Changes in Phytochemical Synthesis, Chalcone Synthase Activity and Pharmaceutical Qualities of Sabah Snake Grass (Clinacanthus nutans L.) in Relation to Plant Age
Abstract
:1. Introduction
2. Results and Discussion
2.1. Changes in Total Flavonoid (TF) and Individual Flavonoid Concentration during the Plant Growth Period
| Samples | TF | Catechin | Kaempferol | Luteolin | Quercetin |
|---|---|---|---|---|---|
| 1-month-old buds | 3.270 ± 0.331 d | 0.968 ± 0.014 e | 0.667 ± 0.060 c | 0.173 ± 0.046 b | 1.121 ± 0.007 b |
| 1-month-old leaves | 3.790 ± 0.293 d | 1.244 ± 0.061 d | 0.475 ± 0.039 d | ND | 1.156 ± 0.189 b |
| 6-month-old buds | 6.320 ± 0.740 a | 1.582 ± 0.072 c | 1.396 ± 0.236 a | 0.390 ± 0.179 a | 1.669 ± 0.451 a |
| 6-month-old leaves | 4.660 ± 0.432 c | 1.711 ± 0.031 b | 0.855 ± 0.038 b | ND | 1.189 ± 0.099 b |
| 1-year-old buds | 5.420 ± 0.408 b | 2.023 ± 0.125 a | 0.910 ± 0.034 b | 0.219 ± 0.153 b | 1.297 ± 0.187 b |
| 1-year-old leaves | 4.120 ± 0.435 c | 1.191 ± 0.216 d | 0.632 ± 0.058 c | 0.115 ± 0.427b | 1.203 ± 0.253 b |
2.2. Changes in Total Phenolic Acid (TP) and Individual Phenolic Acid Concentrations during the Plant Growth Period
| Samples | TP | Caffeic Acid | Gallic Acid |
|---|---|---|---|
| 1-month-old buds | 6.840 ± 0.470 d | 0.169 ± 0.034 b | 3.230 ± 0.645 b |
| 1-month-old leaves | 7.290 ± 0.801 d | 0.180 ± 0.028 b | 2.931 ± 0.459 b |
| 6-month-old buds | 18.210 ± 1.125 a | 0.204 ± 0.014 b | 5.963 ± 0.545 a |
| 6-month-old leaves | 11.320 ± 1.280 c | 0.172 ± 0.033 b | 5.339 ± 0.468 a |
| 1-year-old buds | 15.460 ± 1.231 b | 0.307 ± 0.018 a | 2.699 ± 0.380 b |
| 1-year-old leaves | 10.530 ± 1.665 c | 0.282 ± 0.011 a | 2.166 ± 0.367 b |
2.3. The Enzyme Chalcone Synthase (CHS, EC 2.3.1.74) Activity
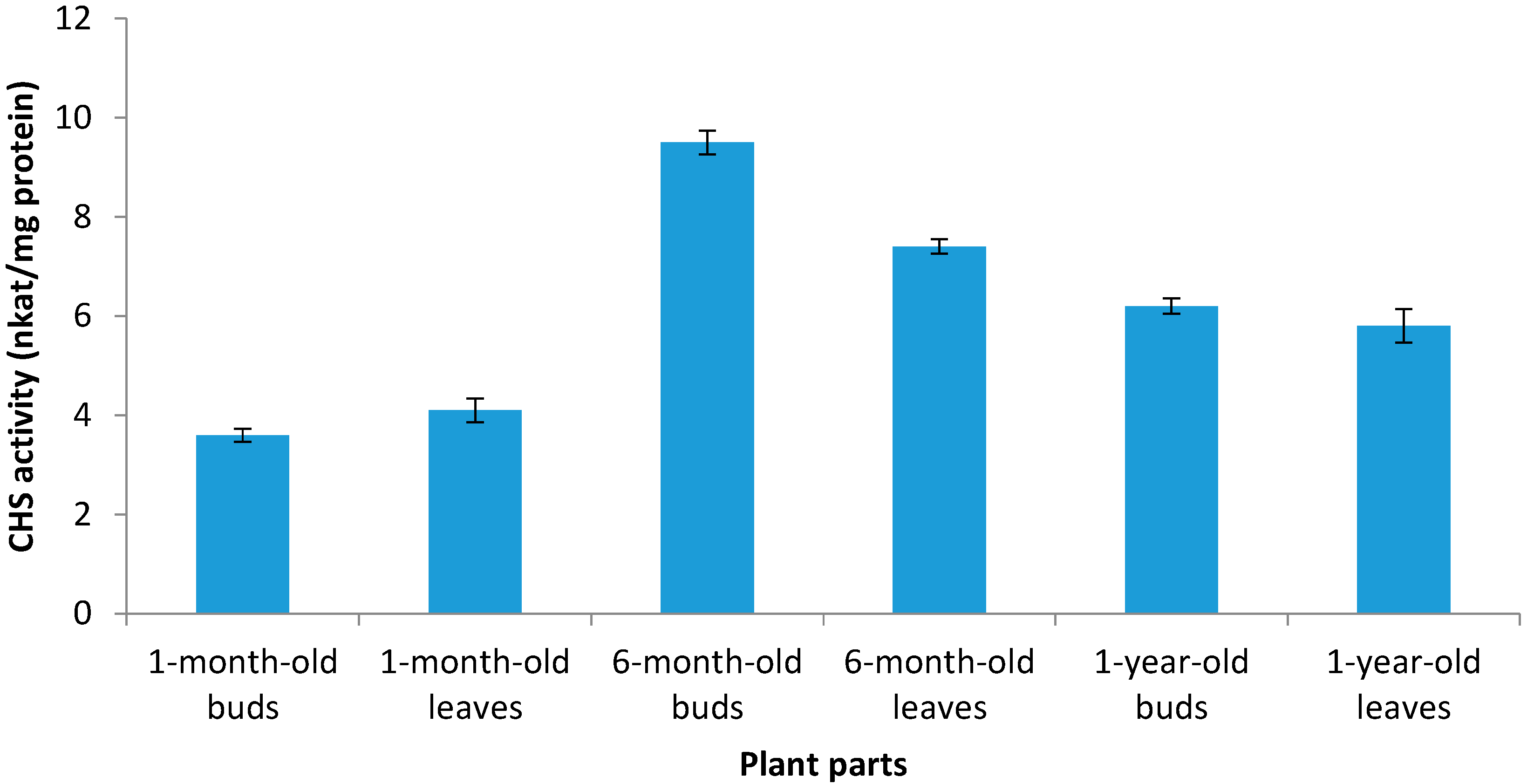
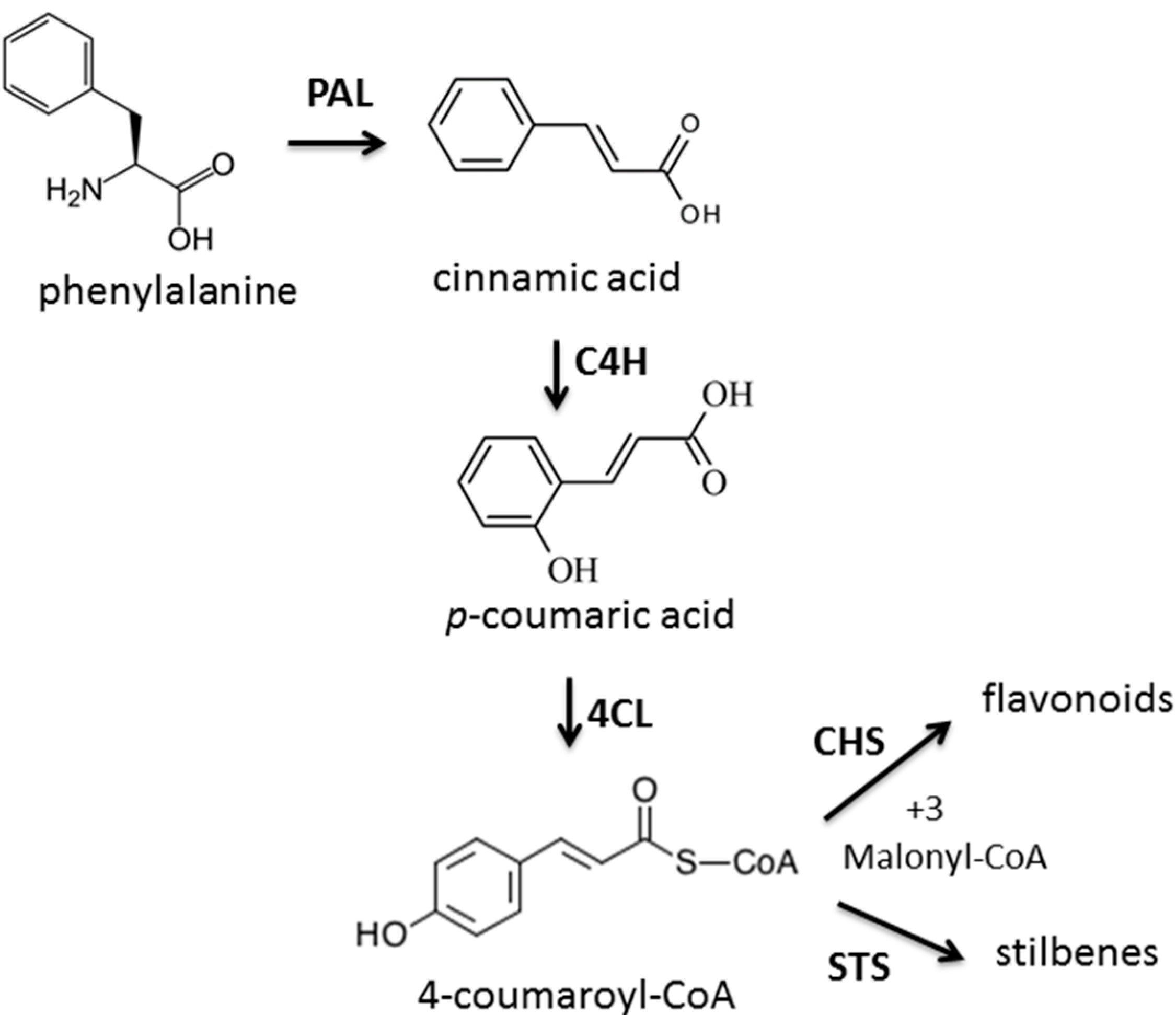
2.4. In Vitro Antioxidant Activity
2.4.1. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Activity of Sabah Snake Grass during the Plant Growth Period
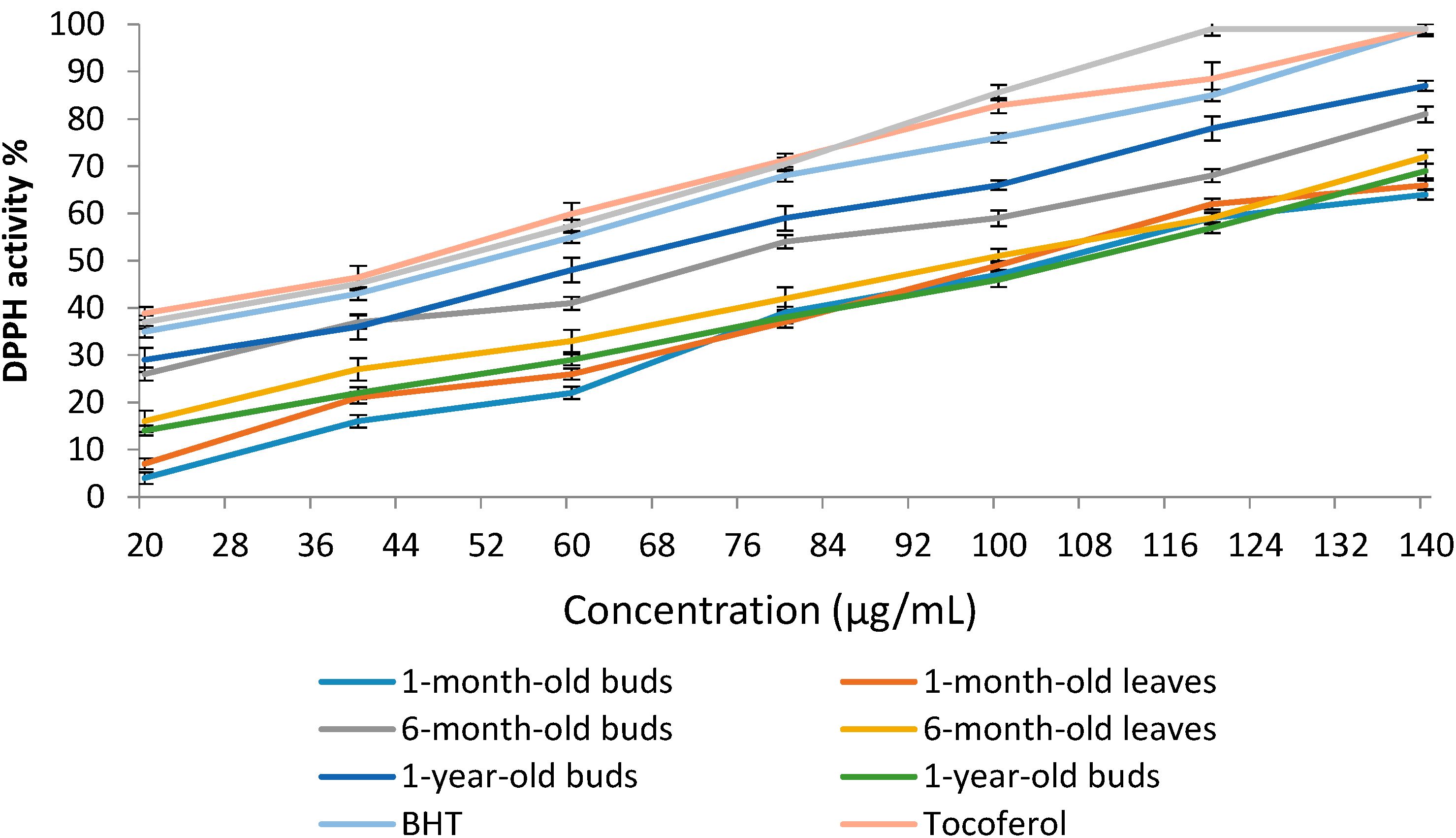
| Extracts | DPPH Activity % | IC50 (µg/mL) |
|---|---|---|
| 1-month-old buds | 47.4 ± 2.45 d | 110.4 ± 0.48 b |
| 1-month-old leaves | 49.1 ± 2.19 d | 106.8 ± 0.82 c |
| 6-month-old buds | 59.6 ± 1.87 b | 73.5 ± 0.45 e |
| 6-month-old leaves | 51.3 ± 2.10 c | 98.2 ± 0.74 d |
| 1-year-old buds | 66.2 ± 1.42 a | 64.6 ± 0.39 f |
| 1-year-old leaves | 46.6 ± 2.77 d | 112.1 ± 0.58 a |
| BHT | 76.5 ± 1.92 | 52.6 ± 0.44 |
| Tocoferol | 82.8 ± 3.15 | 44.0 ± 0.61 |
| Caffeic acid | 85.6 ± 2.74 | 49.2 ± 0.85 |
2.4.2. Ferric Reduction Antioxidant Potential (FRAP) Activity
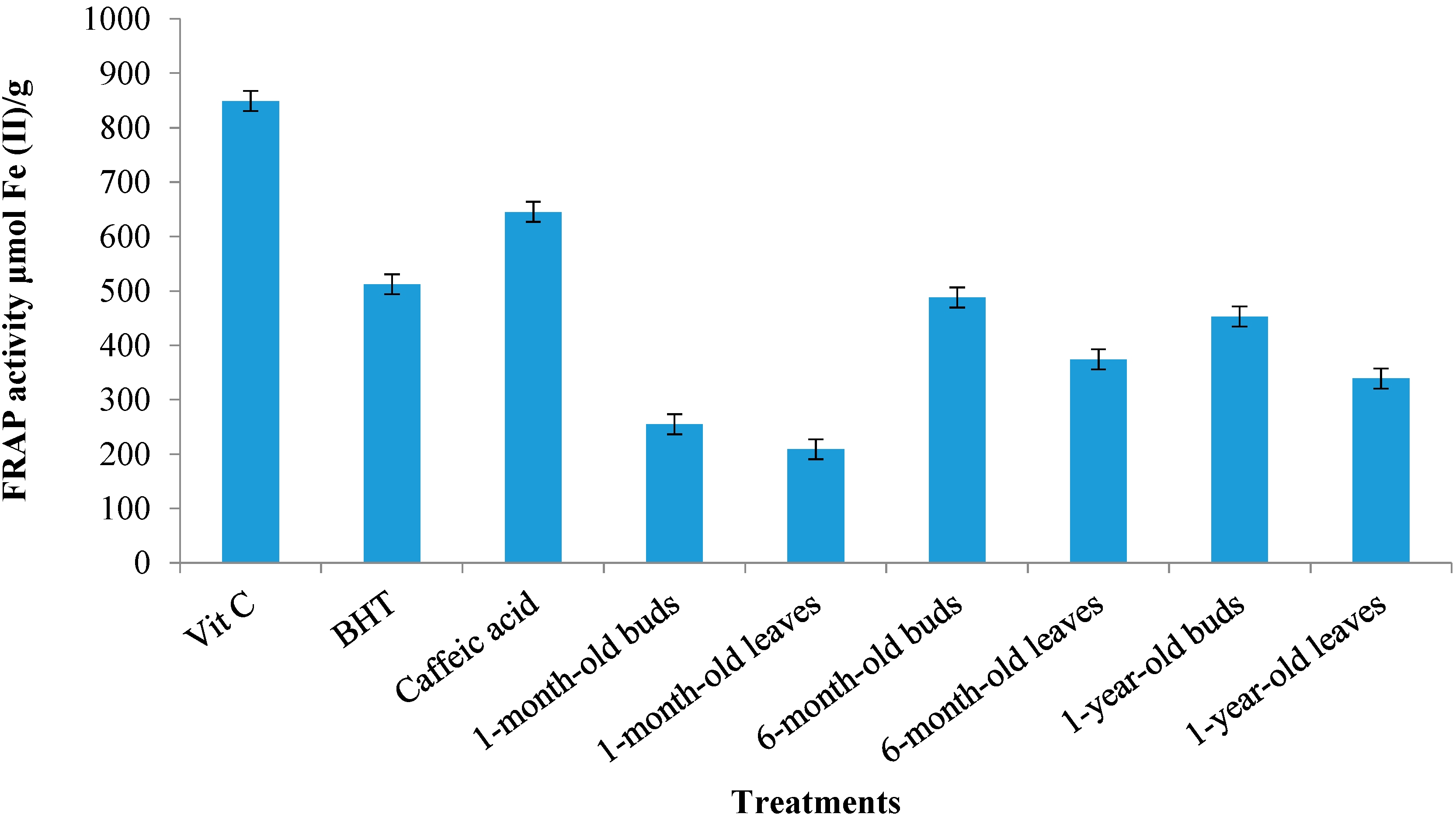
2.5. Correlation between Identified Polyphenolic Compounds and Antioxidant Activity
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 DPPH | 1 | ||||||||||
| 2 FRAP | 0.777 * | 1 | |||||||||
| 3 TF | 0.802 * | 0.932 ** | 1 | ||||||||
| 4 Catechin | 0.875 * | 0.748 | 0.748 | 1 | |||||||
| 5 Kaempferol | 0.644 | 0.883 * | 0.907 * | 0.535 | 1 | ||||||
| 6 Luteolin | 0.073 | 0.353 | 0.16 | −0.202 | 0.217 | 1 | |||||
| 7 Quercetin | 0.649 | 0.921 ** | 0.946 ** | 0.525 | 0.953 ** | 0.386 | 1 | ||||
| 8 TP | 0.811 * | 0.97 ** | 0.985 ** | 0.727 | 0.906 * | 0.305 | 0.96 ** | 1 | |||
| 9 Caffeic Acid | 0.745 | 0.765 | 0.599 | 0.607 | 0.45 | 0.629 | 0.58 | 0.708 | 1 | ||
| 10 Gallic Acid | 0.828 * | 0.996 ** | 0.594 | 0.341 | 0.761 | −0.333 | 0.599 | 0.512 | −0.165 | 1 | |
| 11 CHS | 0.286 | 0.88 * | 0.81 * | 0.79 * | 0.82 * | 0.487 | 0.154 | 0.059 | 0.065 | 0.304 | 1 |
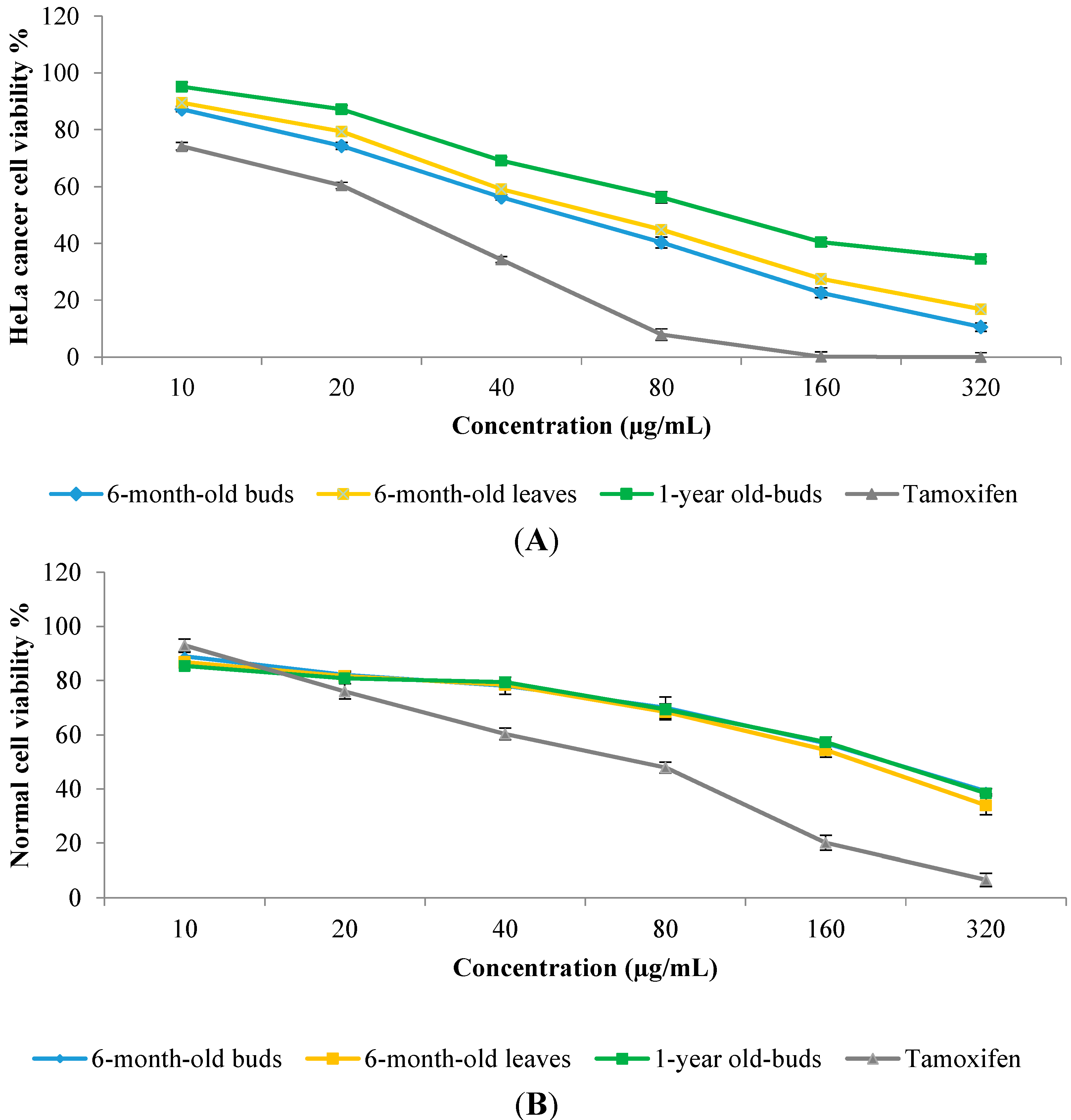
2.6. In Vitro Anticancer Activity
3. Experimental Section
3.1. Plant Material
3.2. Extraction
3.3. Determination of Total Phenolic Content
3.4. Determination of Total Flavonoids
3.5. Separation and Analysis of Flavonoids and Phenolic Acids by UHPLC
3.6. Chalcone Synthase (CHS) Assay
3.7. Evaluation of in Vitro Antioxidant Activity
3.7.1. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Assay
3.7.2. Ferric Reducing Antioxidant Potential (FRAP) Assay
3.8. Determination of in Vitro Anticancer Activity Using MTT Assay
3.8.1. Extract Preparation
3.8.2. MTT Assay
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. WHO Traditional Medicine Strategy 2002–2005. Available online: http://herbalnet.healthrepository.org/handle/123456789/2028 (accessed on 20 June 2014).
- Okwu, D. Phytochemicals, vitamins and mineral contents of two Nigerian medicinal plants. Int. J. Mol. Med. Adv. Sci. 2005, 1, 375–381. [Google Scholar]
- Ao, C.; Li, A.; Elzaawely, A.A.; Xuan, T.D.; Tawata, S. Evaluation of antioxidant and antibacterial activities of Ficus microcarpa L. fil. extract. Food Control 2008, 19, 940–948. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z. Antioxidant potential and anticancer activity of young ginger (Zingiber officinale Roscoe) grown under different CO2 concentration. J. Med. Plants Res. 2011, 5, 3247–3255. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z. Profiling of phenolic compounds and their antioxidant and anticancer activity in pandan (Pandanus amaryllifolius Roxb.) extracts from different locations of Malaysia. BMC Complement. Altern. Med. 2013, 13. [Google Scholar] [CrossRef]
- Amarowicz, R.; Pegg, R.; Rahimi-Moghaddam, P.; Barl, B.; Weil, J. Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem. 2004, 84, 551–562. [Google Scholar] [CrossRef]
- Fritz, R.S.; Hochwender, C.G.; Lewkiewicz, D.A.; Bothwell, S.; Orians, C.M. Seedling herbivory by slugs in a willow hybrid system: Developmental changes in damage, chemical defense, and plant performance. Oecologia 2001, 129, 87–97. [Google Scholar] [CrossRef]
- Pasko, P.; Barto, H.; Zagrodzki, P.; Gorinstein, S.; Fo‚ta, M.; Zachwieja, Z. Anthocyanins, total polyphenols and antioxidant activity in amaranth and quinoa seeds and sprouts during their growth. Food Chem. 2009, 115, 994–998. [Google Scholar] [CrossRef]
- Achakzai, A.K. K.; Achakzai, P.; Masood, A.; Kayani, S.A.; Tareen, R.B. Response of plant parts and age on the distribution of secondary metabolites on plants found in Quetta. Pak. J. Bot. 2009, 41, 2129–2135. [Google Scholar]
- Globinmed. Clinacanthus nutans (Burm.f.) Lindau. Available online: http://www.globinmed.com/index.php?option=com_content&view=article&id=79320:clinacanthus-nutans-burmf-lindau&catid=199:safety-of-herbal&Itemid=139 (accessed on 18 October 2014).
- Sakdarat, S.; Shuyprom, A.; Pientong, C.; Ekalaksananan, T.; Thongchai, S. Bioactive constituents from the leaves of Clinacanthus nutans Lindau. Bioorg. Med. Chem. 2009, 17, 1857–1860. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, and apigenin) content of edible tropical plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef]
- Albach, R.F.; Redman, G.H.; Cruse, R.R. Annual and seasonal changes in naringin concentration of Ruby Red grapefruit juice. J. Agric. Food Chem. 1981, 29, 808–811. [Google Scholar] [CrossRef]
- Berhow, M.A. Effects of early plant growth regulator treatments on flavonoid levels in grapefruit. Plant Growth Regul. 2000, 30, 225–232. [Google Scholar] [CrossRef]
- Behn, H.; Schurr, U.; Ulbrich, A.; Noga, G. Development-dependent UV-B responses in red oak leaf lettuce (Lactuca sativa L.): Physiological mechanisms and significance for hardening. Eur. J. Hortic. Sci. 2011, 76, 33–40. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Identification and concentration of some flavonoid components in Malaysian young ginger (Zingiber officinale Roscoe) varieties by a high performance liquid chromatography method. Molecules 2010, 15, 6231–6243. [Google Scholar] [CrossRef]
- Fernandes, E.F. A.; Meloni, F.; Borella, J.C.; Lopes, N.P. Effect of fertilisation and harvest period on polar metabolites of Calendula oficcinalis. Rev. Bras. Farmacogn. 2013, 23, 731–735. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Galardi, C.; Sani, G.; Cimato, A.; Heimler, D. Polyphenols in greenhouse and open-air-grown lettuce. Food Chem. 2002, 79, 337–342. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O'Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Van der Sluis, A.A.; Dekker, M.; de Jager, A.; Jongen, W.M. Activity and concentration of polyphenolic antioxidants in apple: Effect of cultivar, harvest year, and storage conditions. J. Agric. Food Chem. 2001, 49, 3606–3613. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid biosynthesis: A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Ozeki, Y.; Komamine, A.; Noguchi, H.; Sankawa, U. Changes in activities of enzymes involved in flavonoid metabolism during the initiation and suppression of anthocyanin synthesis in carrot suspension cultures regulated by 2, 4-dichlorophenoxyacetic acid. Physiol. Plantarum 1987, 69, 123–128. [Google Scholar] [CrossRef]
- Cheng, S.; Xu, F.; Li, L.; Cheng, H.; Zhang, W. Seasonal Pattern of Flavonoid Content and Related Enzyme Activities in Leaves of Ginkgo biloba L. Not. Bot. Hort. Agrobot. Cluj-Napoca 2012, 40, 98–106. [Google Scholar]
- Hu, G.; Yuan, Y.; Wu, C.; Jiang, C.; Wang, Z.; Lin, S.; Wu, Z. Effect of different developmental stage on plant growth and active compounds in Scutellaria baicalensis. Zhongguo Zhong Yao Za Zhi 2012, 37, 3793–3798. [Google Scholar]
- Nicholson, R.L.; Hammerschmidt, R. Phenolic compounds and their role in disease resistance. Annu. Rev. Phytopathol. 1992, 30, 369–389. [Google Scholar] [CrossRef]
- Saslowsky, D.; Winkel-Shirley, B. Localization of flavonoid enzymes in Arabidopsis roots. Plant J. 2001, 27, 37–48. [Google Scholar] [CrossRef]
- Flamini, R.; Mattivi, F.; Rosso, M.D.; Arapitsas, P.; Bavaresco, L. Advanced knowledge of three important classes of grape phenolics: Anthocyanins, Stilbenes and Flavonols. Int. J. Molecul. Sci. 2013, 14, 19651–19669. [Google Scholar] [CrossRef]
- Yong, Y.K.; Tan, J.J.; Teh, S.S.; Mah, S.H.; Ee, G.C.L.; Chiong, H.S.; Ahmad, Z. Clinacanthus nutans Extracts Are Antioxidant with Antiproliferative Effect on Cultured Human Cancer Cell Lines. Evid. Based Complement. Alternat. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Pannangpetch, P.; Laupattarakasem, P.; Kukongviriyapan, V.; Kukongviriyapan, U.; Kongyingyoes, B.; Aromdee, C. Antioxidant activity and protective effect against oxidative hemolysis of Clinacanthus nutans (Burm. f) Lindau. Songklanakarin J. Sci. Technol. 2007, 29, 1–9. [Google Scholar]
- Lamson, D.W.; Brignall, M.S. Antioxidants and cancer, part 3: Quercetin. Altern. Med. Rev. 2000, 5, 196–208. [Google Scholar]
- Wojdyo, A.; Oszmiaski, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Karimi, E.; Ibrahim, M.H. Combined effect of CO2 enrichment and foliar application of salicylic acid on the production and antioxidant activities of anthocyanin, flavonoids and isoflavonoids from ginger. BMC Complement. Altern. Med. 2012, 12. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Katalinic, V.; Milos, M.; Kulisic, T.; Jukic, M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006, 94, 550–557. [Google Scholar] [CrossRef]
- Wong, C.-C.; Li, H.-B.; Cheng, K.-W.; Chen, F. A systematic survey of antioxidant activity of 30 Chinese medicinal plants using the ferric reducing antioxidant power assay. Food Chem. 2006, 97, 705–711. [Google Scholar] [CrossRef]
- Sun, T.; Powers, J.R.; Tang, J. Evaluation of the antioxidant activity of asparagus, broccoli and their juices. Food Chem. 2007, 105, 101–106. [Google Scholar] [CrossRef]
- Png, X.W.; Akowuah, G.A.; Chin, J.H. Acute oral toxicity study of Clinacanthus nutans in mice. Int. J. Pharm. Sci. Res. 2012, 3, 4202–4205. [Google Scholar]
- Sylvestre, M.; Pichette, A.; Longtin, A.; Nagau, F.; Legault, J. Essential oil analysis and anticancer activity of leaf essential oil of Croton flavens L. from Guadeloupe. J. Ethnopharmacol. 2006, 103, 99–102. [Google Scholar] [CrossRef]
- Shoemaker, M.; Hamilton, B.; Dairkee, S.H.; Cohen, I.; Campbell, M.J. In vitro anticancer activity of twelve Chinese medicinal herbs. Phytother. Res. 2005, 19, 649–651. [Google Scholar] [CrossRef]
- Chang, J.-Y.; Guo, X.; Chen, H.-X.; Jiang, Z.; Fu, Q.; Wang, H.-K.; Bastow, K.F.; Zhu, X.-K.; Guan, J.; Lee, K.-H. Unique biochemical, cytotoxic, and antitumor activity of camptothecin and 4β-amino-4'-O-demethylepipodophyllotoxin conjugates. Biochem. Pharmacol. 2000, 59, 497–508. [Google Scholar] [CrossRef]
- Jayaprakasha, G.; Patil, B.S. In vitro evaluation of the antioxidant activities in fruit extracts from citron and blood orange. Food Chem. 2007, 101, 410–418. [Google Scholar] [CrossRef]
- Jia, Z.S.; Tang, M.C.; Wu, J.M. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Hsu, C.F.; Zhang, L.; Peng, H.; Travas-Sejdic, J.; Kilmartin, P.A. Scavenging of DPPH free radicals by polypyrrole powders of varying levels of overoxidation and/or reduction. Synth. Met. 2008, 158, 946–952. [Google Scholar] [CrossRef]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Mrillon, J.-M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the sabah snake grass are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghasemzadeh, A.; Nasiri, A.; Jaafar, H.Z.E.; Baghdadi, A.; Ahmad, I. Changes in Phytochemical Synthesis, Chalcone Synthase Activity and Pharmaceutical Qualities of Sabah Snake Grass (Clinacanthus nutans L.) in Relation to Plant Age. Molecules 2014, 19, 17632-17648. https://doi.org/10.3390/molecules191117632
Ghasemzadeh A, Nasiri A, Jaafar HZE, Baghdadi A, Ahmad I. Changes in Phytochemical Synthesis, Chalcone Synthase Activity and Pharmaceutical Qualities of Sabah Snake Grass (Clinacanthus nutans L.) in Relation to Plant Age. Molecules. 2014; 19(11):17632-17648. https://doi.org/10.3390/molecules191117632
Chicago/Turabian StyleGhasemzadeh, Ali, Alireza Nasiri, Hawa Z. E. Jaafar, Ali Baghdadi, and Izham Ahmad. 2014. "Changes in Phytochemical Synthesis, Chalcone Synthase Activity and Pharmaceutical Qualities of Sabah Snake Grass (Clinacanthus nutans L.) in Relation to Plant Age" Molecules 19, no. 11: 17632-17648. https://doi.org/10.3390/molecules191117632
APA StyleGhasemzadeh, A., Nasiri, A., Jaafar, H. Z. E., Baghdadi, A., & Ahmad, I. (2014). Changes in Phytochemical Synthesis, Chalcone Synthase Activity and Pharmaceutical Qualities of Sabah Snake Grass (Clinacanthus nutans L.) in Relation to Plant Age. Molecules, 19(11), 17632-17648. https://doi.org/10.3390/molecules191117632







