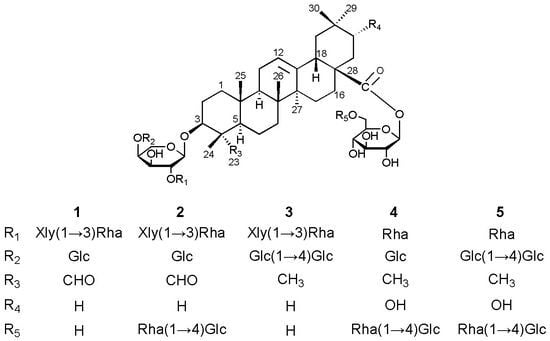
Cytotoxic Oleanane-Type Triterpenoid Saponins from the Rhizomes of Anemone rivularis var. flore-minore
Abstract
:1. Introduction

2. Results and Discussion
| C | 1 | 2 | 3 | 4 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| δC | δH (m, J Hz) | δC | δH (m, J Hz) | δC | δH (m, J Hz) | δC | δH (m, J Hz) | δC | δH (m, J Hz) | |
| 1 | 38.2 | 0.98, 1.56 m | 38.3 | 1.00, 1.60 m | 38.8 | 1.44, 0.88 m | 38.9 | 0.92, 1.46 m | 39.0 | 0.95, 1.48 m |
| 2 | 25.5 | 1.85, 2.09 m | 25.5 | 1.89, 2.09 m | 26.7 | 2.02, 1.81 m | 26.7 | 1.85, 2.06 m | 26.7 | 1.88, 2.10 m |
| 3 | 81.6 | 4.05 m | 81.7 | 4.08 m | 88.6 | 3.23 dd (4.0, 11.6) | 88.7 | 3.27 dd (3.9, 11.6) | 88.7 | 3.29 dd (3.8, 11.6) |
| 4 | 55.5 | − | 55.7 | − | 39.5 | − | 39.5 | − | 39.5 | − |
| 5 | 47.9 | 1.36 m | 48.0 | 1.37 m | 56.0 | 0.75 d (11.5) | 56.0 | 0.78 d (11.5) | 56.0 | 0.79 d (11.5) |
| 6 | 20.6 | 0.96, 1.38 m | 20.6 | 0.98, 1.42 m | 18.5 | 1.42, 1.24 m | 18.5 | 1.25, 1.43 m | 18.5 | 1.26, 1.47 m |
| 7 | 32.5 | 1.18, 1.42 m | 32.5 | 1.19, 1.42 m | 33.1 | 1.40, 1.23 m | 33.1 | 1.24, 1.40 m | 33.2 | 1.25, 1.41 m |
| 8 | 40.2 | − | 40.3 | − | 39.8 | − | 39.8 | − | 39.8 | − |
| 9 | 48.2 | 1.68 m | 48.2 | 1.71 m | 48.0 | 1.60 m | 48.1 | 1.66 m | 48.1 | 1.67 m |
| 10 | 36.3 | − | 36.3 | − | 37.0 | − | 37.0 | − | 37.1 | − |
| 11 | 23.4 | 1.91, 1.99 m | 23.5 | 1.90, 2.02 m | 23.7 | 1.93, 1.86 m | 23.7 | 1.87, 1.93 m | 23.7 | 1.86, 1.94 m |
| 12 | 122.6 | 5.40 br s | 122.6 | 5.41 br s | 122.8 | 5.39 br s | 122.8 | 5.43 br s | 122.9 | 5.45 br s |
| 13 | 144.1 | − | 144.2 | − | 144.1 | − | 144.2 | − | 144.3 | − |
| 14 | 42.2 | − | 42.2 | − | 42.1 | − | 42.3 | − | 42.3 | − |
| 15 | 28.3 | 1.14, 2.03 m | 28.3 | 1.15, 2.06 m | 28.3 | 2.27, 1.14 m | 28.5 | 1.16, 2.30 m | 28.6 | 1.18, 2.30 m |
| 16 | 23.4 | 1.76, 2.01 m | 23.4 | 1.77, 2.04 m | 23.4 | 2.05, 1.91 m | 27.0 | 2.36, 3.08 m | 27.0 | 2.33, 3.10 m |
| 17 | 47.1 | − | 47.2 | − | 46.9 | − | 47.3 | − | 47.3 | − |
| 18 | 41.7 | 3.13 dd (3.8, 13.5) | 41.7 | 3.15 dd (3.9, 13.5) | 41.6 | 3.16 dd (3.8, 13.4) | 41.7 | 3.36 dd (3.2, 14.0) | 41.8 | 3.35 dd (3.3, 13.9) |
| 19 | 46.1 | 1.23, 1.74 m | 46.1 | 1.23, 1.75 m | 46.2 | 1.71, 1.21 m | 41.3 | 1.71, 1.20 m | 41.5 | 1.75, 1.22 m |
| 20 | 30.6 | − | 30.7 | − | 30.7 | − | 35.7 | − | 35.7 | − |
| 21 | 33.9 | 1.11, 1.34 m | 34.0 | 1.13, 1.36 m | 33.9 | 1.30, 1.08 m | 73.3 | 3.65 br s | 73.3 | 3.66 br s |
| 22 | 32.5 | 1.72, 1.89 m | 32.5 | 1.72, 1.90 m | 32.5 | 1.82, 1.74 m | 39.6 | 2.25, 2.28 m | 39.7 | 2.23, 2.29 m |
| 23 | 206.3 | 9.61 s | 206.4 | 9.66 s | 28.1 | 1.30 s | 28.1 | 1.29 s | 28.2 | 1.31 s |
| 24 | 10.5 | 1.32 s | 10.6 | 1.34 s | 17.2 | 1.15 s | 17.2 | 1.16 s | 17.2 | 1.17 s |
| 25 | 15.6 | 0.89 s | 15.7 | 0.89 s | 15.5 | 0.83 s | 15.6 | 0.87 s | 15.7 | 0.88 s |
| 26 | 17.5 | 1.05 s | 17.5 | 1.06 s | 17.4 | 1.06 s | 17.4 | 1.09 s | 17.4 | 1.10 s |
| 27 | 26.2 | 1.21 s | 26.2 | 1.21 s | 26.1 | 1.23 s | 25.6 | 1.33 s | 25.7 | 1.35 s |
| 28 | 176.6 | − | 176.5 | − | 176.5 | − | 176.5 | − | 176.5 | − |
| 29 | 33.1 | 0.86 s | 33.1 | 0.86 s | 33.1 | 0.86 s | 28.3 | 1.14 s | 28.4 | 1.15 s |
| 30 | 23.7 | 0.88 s | 23.7 | 0.87 s | 23.7 | 0.87 s | 24.9 | 1.00 s | 25.0 | 1.01 s |
| C | 1 | 2 | 3 | 4 | 5 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | δC | δH | δC | δH | |||
| 3-O-sugar (Confirm here) | ||||||||||||
| Ara | ||||||||||||
| 1 | 104.6 | 4.92 d (7.1) | 104.6 | 4.91 d (7.2) | 105.3 | 4.72 d (6.9) | 105.0 | 4.77 d (6.2) | 105.2 | 4.74 d (6.2) | ||
| 2 | 75.6 | 4.48 m | 75.5 | 4.50 m | 75.5 | 4.50 m | 76.4 | 4.50 m | 76.1 | 4.49 m | ||
| 3 | 75.5 | 3.91 d (10.3) | 75.4 | 3.90 m | 75.0 | 4.18 m | 74.1 | 4.26 m | 74.6 | 4.25 m | ||
| 4 | 80.7 | 4.10 m | 80.8 | 4.08 m | 80.3 | 4.21 m | 79.7 | 4.27 m | 81.0 | 4.26 m | ||
| 5 | 65.8 | 3.58, 4.35 m | 65.9 | 3.56, 4.32 m | 65.3 | 3.74, 4.38 m | 64.5 | 4.40, 3.80 m | 64.9 | 3.78, 4.41 m | ||
| Rha I | ||||||||||||
| 1 | 101.3 | 6.33 s | 101.2 | 6.34 s | 101.4 | 6.30 s | 101.8 | 6.15 s | 101.7 | 6.26 s | ||
| 2 | 72.0 | 4.88 br s | 71.9 | 4.87 br s | 71.9 | 4.89 m | 72.3 | 4.71 m | 72.2 | 4.67 m | ||
| 3 | 82.9 | 4.73 m | 82.9 | 4.75 m | 82.9 | 4.72 m | 72.6 | 4.59 m | 72.4 | 4.60 m | ||
| 4 | 72.9 | 4.45 m | 73.0 | 4.47 m | 73.0 | 4.48 m | 74.1 | 4.28 m | 74.0 | 4.25 m | ||
| 5 | 69.5 | 4.71 m | 69.6 | 4.72 m | 69.7 | 4.66 m | 69.8 | 4.62 m | 69.7 | 4.72 m | ||
| 6 | 18.5 | 1.56 d (6.1) | 18.5 | 1.55 d (6.2) | 18.5 | 1.55 d (6.2) | 18.6 | 1.63 d (6.2) | 18.6 | 1.64 d (6.2) | ||
| Xyl | ||||||||||||
| 1 | 107.7 | 5.34 d (7.6) | 107.6 | 5.35 d (7.7) | 107.6 | 5.34 d (7.7) | ||||||
| 2 | 75.6 | 4.03 m | 75.5 | 4.04 m | 75.6 | 4.05 m | ||||||
| 3 | 78.5 | 4.13 m | 78.5 | 4.15 m | 78.5 | 4.14 m | ||||||
| 4 | 71.1 | 4.12 m | 71.0 | 4.13 m | 71.1 | 4.16 m | ||||||
| 5 | 67.4 | 3.68, 4.45 m | 67.4 | 3.68, 4.23 m | 67.5 | 3.73, 4.32 m | ||||||
| Glc I | ||||||||||||
| 1 | 106.9 | 5.08 d (7.9) | 106.9 | 5.08 d (7.9) | 106.2 | 5.03 d (8.0) | 106.4 | 5.13 d (7.9) | 106.3 | 5.04 d (8.0) | ||
| 2 | 75.5 | 3.99 m | 75.4 | 4.01 m | 74.9 | 4.00 m | 75.5 | 4.02 m | 74.9 | 3.99 m | ||
| 3 | 78.5 | 4.18 m | 78.5 | 4.16 m | 76.7 | 4.18 m | 78.6 | 4.19 m | 76.7 | 4.17 m | ||
| 4 | 71.2 | 4.20 m | 71.1 | 4.22 m | 81.3 | 4.29 m | 71.3 | 4.23 m | 81.3 | 4.28 m | ||
| 5 | 78.8 | 3.85 m | 78.8 | 3.86 m | 76.7 | 3.83 m | 78.8 | 3.89 m | 76.7 | 3.82 m | ||
| 6 | 62.5 | 4.33, 4.45 m | 62.4 | 4.36, 4.46 m | 61.8 | 4.41, 4.51 m | 62.6 | 4.37, 4.48 m | 61.8 | 4.40, 4.51 m | ||
| Glc IV | ||||||||||||
| 1 | 105.1 | 5.15 d (7.8) | 105.1 | 5.15 d (7.8) | ||||||||
| 2 | 74.9 | 4.06 m | 74.9 | 4.05 m | ||||||||
| 3 | 78.3 | 4.19 m | 78.3 | 4.18 m | ||||||||
| 4 | 71.6 | 4.17 m | 71.5 | 4.17 m | ||||||||
| 5 | 78.4 | 3.99 m | 78.4 | 3.98 m | ||||||||
| 6 | 62.6 | 4.26, 4.52 m | 62.5 | 4.27, 4.53 m | ||||||||
| 28-O-sugar | ||||||||||||
| Glc II | ||||||||||||
| 1 | 95.6 | 6.31 d (8.2) | 95.6 | 6.23 d (8.1) | 95.7 | 6.32 d (8.2) | 95.6 | 6.25 d (8.2) | 95.7 | 6.23 d (8.1) | ||
| 2 | 74.1 | 4.19 m | 73.9 | 4.10 m | 74.2 | 4.20 m | 73.8 | 4.08 m | 73.8 | 4.11 m | ||
| 3 | 79.3 | 4.03 m | 78.7 | 4.18 m | 79.3 | 4.02 m | 78.7 | 4.16 m | 78.7 | 4.17 m | ||
| 4 | 71.2 | 4.35 m | 70.8 | 4.28 m | 71.2 | 4.37 m | 70.8 | 4.23 m | 70.7 | 4.29 m | ||
| 5 | 78.9 | 4.27 m | 78.0 | 4.08 m | 78.9 | 4.28 m | 78.0 | 4.06 m | 78.0 | 4.08 m | ||
| 6 | 62.2 | 4.41, 4.44 m | 69.2 | 4.31, 4.64 m | 62.3 | 4.41, 4.46 m | 69.1 | 4.28, 4.62 m | 69.0 | 4.31, 4.63 m | ||
| Glc III | ||||||||||||
| 1 | 104.9 | 4.97 d (7.7) | 104.8 | 4.97 d (7.8) | 104.9 | 4.98 d (7.8) | ||||||
| 2 | 75.3 | 3.92 m | 75.3 | 3.93 m | 75.3 | 3.93 m | ||||||
| 3 | 76.5 | 4.12 m | 76.5 | 4.12 m | 76.4 | 4.14 m | ||||||
| 4 | 78.2 | 4.40 m | 78.2 | 4.39 m | 78.0 | 4.39 m | ||||||
| 5 | 77.1 | 3.62 m | 77.1 | 3.64 m | 77.1 | 3.63 m | ||||||
| 6 | 61.2 | 4.07, 4.18 m | 61.2 | 4.07, 4.17 m | 61.1 | 4.08, 4.18 m | ||||||
| Rha II | ||||||||||||
| 1 | 102.7 | 5.84 s | 102.7 | 5.84 s | 102.7 | 5.85 s | ||||||
| 2 | 72.6 | 4.66 m | 72.5 | 4.65 m | 72.6 | 4.65 m | ||||||
| 3 | 72.7 | 4.53 m | 72.7 | 4.52 m | 72.7 | 4.53 m | ||||||
| 4 | 74.0 | 4.31 m | 74.0 | 4.30 m | 74.0 | 4.32 m | ||||||
| 5 | 70.3 | 4.95 m | 70.3 | 4.94 m | 70.2 | 4.95 m | ||||||
| 6 | 18.5 | 1.68 d (6.2) | 18.5 | 1.68 d (6.2) | 18.5 | 1.62 d (6.2) | ||||||
| Compounds a | Cytotoxic activity (IC50, μM; mean ± SD, n = 3) | |||
|---|---|---|---|---|
| HL-60 | HepG2 | A549 | HeLa | |
| 1 | 82.16 ± 2.79 | >100 | >100 | >100 |
| 3 | 45.32 ± 1.74 | 55.79 ± 2.57 | 75.68 ± 2.79 | >100 |
| 6 | 12.32 ± 0.41 | 13.25 ± 0.38 | 19.42 ± 0.67 | 22.20 ± 0.42 |
| 7 | 9.57 ± 0.54 | 10.64 ± 0.47 | 22.38 ± 0.72 | 17.31 ± 0.81 |
| 8 | 7.25 ± 0.31 | 12.11 ± 0.87 | 9.89 ± 0.57 | 15.47 ± 0.73 |
| Doxorubicin b | 0.35 ± 0.05 | 0.50 ± 0.04 | 0.68 ± 0.10 | 0.66 ± 0.07 |
3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
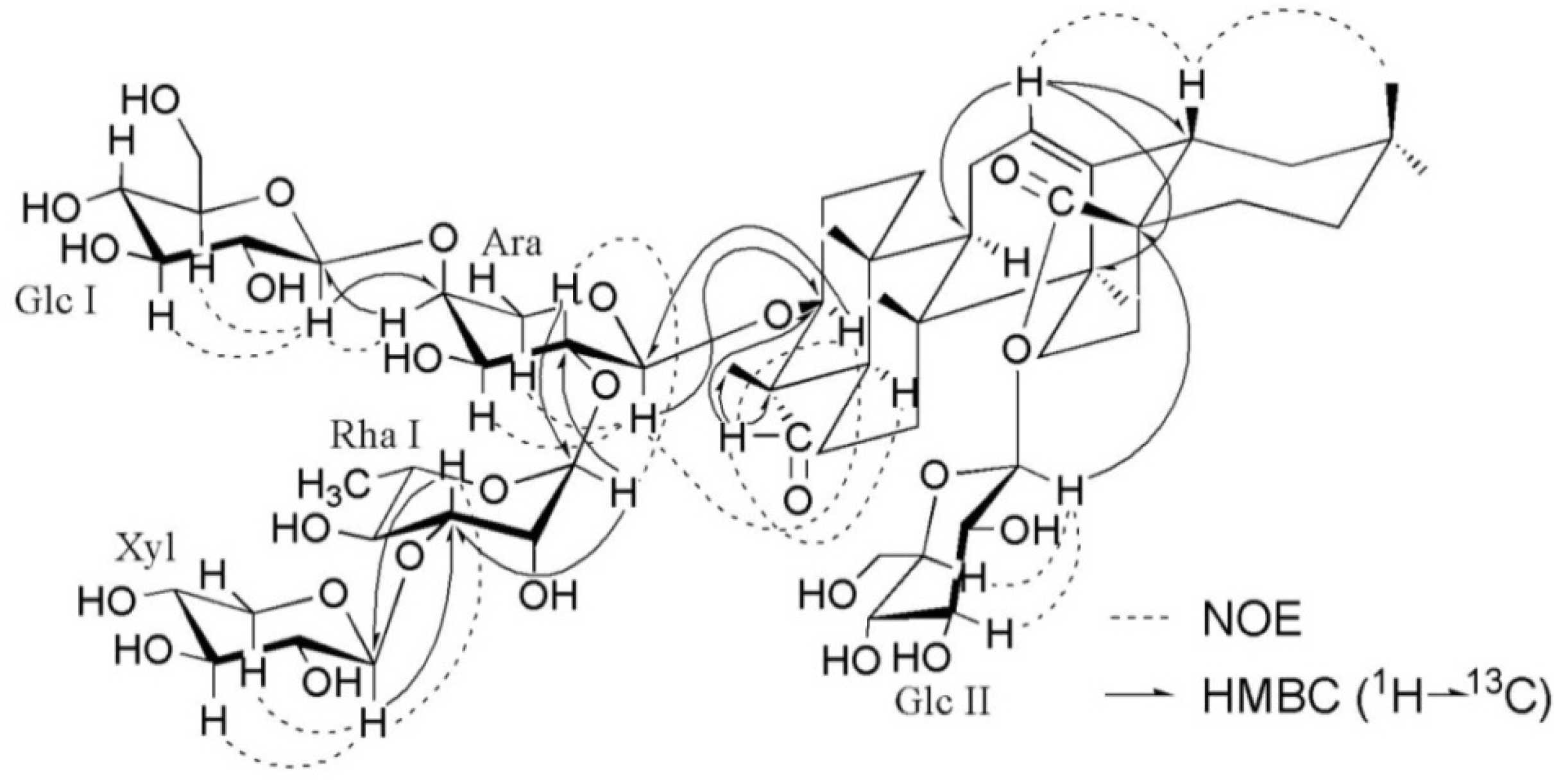
 +12.3 (c 0.16, MeOH); 1H- and 13C-NMR data, see Table 1 and Table 2; key HMBC and NOESY correlations, see Figure 2; ESIMS (pos. ion mode) m/z 1227 [M+Na]+; ESIMS (neg. ion mode) m/z 1203 [M−H]−, 1071 [1203−132]−, 1041 [1203−162]−, 925 [1071−146]−, 909 [1071−162]−, 879 [1041−162]−; HRESIMS (pos. ion mode) m/z 1227.5779 [M+Na]+ (calcd. for C58H92NaO26, 1227.5775).
+12.3 (c 0.16, MeOH); 1H- and 13C-NMR data, see Table 1 and Table 2; key HMBC and NOESY correlations, see Figure 2; ESIMS (pos. ion mode) m/z 1227 [M+Na]+; ESIMS (neg. ion mode) m/z 1203 [M−H]−, 1071 [1203−132]−, 1041 [1203−162]−, 925 [1071−146]−, 909 [1071−162]−, 879 [1041−162]−; HRESIMS (pos. ion mode) m/z 1227.5779 [M+Na]+ (calcd. for C58H92NaO26, 1227.5775). −8.2 (c 0.18, MeOH); 1H- and 13C-NMR data, see Table 1 and Table 2; key HMBC and NOESY correlations, see Figure S1 in the Supplementary Data; ESIMS (pos. ion mode) m/z 1535 [M+Na]+, 1065 [1535−146−162−162]+; ESIMS (neg. ion mode) m/z 1511 [M−H]−, 1379 [1511−132]−, 1349 [1511−162]−, 1041 [1511−146−162−162]−; HRESIMS (pos. ion mode) m/z 1535.6885 [M+Na]+ (calcd. for C70H112NaO35, 1535.6882).
−8.2 (c 0.18, MeOH); 1H- and 13C-NMR data, see Table 1 and Table 2; key HMBC and NOESY correlations, see Figure S1 in the Supplementary Data; ESIMS (pos. ion mode) m/z 1535 [M+Na]+, 1065 [1535−146−162−162]+; ESIMS (neg. ion mode) m/z 1511 [M−H]−, 1379 [1511−132]−, 1349 [1511−162]−, 1041 [1511−146−162−162]−; HRESIMS (pos. ion mode) m/z 1535.6885 [M+Na]+ (calcd. for C70H112NaO35, 1535.6882). −15.4 (c 0.20, MeOH); 1H- and 13C-NMR data, see Table 1 and Table 2; key HMBC and NOESY correlations, see Figure S2 in the Supplementary Data; ESIMS (pos. ion mode) m/z 1375 [M+Na]+, 1243 [1375−132]+, 1213 [1375−162]+, 1081 [1243−162]+, 1051 [1213−162]+; ESIMS (neg. ion mode) m/z 1351 [M−H]−, 1219 [1351−132]−, 1189 [1351−162]−, 1027 [1189−162]−; HRESIMS (pos. ion mode) m/z 1375.6507 [M+Na]+ (calcd. for C70H114NaO34, 1375.6510).
−15.4 (c 0.20, MeOH); 1H- and 13C-NMR data, see Table 1 and Table 2; key HMBC and NOESY correlations, see Figure S2 in the Supplementary Data; ESIMS (pos. ion mode) m/z 1375 [M+Na]+, 1243 [1375−132]+, 1213 [1375−162]+, 1081 [1243−162]+, 1051 [1213−162]+; ESIMS (neg. ion mode) m/z 1351 [M−H]−, 1219 [1351−132]−, 1189 [1351−162]−, 1027 [1189−162]−; HRESIMS (pos. ion mode) m/z 1375.6507 [M+Na]+ (calcd. for C70H114NaO34, 1375.6510). +7.2 (c 0.11, MeOH); 1H- and 13C-NMR data, see Table 1 and Table 2; key HMBC and NOESY correlations, see Figure S3 in the Supplementary Data; ESIMS (pos. ion mode) m/z 1405 [M+Na]+, 1259 [1405−146]+, 1243 [1405−162]+, 935 [1405−146−162−162]+; ESIMS (neg. ion mode) m/z 1381 [M−H]−, 911 [1381−146−162−162]−; HRESIMS (pos. ion mode) m/z 1405.6612 [M+Na]+ (calcd. for C65H106NaO31, 1405.6616).
+7.2 (c 0.11, MeOH); 1H- and 13C-NMR data, see Table 1 and Table 2; key HMBC and NOESY correlations, see Figure S3 in the Supplementary Data; ESIMS (pos. ion mode) m/z 1405 [M+Na]+, 1259 [1405−146]+, 1243 [1405−162]+, 935 [1405−146−162−162]+; ESIMS (neg. ion mode) m/z 1381 [M−H]−, 911 [1381−146−162−162]−; HRESIMS (pos. ion mode) m/z 1405.6612 [M+Na]+ (calcd. for C65H106NaO31, 1405.6616). −9.4 (c 0.18, MeOH); 1H- and 13C-NMR data, see Table 1 and Table 2; key HMBC and NOESY correlations, see Figure S4 in the Supplementary Data; ESIMS (pos. ion mode) m/z 1567 [M+Na]+, 1421 [1567−146]+, 1405 [1567−162]+, 1097 [1567−146−162−162]+; ESIMS (neg. ion mode) m/z 1543 [M−H]−, 1073 [1543−146−162−162]−; HRESIMS (pos. ion mode) m/z 1567.7150 [M+Na]+ (calcd. for C71H116NaO36,1567.7144).
−9.4 (c 0.18, MeOH); 1H- and 13C-NMR data, see Table 1 and Table 2; key HMBC and NOESY correlations, see Figure S4 in the Supplementary Data; ESIMS (pos. ion mode) m/z 1567 [M+Na]+, 1421 [1567−146]+, 1405 [1567−162]+, 1097 [1567−146−162−162]+; ESIMS (neg. ion mode) m/z 1543 [M−H]−, 1073 [1543−146−162−162]−; HRESIMS (pos. ion mode) m/z 1567.7150 [M+Na]+ (calcd. for C71H116NaO36,1567.7144).3.4. Acid Hydrolysis and GC Analysis of the Sugar Moieties in 1–5
3.5. Assays for in Vitro Antitumor Activity
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- National Pharmacopoeia Committee. Pharmacopoeia of People’s Republic of China; Chemical Industry Press: Beijing, China, 2010; p. 157. [Google Scholar]
- Ye, W.C.; Zhang, Q.W.; Zhao, S.X.; Che, C.T. Four new oleanane saponins from Anemone anhuiensis. Chem. Pharm. Bull. 2001, 49, 632–634. [Google Scholar] [CrossRef]
- Lu, J.C.; Xu, B.B.; Gao, S.; Fan, L.; Zhang, H.F.; Liu, R.X.; Kodama, H. Structure elucidation of two triterpenoid saponins from rhizome of Anemone raddeana. Regel. Fitoterapia 2009, 80, 345–348. [Google Scholar] [CrossRef]
- Wang, M.K.; Wu, F.E.; Chen, Y.Z. Triterpenoid saponins from Anemone hupehensis. Phytochemistry 1997, 44, 333–335. [Google Scholar] [CrossRef]
- Mimaki, Y.; Watanabe, K.; Matsuo, Y.; Sakagami, H. Triterpene glycosides from the tubers of Anemone coronaria. Chem. Pharm. Bull. 2009, 57, 724–729. [Google Scholar] [CrossRef]
- Sun, Y.X.; Liu, J.C.; Liu, D.Y. Phytochemicals and bioactivities of Anemone raddeana Regel: A review. Pharmazie 2011, 66, 813–821. [Google Scholar]
- Zhang, L.T.; Zhang, Y.W.; Takaishi, Y.; Duan, H.Q. Antitumor triterpene saponins from Anemone flaccida. Chin. Chem. Lett. 2008, 19, 190–192. [Google Scholar] [CrossRef]
- Wang, J.L.; Liu, K.; Gong, W.Z.; Wang, Q.; Xu, D.T.; Liu, M.F.; Bi, K.L.; Song, Y.F. Anticancer, antioxidant, and antimicrobial activities of Anemone (Anemone cathayensis). Food Sci. Biotechenol. 2012, 21, 551–557. [Google Scholar] [CrossRef]
- Wang, J.R.; Ma, J.Q.; Peng, L.L.; Feng, J.T.; Ding, L.S. Chemical constituents and antifeeding activity of Anemone tomentosa. Acta Bot. Boreali. Occid. Sin. 1998, 18, 643–644. [Google Scholar]
- Flora of China Editorial Committee of Chinese Academy of Sciences. Flora of China; Science Press: Beijing, China, 1980; Volume 28, p. 24. [Google Scholar]
- Ding, Y.; Tang, H.F.; Wang, J.B.; Liu, D.; Tian, X.R.; Wang, X.Y.; Zhou, X.M. Triterpenoid saponins from Anemone rivularis var. flore-minore. Biochem. Syst. Ecol. 2011, 39, 236–239. [Google Scholar] [CrossRef]
- Ding, Y.; Tian, X.R.; Tang, H.F.; Feng, J.T.; Zhang, W.; Hai, W.L.; Wang, X.Y.; Wang, Y. Two new glycosides from the whole plant of Anemone rivularis var. flore-minore. Phytochem. Lett. 2012, 5, 668–672. [Google Scholar] [CrossRef]
- Wang, Y.; Kang, W.; Hong, L.J.; Hai, W.L.; Wang, X.Y.; Tang, H.F.; Tian, X.R. Triterpenoid saponins from the root of Anemone tomentosa. J. Nat. Med. 2013, 67, 70–77. [Google Scholar] [CrossRef]
- Wang, X.Y.; Chen, X.L.; Tang, H.F.; Gao, H.; Tian, X.R.; Zhang, P.H. Cytotoxic triterpenoid saponins from the rhizomes of Anemone taipaiensis. Planta Med. 2011, 77, 1550–1554. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhang, W.; Gao, K.; Lu, Y.Y.; Tang, H.F.; Sun, X.L. Oleanane-type saponins from the rhizomes of Anemone taipaiensis and their cytotoxic activities. Fitoterapia 2013, 89, 224–230. [Google Scholar] [CrossRef]
- Wang, X.Y.; Gao, H.; Zhang, W.; Li, Y.; Cheng, G.; Sun, X.L.; Tang, H.F. Bioactive oleanane-type saponins from the rhizomes of Anemone taipaiensis. Bioorg. Med. Chem. Lett. 2013, 23, 5714–5720. [Google Scholar] [CrossRef]
- Nie, R.L.; Tanakaa, T.; Miyakoshia, M.; Kasaia, R.; Moritaa, T.; Zhou, J.; Tanaka, O. A triterpenoid saponin from Thladiantha hookeri var. pentadactyla. Phytochemistry 1989, 28, 1711–1715. [Google Scholar] [CrossRef]
- Mahato, S.B.; Kundu, A.P. 13C-NMR Spectra of pentacyclic triterpenoidse—A compilation and some salient features. Phytochemistry 1994, 37, 1517–1575. [Google Scholar] [CrossRef]
- De Marino, S.; Borbone, N.; Iorizzi, M.; Esposito, G.; McClintock, J.B.; Zollo, F. Bioactive asterosaponins from the starfish Luidia quinaria and Psilaster cassiope. Isolation and structure characterization by two-dimensional NMR spectroscopy. J. Nat. Prod. 2003, 66, 515–519. [Google Scholar] [CrossRef]
- Zheng, Q.; Koike, K.; Han, L.K.; Okuda, H.; Nikaido, T. New biologically active triterpenoid saponins from Scabiosatschiliensis. J. Nat. Prod. 2004, 67, 604–613. [Google Scholar] [CrossRef]
- Xu, T.H.; Xu, Y.J.; Li, H.X.; Han, D.; Zhao, H.F.; Xie, S.X.; Xu, D.M. Two new triterpenoid saponins from Pulsatillacernua (Thunb.) Bercht. etOpiz. J. Asian Nat. Prod. Res. 2007, 9, 705–711. [Google Scholar] [CrossRef]
- Werner, S.; Nebojsa, S.; Robert, W.; Robert, S.; Olaf, K. Complete assignments of 1H and 13C-NMR resonances of oleanolic acid, 18α-oleanolic acid, ursolic acid and their 11-oxo derivatives. Magn. Reson. Chem. 2003, 41, 636–638. [Google Scholar] [CrossRef]
- Zhang, W.; Yao, M.N.; Tang, H.F.; Tian, X.R.; Wang, M.C.; Ji, L.J.; Xi, M.M. Triterpenoid saponins with anti-myocardial ischemia activity from the whole plants of Clematis tangutica. Planta Med. 2013, 79, 673–679. [Google Scholar] [CrossRef]
- Mimaki, Y.; Yokosuka, A.; Hamanaka, M.; Sakuma, C.; Yamori, T.; Sashida, Y. Triterpene saponins from the roots of Clematis chinensis. J. Nat. Prod. 2004, 67, 1511–1516. [Google Scholar] [CrossRef]
- Lavaud, C.; Crublet, M.L.; Pouny, I.; Litaudon, M.; Sdonet, T. Triterpeniod saponins from the stem bark of Elattostachys apetala. Phytochmistry 2001, 57, 469–478. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Ye, W.C.; Che, C.T.; Zhao, S.X. Triterpene saponins from Pulsatilla cernua. Acta Pharm. Sin. 2000, 35, 756–759. [Google Scholar]
- Schenkel, E.P.; Werner, W.; Schulte, K.E. Saponins from Thinouia coriaceae. Planta Med. 1991, 57, 463–467. [Google Scholar] [CrossRef]
- Sawada, H.; Miyakoshi, M.; Isoda, S.; Ida, Y.; Shoji, J. Saponins from leaves of Acanthopanax sieboldianus. Phytochemistry 1993, 34, 1117–1121. [Google Scholar] [CrossRef]
- Bang, S.C.; Lee, J.H.; Song, G.Y.; Kim, D.H.; Yoon, M.Y.; Ahn, B.Z. Antitumor activity of Pulsatilla koreana saponins and their structure-activity relationship. Chem. Pharm. Bull. 2005, 53, 1451–1454. [Google Scholar] [CrossRef]
- Yokosuka, A.; Sano, T.; Hashimoto, K.; Sakagami, H.; Mimaki, Y. Triterpene glycosides from the whole plant of Anemone hupehensis var. japonica and their cytotoxic activity. Chem. Pharm. Bull. 2009, 57, 1425–1430. [Google Scholar] [CrossRef]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar]
- Sample Availability: Samples of the compounds 1–10 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, X.; Wang, M.; Xu, M.; Wang, Y.; Tang, H.; Sun, X. Cytotoxic Oleanane-Type Triterpenoid Saponins from the Rhizomes of Anemone rivularis var. flore-minore. Molecules 2014, 19, 2121-2134. https://doi.org/10.3390/molecules19022121
Wang X, Wang M, Xu M, Wang Y, Tang H, Sun X. Cytotoxic Oleanane-Type Triterpenoid Saponins from the Rhizomes of Anemone rivularis var. flore-minore. Molecules. 2014; 19(2):2121-2134. https://doi.org/10.3390/molecules19022121
Chicago/Turabian StyleWang, Xiaoyang, Minchang Wang, Min Xu, Yi Wang, Haifeng Tang, and Xiaoli Sun. 2014. "Cytotoxic Oleanane-Type Triterpenoid Saponins from the Rhizomes of Anemone rivularis var. flore-minore" Molecules 19, no. 2: 2121-2134. https://doi.org/10.3390/molecules19022121
APA StyleWang, X., Wang, M., Xu, M., Wang, Y., Tang, H., & Sun, X. (2014). Cytotoxic Oleanane-Type Triterpenoid Saponins from the Rhizomes of Anemone rivularis var. flore-minore. Molecules, 19(2), 2121-2134. https://doi.org/10.3390/molecules19022121