchiol, reduce bone loss in ovariectomised Sprague–Dawley rats. Br. J. Nutr. 2009, 101, 1031–1039. [Google Scholar] [CrossRef]
- Choi, Y.H.; Yon, G.H.; Hong, K.S.; Yoo, D.S.; Choi, C.W.; Park, W.-K.; Kong, J.Y.; Kim, Y.S.; Ryu, S.Y. In vitro BACE-1 inhibitory phenolic components from the seeds of Psoralea corylifolia. Planta Med. 2008, 74, 1405–1408. [Google Scholar] [CrossRef]
- Wu, C.-Z.; Hong, S.S.; Cai, X.F.; Dat, N.T.; Nan, J.-X.; Hwang, B.Y.; Lee, J.J.; Lee, D. Hypoxia-inducible factor-1 and nuclear factor-κB inhibitory meroterpene analogues of bakuchiol, a constituent of the seeds of Psoralea corylifolia. Bioorg. Med. Chem. Lett. 2008, 18, 2619–2623. [Google Scholar] [CrossRef]
- Kim, Y.-C.; Oh, H.; Kim, B.S.; Kang, T.-H.; Ko, E.-K.; Han, Y.M.; Kim, B.Y.; Ahn, J.S. In vitro Protein Tyrosine Phosphatase 1B inhibitory phenols from the seeds of Psoralea corylifolia. Planta Med. 2005, 71, 87–89. [Google Scholar] [CrossRef]
- Park, E.-J.; Zhao, Y.-Z.; Kim, Y.-C.; Sohn, D.H. Bakuchiol-induced caspase-3-dependent apoptosis occurs through c-Jun NH2-terminal kinase-mediated mitochondrial translocation of Bax in rat liver myofibroblasts. Eur. J. Pharmacol. 2007, 559, 115–123. [Google Scholar] [CrossRef]
- Adhikari, S.; Joshi, R.; Patro, B.S.; Ghanty, T.K.; Chintalwar, G.J.; Sharma, A.; Chattopadhyay, S.; Mukherjee, T. Antioxidant activity of bakuchiol: Experimental evidences and theoretical treatments on the possible involvement of the terpenoid chain. Chem. Res. Toxicol. 2003, 16, 1062–1069. [Google Scholar] [CrossRef]
- Haraguchi, H.; Inoue, J.; Tamura, Y.; Mizutani, K. Antioxidative components of Psoralea corylifolia (Leguminosae). Phytother. Res. 2002, 16, 539–544. [Google Scholar] [CrossRef]
- Cho, H.; Jun, J.-Y.; Song, E.-K.; Kang, K.-H.; Baek, H.-Y.; Ko, Y.-S.; Kim, Y.-C. Bakuchiol: A hepatoprotective compound of Psoralea corylifolia on tacrine-induced cytotoxicity in Hep G2 Cells. Planta Med. 2001, 67, 750–751. [Google Scholar] [CrossRef]
- Haraguchi, H.; Inoue, J.; Tamura, Y.; Mizutani, K. Inhibition of mitochondrial lipid peroxidation by bakuchiol, a meroterpene from Psoralea corylifolia. Planta Med. 2000, 66, 569–571. [Google Scholar] [CrossRef]
- Krenisky, J.M.; Luo, J.; Reed, M.J.; Carney, J.R. Isolation and antihyperglycemic activity of bakuchiol from Otholobium pubescens (Fabaceae), a peruvian medicinal plant used for the treatment of diabetes. Biol. Pharm. Bull. 1999, 22, 1137–1140. [Google Scholar] [CrossRef]
- Labbé, C.; Faini, F.; Coll, J.; Connolly, J.D. Bakuchiol derivatives from the leaves of Psoralea glandulosa. Phytochemistry 1996, 42, 1299–1303. [Google Scholar] [CrossRef]
- Shah, C.C.; Bhalla, V.K.; Dev, S. Meroterpenoids-V: Psoralea Corylifolia Linn. - 4. 2,3-Epoxybakuchiol, Delta 1,3-Hydroxybakuchiol, and Delta 3,2-Hydroxybakuchiol. J. Indian Chem. Soc. 1997, 74, 907–973. [Google Scholar]
- Zhao, G.; Zang, S.-Y.; Zheng, X.-W.; Zhang, X.-H.; Guo, L.-H. Bakuchiol analogs inhibit monoamine transporters and regulate monoaminergic functions. Biochem. Pharmacol. 2008, 75, 1835–1847. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, C.; Fang, L.; Xu, W.; Zhao, Q.; Zhang, J.; Wang, Y.; Lei, X. Synthesis and Evaluation of the Analogues of Penicillide against Cholesterol Ester Transfer Protein. Chin. J. Chem. 2013, 31, 355–370. [Google Scholar] [CrossRef]
- Deng, C.-L.; Zhang, Q.; Fang, L.-S.; Lei, X.; Lin, G.-Q. A Convergent Approach to Dibenzodioxocinones: Synthesis of Racemic Penicillide. Helv. Chim. Acta 2012, 95, 626–635. [Google Scholar] [CrossRef]
- Fang, L.-S.; Zhang, Q.; Deng, C.-L.; Lei, X.; Lin, G.-Q. Regio-selective chlorination of vinca alkaloids catalyzed by Lewis acid. Sci. China Chem. 2011, 54, 1039–1043. [Google Scholar] [CrossRef]
- Lei, X.; Yu, X.; Yin, L.; Liu, Z.; Tang, P.C. Synthesis and biological evaluation of C-12' substituted vinflunine derivatives. Bioorg. Med. Chem. Lett. 2008, 18, 4602–4605. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.; Lei, X.; Lin, G. Synthesis of racemic Δ3-2-Hydroxybakuchiol and its analogues. Helv. Chim. Acta 2010, 93, 555–564. [Google Scholar] [CrossRef]
- Xu, Q.-Q.; Zhao, Q.; Shang, G.-S.; Yang, X.-C.; Lei, X. A facile asymmetric synthesis of Δ3-2-Hydroxybakuchiol, Bakuchiol and ent-Bakuchiol. Tetrahedron 2013, 69, 10739–10746. [Google Scholar]
- Cha, M.-R.; Choi, C.W.; Lee, J.Y.; Kim, Y.S.; Yon, G.H.; Choi, S.-U.; Ryn, S.Y. Anti-proliferative effect of synthesized bakuchiol analogues on cultured human tumor cell lines. Bull. Korean Chem. Soc. 2012, 33, 2378–2380. [Google Scholar]
- Zheng, C.; Hua, C.; Ma, X.; Peng, C.; Zhang, H.; Qin, L. Cytotoxic phenylpropanoid glycosides from Fagopyrum tataricum (L.) Gaertn. Food Chem. 2012, 132, 433–438. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhao, Q.; Xu, Q.; Shan, G.; Dong, C.; Zhang, H.; Lei, X. Synthesis of ∆3-2-Hydroxybakuchiol Analogues and Their Growth Inhibitory Activity against Rat UMR106 Cells. Molecules 2014, 19, 2213-2225. https://doi.org/10.3390/molecules19022213
Zhao Q, Xu Q, Shan G, Dong C, Zhang H, Lei X. Synthesis of ∆3-2-Hydroxybakuchiol Analogues and Their Growth Inhibitory Activity against Rat UMR106 Cells. Molecules. 2014; 19(2):2213-2225. https://doi.org/10.3390/molecules19022213
Chicago/Turabian StyleZhao, Qun, Qianqian Xu, Guangsheng Shan, Chao Dong, Hong Zhang, and Xinsheng Lei. 2014. "Synthesis of ∆3-2-Hydroxybakuchiol Analogues and Their Growth Inhibitory Activity against Rat UMR106 Cells" Molecules 19, no. 2: 2213-2225. https://doi.org/10.3390/molecules19022213
APA StyleZhao, Q., Xu, Q., Shan, G., Dong, C., Zhang, H., & Lei, X. (2014). Synthesis of ∆3-2-Hydroxybakuchiol Analogues and Their Growth Inhibitory Activity against Rat UMR106 Cells. Molecules, 19(2), 2213-2225. https://doi.org/10.3390/molecules19022213