Carapanolides J–L from the Seeds of Carapa guianensis (Andiroba) and Their Effects on LPS-Activated NO Production
Abstract
:1. Introduction
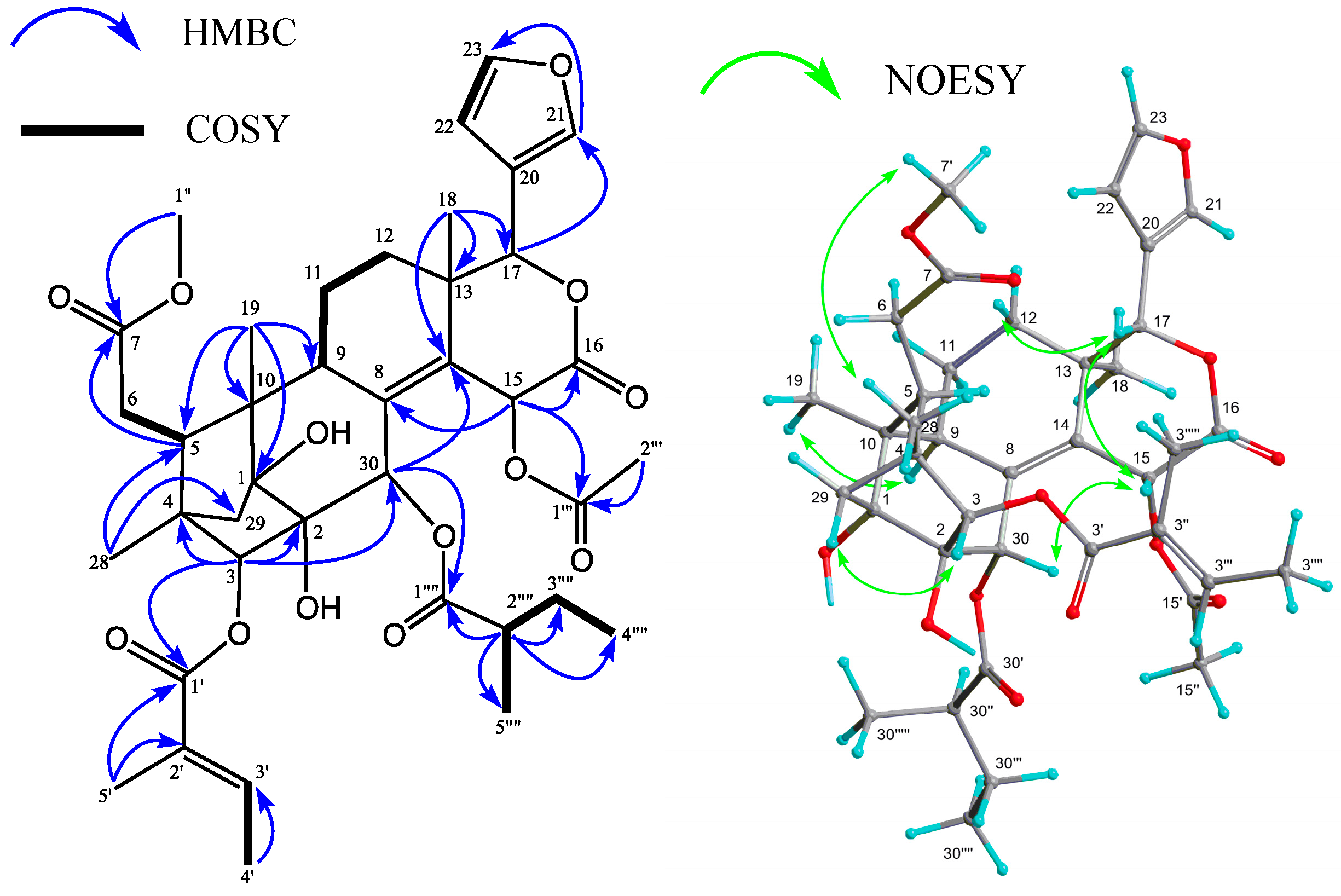
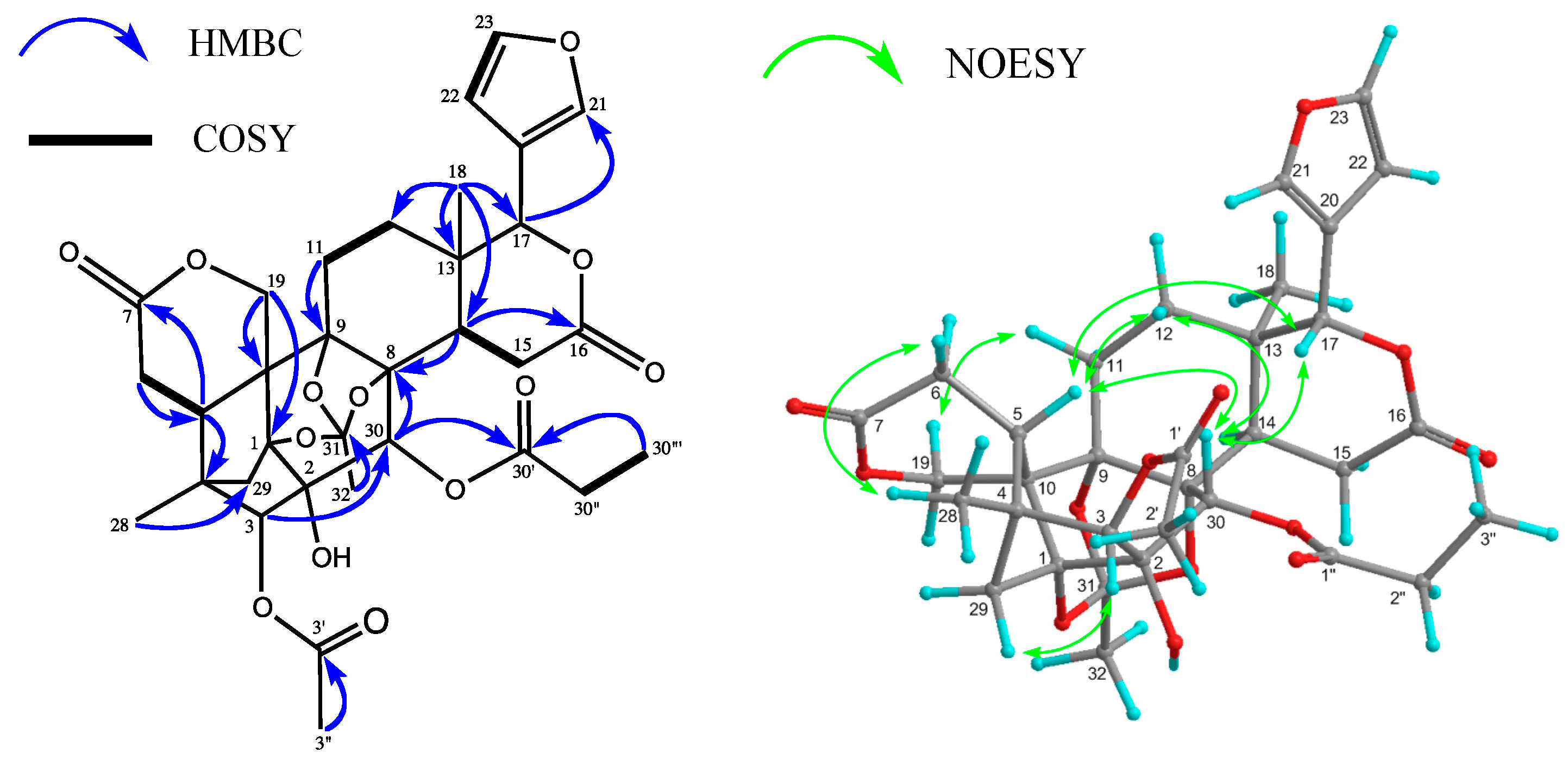
2. Results and Discussion

| Position | 1 | Position | 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1H a (J, Hz) | 13C b | 1H a (J, Hz) | 13C b | |||||||
| 1 | 8.24 | d 10.3 (2) | 160.2 | 14 | 65.4 | |||||
| 2 | 5.84 | d 10.3 (1) | 124.9 | 15 | 3.88 | s | 54.1 | |||
| 3 | 203.0 | 16 | 166.4 | |||||||
| 4 | 45.6 | 17 | 5.49 | s | 77.6 | |||||
| 5 | 2.21 | dd 3.2 (6α), 14.6 (6β) | 53.9 | 18 | 1.21 | s | 20.6 | |||
| 6 | α | 2.38 | dd 3.2 (5), 13.8 (6β) | 36.3 | 19 | 1.56 | s | 20.9 | ||
| β | 2.93 | dd 13.8 (6α), 14.6 (5) | 20 | 120.0 | ||||||
| 7 | 207.7 | 21 | 7.44 | m | 141.1 | |||||
| 8 | 53.4 | 22 | 6.39 | dd 0.6 (21), 1.7(23) | 109.7 | |||||
| 9 | 2.45 | d 10.2 (11) | 51.3 | 23 | 7.42 | t 1.7 (21, 22) | 143.3 | |||
| 10 | 40.9 | 28 | 1.17 | s | 20.7 | |||||
| 11 | β | 4.47 | ddd 7.9 (12β), 10.2 (9), 13.5 (12α) | 67.3 | 29 | 1.16 | s | 27.4 | ||
| 12 | α | 1.46 | dd 13.5 (11), 13.8 (12β) | 44.6 | 30 | 1.28 | s | 18.2 | ||
| β | 2.21 | dd 7.9 (11), 13.8 (12α) | 11-OH | 1.83 | s | |||||
| 13 | 38.0 | |||||||||
| Position | 2 | 3 | |||||
|---|---|---|---|---|---|---|---|
| 1H a (J, Hz) | 13C b | 1H a (J, Hz) | 13C b | ||||
| 1 | 83.6 | 85.4 | |||||
| 2 | 77.0 | 79.5 | |||||
| 3 | 4.73 | s | 88.2 | 4.66 | s | 83.9 | |
| 4 | 43.1 | 45.2 | |||||
| 5 | 2.88 | dd 1.2 (6B), 5.3 (6A) | 37.5 | 2.68 | dd 3.5 (6B), 5.5 (6A) | 33.8 | |
| 6 | A | 2.32 | d 5.3 (5) | 33.7 | 2.46 | dd 5.5 (5), 17.6 (6B) | 31.0 |
| B | 2.33 | d 1.2 (5) | 2.66 | dd 3.5 (5), 17.6 (6A) | |||
| 7 | 174.2 | 171.1 | |||||
| 8 | 134.9 | 86.4 | |||||
| 9 | 2.73 | d 7.7 | 35.9 | 86.3 | |||
| 10 | 47.5 | 44.7 | |||||
| 11 | Α | 1.70 | m | 18.3 | 1.85 | dt 2.9 (11α), 14.7 (12α,β) | 25.7 |
| Β | 1.89 | m | 2.27 | m | |||
| 12 | α | 1.05 | m | 28.5 | 1.48 | m | 29.4 |
| β | 1.4 | dt 3.2 (12α), 14.1 (11β) | 1.38 | m | |||
| 13 | 38.9 | 34.5 | |||||
| 14 | 135.4 | 2.02 | dd 2.0 (15β), 10.5 (15α) | 42.8 | |||
| 15 | α | 2.70 | dd 10.5 (14), 20.0 (15β) | 26.4 | |||
| β | 6.28 | d 2.4 | 64.2 | 3.19 | dd 2.0 (14), 20.0 (15α) | ||
| 16 | 167.8 | 169.8 | |||||
| 17 | 5.36 | s | 80.3 | 5.35 | s | 78.4 | |
| 18 | 1.09 | s | 16.7 | 1.13 | s | 20.0 | |
| 19 | α | 1.14 | 3H, s | 17.3 | 4.77 | d 13.8 (19β) | 68.8 |
| β | 4.38 | d 13.8 (19α) | |||||
| 20 | 120.5 | 120.8 | |||||
| 21 | 7.58 | t 0.8 (22) | 142.0 | 7.48 | t 0.8 (22) | 140.8 | |
| 22 | 6.47 | dd 0.8 (21), 1.6 (23) | 109.9 | 6.41 | dd 0.8 (21), 1.8 (23) | 109.6 | |
| 23 | 7.41 | t 1.6 (22) | 143 | 7.44 | t 1.8 (22) | 143.4 | |
| 28 | 0.83 | s | 14.8 | 1.00 | s | 13.6 | |
| 29 | pro-R | 1.58 | d 11.0 (29 pro-S) | 39.8 | 1.80 | d 11.1 (29 pro-S) | 38.3 |
| pro-S | 1.86 | d 11.0 (29 pro-R) | 2.25 | d 11.1 (29 pro-R) | |||
| 30 | 5.41 | s | 69.7 | 5.71 | s | 70.0 | |
| 31 | 119.6 | ||||||
| 32 | 1.70 | s | 21.0 | ||||
| 1' | 168.5 | 170.4 | |||||
| 2' | 130.0 | 2.19 | s | 21.6 | |||
| 3' | 7.14 | qq 7.0 (4'), 1.1 (5') | 12.2 | ||||
| 4' | 1.77 | dd 1.1 (5'), 7.0 (3') | 139.2 | ||||
| 5' | 1.98 | t 1.1 (3', 4') | 14.5 | ||||
| 1'' | 3.72 | s | 52.0 | 172.8 | |||
| 2'' | A | 2.36 | dq 7.5 (3''), 9.7 (2''B) | 27.8 | |||
| B | 2.39 | dq 7.5 (3''), 9.7 (2''A) | |||||
| 3'' | 1.09 | 3H, t 7.5 (2''A, 2"B) | 8.6 | ||||
| 1''' | 169.9 | ||||||
| 2''' | 2.07 | s | 21.1 | ||||
| 1'''' | 176.3 | ||||||
| 2'''' | A | 2.38 | m | 40.9 | |||
| B | |||||||
| 3'''' | A | 1.48 | m | 26.5 | |||
| B | 1.64 | m | |||||
| 4'''' | 0.90 | t 7.3 (3''''A, 3''''B) | 16.4 | ||||
| 5'''' | 1.13 | d 7.0 (2'''') | 11.3 | ||||
| Compound | Concentration (μM) | |||||
|---|---|---|---|---|---|---|
| 3 | 10 | 30 | 100 | |||
| 1 | Produced NO (%) a | 92.1 ± 1.5 | 83.4 ± 3.1 | 61.8 ± 1.8 | 16.8 ± 0.0 | 37.4 |
| Cell viability (%) a | 102.4 ± 0.8 | 101.0 ± 1.7 | 102.8 ± 0.6 | 103.4 ± 1.8 | >100 | |
| 2 | Produced NO (%) | 78.6 ± 1.9 | 58.3 ± 2.8 | 25.8 ± 7.0 | 7.1 ± 1.2 | 12.0 |
| Cell viability (%) | 81.4 ± 0.8 | 65.6 ± 0.2 | 33.6 ± 6.3 | 0.4 ± 0.4 | 15.2 | |
| 3 | Produced NO (%) | 95.6 ± 2.5 | 95.4 ± 1.2 | 95.4 ± 2.9 | 78.4 ± 2.3 | >100 |
| Cell viability (%) | 97.6 ± 0.6 | 97.3 ± 1.3 | 100.5 ± 0.4 | 94.4 ± 1.0 | >100 | |
| 4 | Produced NO (%) | 74.0 ± 5.0 | 30.0 ± 2.3 | 7.5 ± 1.0 | 3.9 ± 1.8 | 5.9 |
| Cell viability (%) | 93.6 ± 1.4 | 99.7 ± 0.8 | 6.8 ± 0.3 | 3.3 ± 0.3 | 21.3 | |
| L-NMMA b | Produced NO (%) | 93.0 ± 3.3 | 79.3 ± 0.8 | 58.2 ± 2.4 | 39.9 ± 1.7 | 53.7 |
| Cell viability (%) | 103.5 ± 0.5 | 102.0 ± 1.5 | 94.1 ± 1.4 | 96.5 ± 2.5 | >100 | |
3. Experimental Section
3.1. General Procedures
3.2. Plant Material
3.3. Isolation of Compounds 1–4
3.4. Analytical Data
3.5. Determination of RAW264.7 Cell Proliferation
3.6. Inhibitory Assay of NO Production
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tan, Q.G.; Luo, X.D. Meliaceous limonoids: chemistry and biological activities. Chem. Rev. 2011, 111, 7437–7522. [Google Scholar] [PubMed]
- Prophiro, J.S.; da Silva Mario, A.N.; Kanis, L.A.; da Rocha, L.C.B.P.; Duque-Luna, J.E.; da Silva, O.S. First report on susceptibility of wild Aedes aegypty (Diptera: Culicidae) using Carapa guianensis (Meliaceae) and Copaifera sp. (Leguminosae). Parasitol. Res. 2012, 110, 7699–7705. [Google Scholar]
- Penido, C.; Costa, K.A.; Pennaforte, R.J.; Costa, M.F.S.; Pereira, J.F.G.; Siani, A.C.; Henriques, M.G.M.O. Anti-allergic effects of natural tetranortriterpenoids isolated from Carapa guianensis Aublet on allergen-induced vascular permeability and hyperalgesia. Inflamm. Res. 2005, 54, 295–303. [Google Scholar] [PubMed]
- Bickii, J.; Njifutie, N.; Foyere, J.A.; Basco, L.K.; Ringwald, P.J. In vitro antimalarial activity of limonoids from Khaya grandifoliola C.D.C. (Meliaceae). J. Ethnopharmacol. 2000, 69, 27–33. [Google Scholar] [CrossRef]
- Penido, C.; Conte, F.P.; Chagas, M.S.S.; Rodrigue, C.A.B.; Pereira, J.F.G.; Henriques, M.G.M.O. Antiinflammatory effects of natural tetranortriterpenoids isolated from Carapa guianensis Aublet on zymosan-induced arthritis in mice. Inflamm. Res. 2006, 55, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Ferraris Fausto, K.; Rodrigues, R.; da Silva, V.P.; Figueiredo, R.; Penido, C.; Henriques, M.G.M.O. Modulation of T lymphocyte and eosinophil functions in vitro by natural tetranortriterpenoids isolated from Carapa guianensis Aublet. Int. Immunopharmacol. 2011, 11, 1–11. [Google Scholar]
- Miranda Junior, R.N.C.; Dolabela, M.F.; da Silva, M.N.; Povoa, M.M.; Maia, J.G.S. Antiplasmoidal activity of the andiroba (Carapa guianensis Aublet., Meliaceae) oil and its limonoid-rich fraction. J. Ethnopharmacol. 2012, 142, 679–683. [Google Scholar]
- Costa-Silva, H.; Lima, C.R.; Silva, E.J.R.; Araujo, A.V.; Fraga, M.C.C.R.; Ribeiro, E.; Ribwiro, A.; Arruda, A.C.; Lafayette, S.S.L.; Wanderley, J. Acute and subacute toxicity of the Carapa guianensis Aublet (Meliaceae) seed oil. J. Ethnopharmacol. 2008, 116, 495–500. [Google Scholar] [CrossRef]
- Inoue, T.; Nagai, Y.; Mitooka, A.; Ujike, R.; Muraoka, O.; Yamada, T.; Tanaka, R. Carapanolides A and B: unusual 9,10-seco-mexicanolides having a 2R,9S-oxygen bridge from the seeds of Carapa guianensis. Tetrahedron Lett. 2012, 53, 6685–6688. [Google Scholar] [CrossRef]
- Inoue, T.; Matsui, Y.; Kikuchi, T.; In, Y.; Yamada, T.; Muraoka, O.; Matsunaga, S.; Tanaka, R. Guianolides A and B, New Carbon Skeletal Limonoids from the seeds of Carapa guianensis. Org. Lett. 2013, 15, 3018–3021. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Matsui, Y.; Kikuchi, T.; In, Y.; Muraoka, O.; Yamada, T.; Tanaka, R. Carapanolides C–I from the seeds of andiroba (Carapa guianensis, Meliaceae). Fitoterapia 2014, 96, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Malathi, R.; Rajan, S.S.; Mohan Kumar, R.; Narasimhan, S.; Ravikumar, K. Epoxyazadiradione. Acta Crystallogr. 2007, E63, 2483–2485. [Google Scholar]
- Tanaka, Y.; Yamada, T.; In, Y.; Muraoka, O.; Kajimito, T.; Tanaka, R. Absolute stereostructure of Andirolides A-G from the flower of Carapa guianensis (Meliaceae). Tetrahedron 2011, 67, 782–792. [Google Scholar] [CrossRef]
- Ravangpai, W.; Sommit, D.; Teerawatananond, T.; Sinpranee, N.; Palaga, T.; Pengpreecha, S.; Muangsin, N.; Pudhom, K. Limonoids from seeds of Thai Xylocarpus moluccensis. Bioorg. Med. Chem. Lett. 2011, 21, 4485–4489. [Google Scholar] [CrossRef] [PubMed]
- Kirkeboen, K.A.; Strand, O.A. The role of nitric oxide in sepsis–an overview. Acta Anaesthesiol. Scand. 1999, 43, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Muroga, Y.; Jinno, M.; Kajimoto, T.; Usami, Y.; Numata, A.; Tanaka, R. New class azaphilone produced by a marine fish-derived Chaetomium globosum. The stereochemistry and biological activities. Bioorg. Med. Chem. 2011, 19, 4106–4113. [Google Scholar] [CrossRef]
- Yamasaki, F.; Machida, S.; Nakata, A.; Nugroho, A.E.; Hirasawa, Y.; Kaneda, T.; Morita, H. Haworforbins A-C, new phenolics from Haworthia cymbiformis. J. Nat. Med. 2013, 67, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsui, Y.; Kikuchi, T.; Inoue, T.; Muraoka, O.; Yamada, T.; Tanaka, R. Carapanolides J–L from the Seeds of Carapa guianensis (Andiroba) and Their Effects on LPS-Activated NO Production. Molecules 2014, 19, 17130-17140. https://doi.org/10.3390/molecules191117130
Matsui Y, Kikuchi T, Inoue T, Muraoka O, Yamada T, Tanaka R. Carapanolides J–L from the Seeds of Carapa guianensis (Andiroba) and Their Effects on LPS-Activated NO Production. Molecules. 2014; 19(11):17130-17140. https://doi.org/10.3390/molecules191117130
Chicago/Turabian StyleMatsui, Yuuki, Takashi Kikuchi, Takanobu Inoue, Osamu Muraoka, Takeshi Yamada, and Reiko Tanaka. 2014. "Carapanolides J–L from the Seeds of Carapa guianensis (Andiroba) and Their Effects on LPS-Activated NO Production" Molecules 19, no. 11: 17130-17140. https://doi.org/10.3390/molecules191117130
APA StyleMatsui, Y., Kikuchi, T., Inoue, T., Muraoka, O., Yamada, T., & Tanaka, R. (2014). Carapanolides J–L from the Seeds of Carapa guianensis (Andiroba) and Their Effects on LPS-Activated NO Production. Molecules, 19(11), 17130-17140. https://doi.org/10.3390/molecules191117130





