Design of Composite Photocatalyst of TiO2 and Y-Zeolite for Degradation of 2-Propanol in the Gas Phase under UV and Visible Light Irradiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of TiO2/Y-Zeolite Composite Photocatalysts
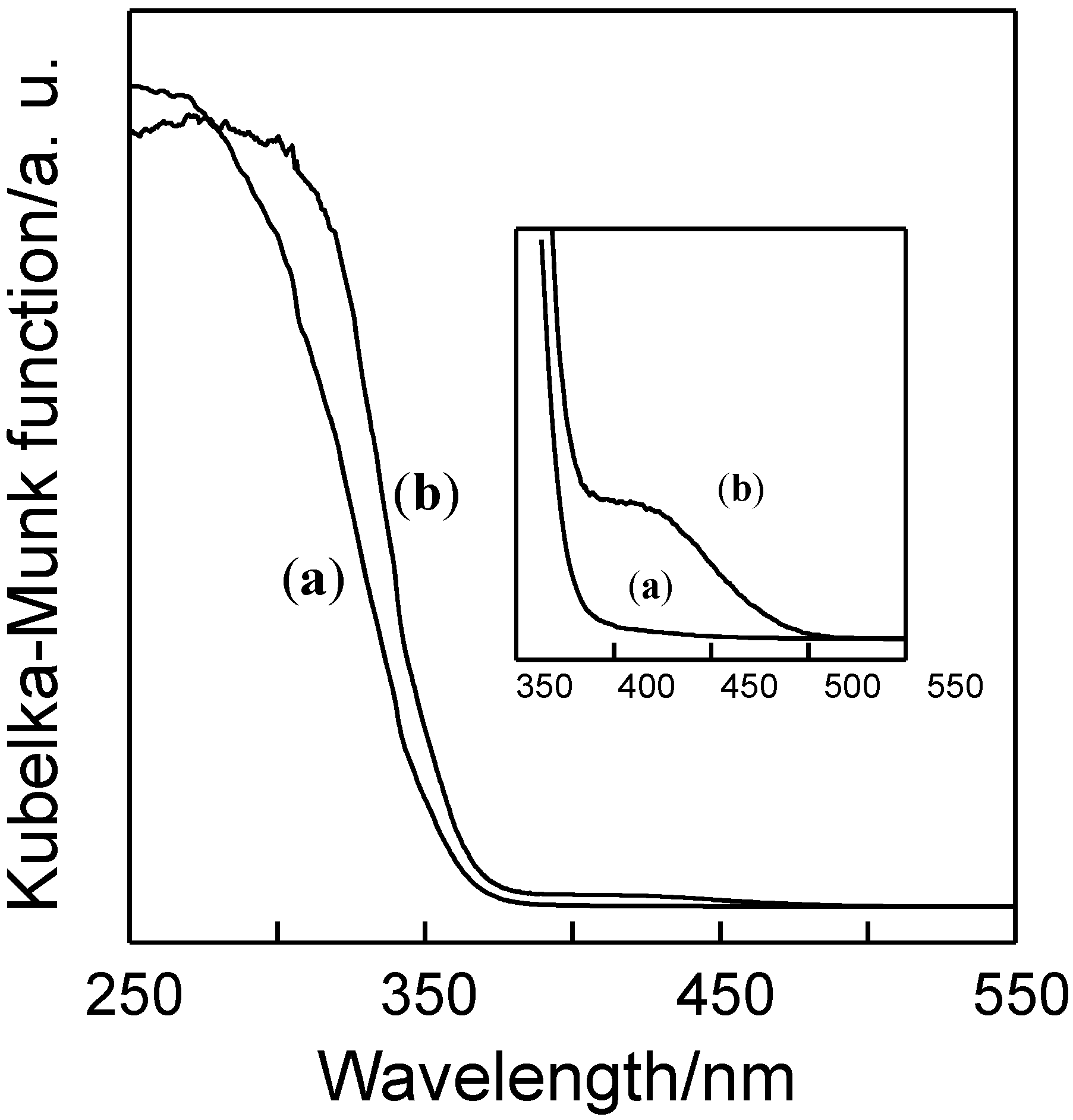
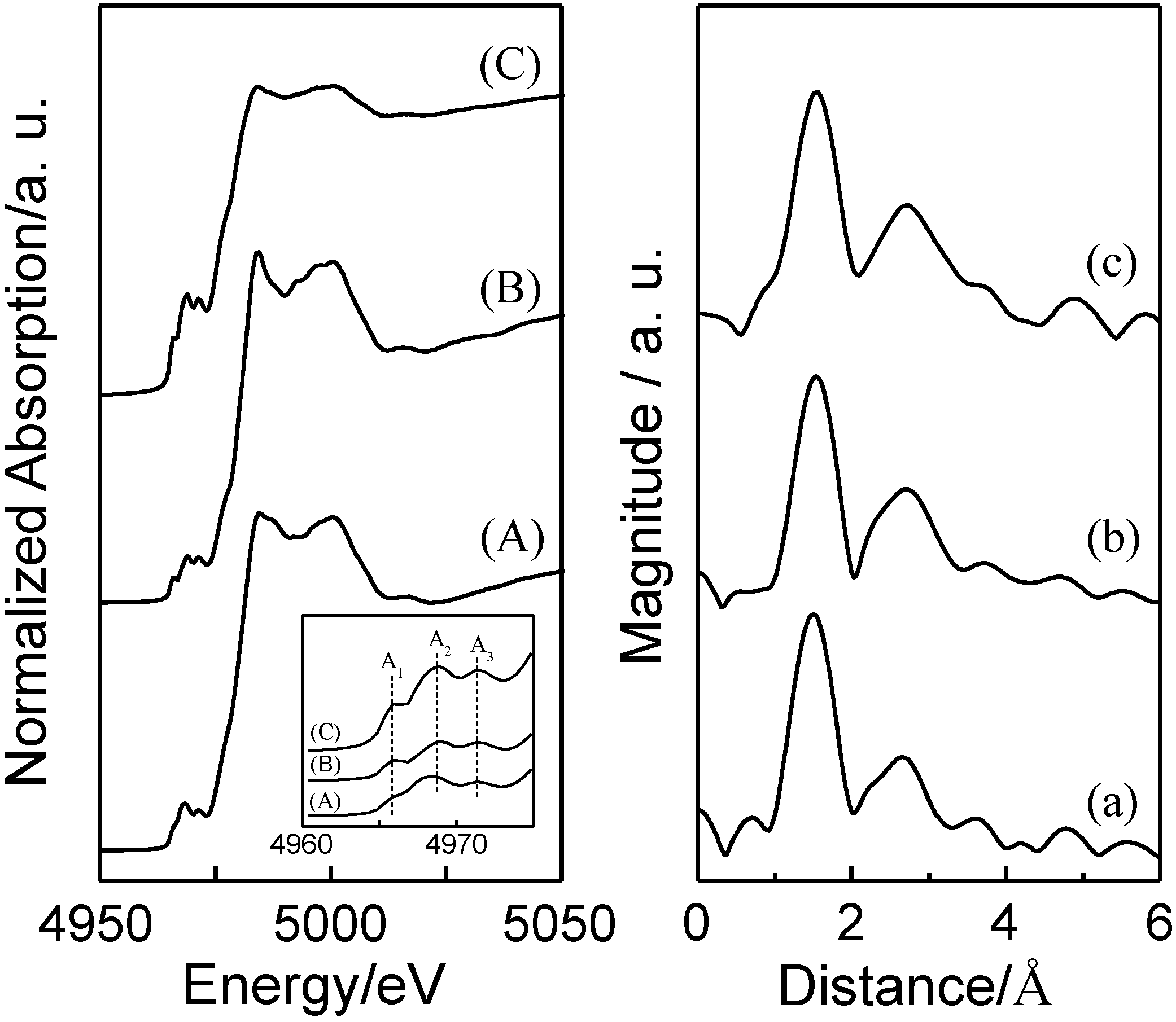
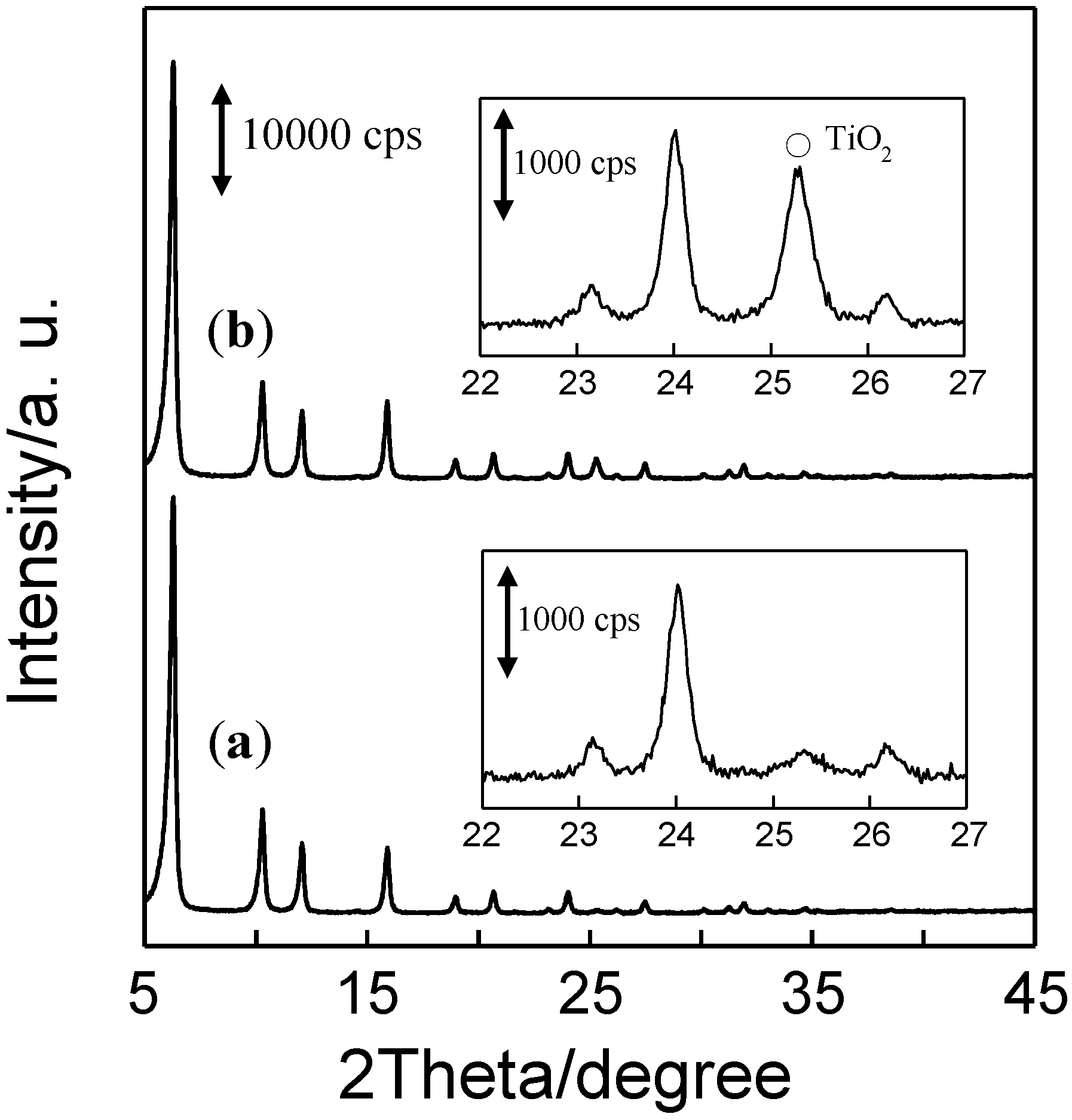
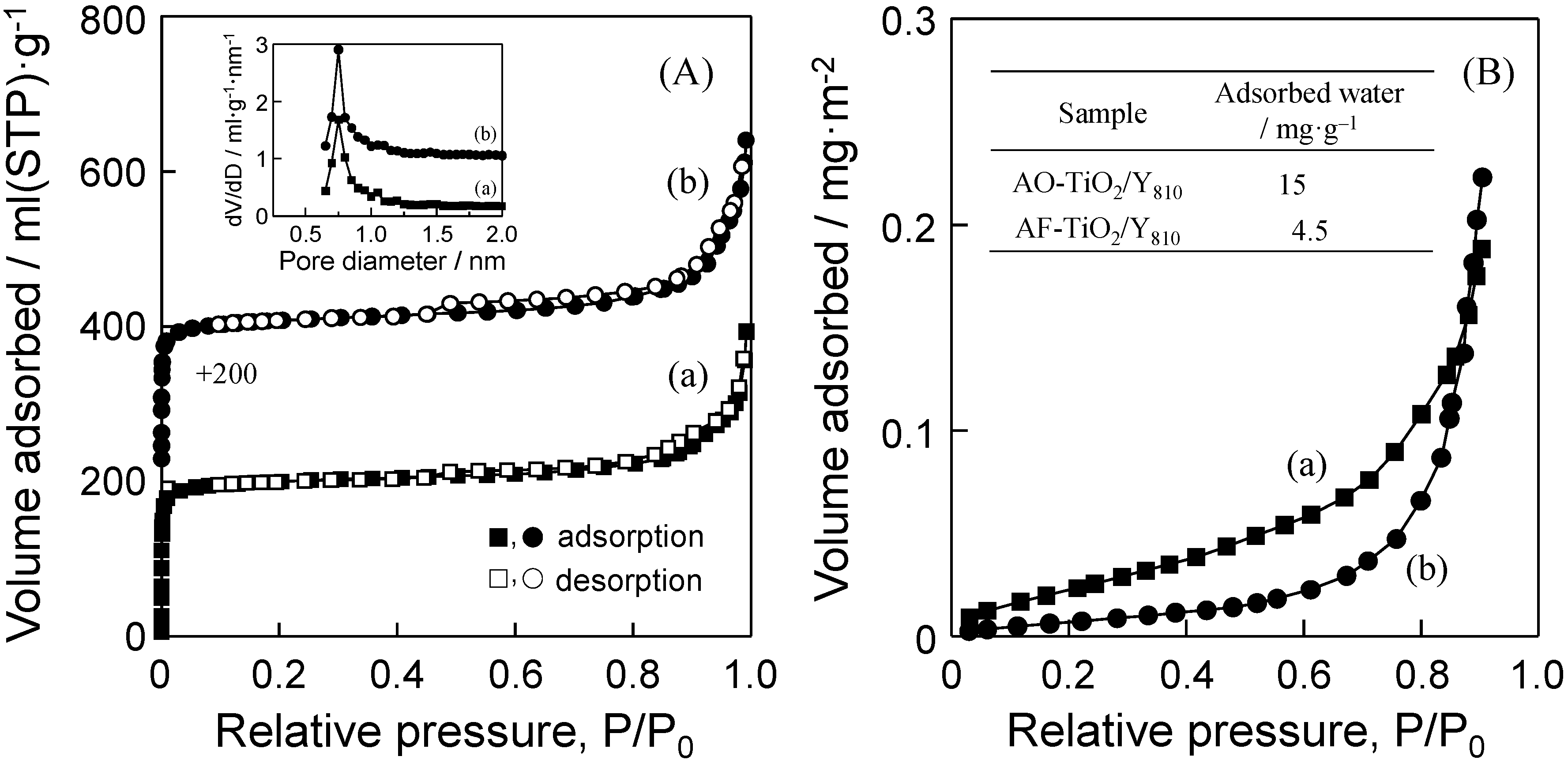

2.2. Photocatalytic Performance of TiO2/Y-Zeolite Composite Photocatalysts

3. Experimental Section
3.1. Materials
3.2. Sample Preparation
3.3. Characterization Techniques
3 4. Photocatalytic Reaction
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fox, M.A.; Dulay, M.T. Heterogeneous Photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium Dioxide Photocatalysis. J. Photochem. Photobiol. C: Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, Y.; Kamegawa, T.; Mori, K.; Yamashita, H. Design of New Functional Titanium Oxide-based Photocatalysts for Degradation of Organics Diluted in Water and Air. Curr. Org. Chem. 2010, 14, 616–629. [Google Scholar] [CrossRef]
- Qian, X.; Fuku, K.; Kuwahara, Y.; Kamegawa, T.; Mori, K.; Yamashita, H. Design and Functionalization of Photocatalytic Systems within Mesoporous Silica. ChemSusChem 2014, 7, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Kubacka, A.; Fernández-García, M.; Colón, G. Advanced Nanoarchitectures for Solar Photocatalytic Applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.; Ma, W.; Chen, C.; Ji, H.; Zhao, J. Selective Aerobic Oxidation Mediated by TiO2 Photocatalysis. Acc. Chem. Res. 2014, 47, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Palmisano, G.; García-López, E.; Marcí, G.; Loddo, V.; Yurdakal, S.; Augugliaro, V.; Palmisano, L. Advances in Selective Conversions by Heterogeneous Photocatalysis. Chem. Commun. 2010, 46, 7074–7089. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Sugano, Y.; Tanaka, S.; Hirai, T. One-Pot Synthesis of Benzimidazoles by Simultaneous Photocatalytic and Catalytic Reactions on Pt@TiO2 Nanoparticles. Angew. Chem. Int. Ed. 2010, 49, 1656–1660. [Google Scholar] [CrossRef]
- Kominami, H.; Yamamoto, S.; Imamura, K.; Tanaka, A.; Hashimoto, K. Photocatalytic Chemoselective Reduction of Epoxides to Alkenes Along with Formation of Ketones in Alcoholic Suspensions of Silver-loaded Titanium(IV) Oxide at Room Temperature without the Use of Reducing Gasses. Chem. Commun. 2014, 50, 4558–4560. [Google Scholar] [CrossRef]
- Kamegawa, T.; Seto, H.; Matsuura, S.; Yamashita, H. Preparation of Hydroxynaphthalene-Modified TiO2 via Formation of Surface Complexes and their Applications in the Photocatalytic Reduction of Nitrobenzene under Visible-Light Irradiation. ACS Appl. Mater. Interfaces 2012, 4, 6635–6639. [Google Scholar] [CrossRef] [PubMed]
- Kamegawa, T.; Matsuura, S.; Seto, H.; Yamashita, H. A Visible-Light-Harvesting Assembly with a Sulfocalixarene Linker between Dyes and a Pt-TiO2 Photocatalyst. Angew. Chem. Int. Ed. 2013, 52, 916–919. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced Amphiphilic Surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Photogeneration of Highly Amphiphilic TiO2 Surfaces. Adv. Mater. 1998, 10, 135–138. [Google Scholar] [CrossRef]
- Kamegawa, T.; Suzuki, N.; Yamashita, H. Design of Macroporous TiO2 Thin Film Photocatalysts with Enhanced Photofunctional Properties. Energy Environ. Sci. 2011, 4, 1411–1416. [Google Scholar] [CrossRef]
- Kamegawa, T.; Shimizu, Y.; Yamashita, H. Superhydrophobic Surfaces with Photocatalytic Self-Cleaning Properties by Nanocomposite Coating of TiO2 and Polytetrafluoroethylene. Adv. Mater. 2012, 24, 3697–3700. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Termin, A.; Hoffmann, M.R. The Role of Metal Ion Dopants in Quantum-Sized TiO2: Correlation between Photoreactivity and Charge Carrier Recombination Dynamics. J. Phys. Chem. 1994, 98, 13669–13679. [Google Scholar] [CrossRef]
- Yamashita, H.; Harada, M.; Misaka, M.; Takeuchi, M.; Neppolian, B.; Anpo, M. Photocatalytic Degradation of Organic Compounds Diluted in Water Using Visible Light-responsive Metal Ion-Implanted TiO2 Catalysts: Fe Ion-implanted TiO2. Catal. Today 2003, 84, 191–196. [Google Scholar] [CrossRef]
- Yamashita, H.; Harada, M.; Misaka, J.; Takeuchi, M.; Ikeue, K.; Anpo, M. Degradation of Propanol Diluted in Water under Visible Light Irradiation using Metal Ion-implanted Titanium Dioxide Photocatalysts. J. Photochem. Photobiol. A-Chem. 2002, 148, 257–261. [Google Scholar] [CrossRef]
- Kamegawa, T.; Sonoda, J.; Sugimura, K.; Mori, K.; Yamshita, H. Degradation of Isobutanol Diluted in Water over Visible Light Sensitive Vanadium Doped TiO2 Photocatalyst. J. Alloys Compd. 2009, 486, 685–688. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, S.; Kisch, H. Daylight Photocatalysis by Carbon-Modified Titanium Dioxide. Angew. Chem. Int. Ed. 2003, 42, 4908–4911. [Google Scholar] [CrossRef]
- Ohno, T.; Akiyoshi, M.; Umebayashi, T.; Asai, K.; Mitui, T.; Matsumura, M. Preparation of S-doped TiO2 Photocatalysts and their Photocatalytic Activities under Visible Light. Appl. Catal. A: Gen. 2004, 265, 115–121. [Google Scholar] [CrossRef]
- Maeda, K.; Shimodaira, Y.; Lee, B.; Teramura, K.; Lu, D.; Kobayashi, H.; Domen, K. Studies on TiNxOyFz as a Visible-Light-Responsive Photocatalyst. J. Phys. Chem. C 2007, 111, 18264–19270. [Google Scholar] [CrossRef]
- Kisch, H.; Zang, L.; Lange, C.; Maier, W.F.; Antonius, C.; Meissner, D. Modified, Amorphous Titania-A Hybrid Semiconductor for Detoxification and Current Generation by Visible Light. Angew. Chem. Int. Ed. 1998, 37, 30345–3036. [Google Scholar] [CrossRef]
- Irie, H.; Kamiya, K.; Shibanuma, T.; Miura, S.; Tryk, D.A.; Yokouyama, T.; Hashimoto, K. Visible Light-Sensitive Cu(II)-Grafted TiO2 Photocatalysts: Activities and X-ray Absorption Fine Structure Analyses. J. Phys. Chem. C 2009, 113, 10761–10766. [Google Scholar] [CrossRef]
- Kitano, S.; Hashimoto, K.; Kominami, H. Photocatalytic Degradation of 2-propanol over Metal-ion-loaded Titanium(IV) Oxide under Visible Light Irradiation: Effect of Physical Properties of Nano-crystalline Titanium(IV) Oxide. Appl. Catal. B: Environ. 2011, 101, 206–211. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Zeolite-based Photocatalysts. Chem. Commun. 2004, 1443–1459. [Google Scholar]
- Qian, X.F.; Kamegawa, T.; Mori, K.; Li, H.X.; Yamashita, H. Calcium Phosphate Coatings Incorporated in Mesoporous TiO2/SBA-15 by a Facile Inner-pore Sol-gel Process toward Enhanced Adsorption-photocatalysis Performances. J. Phys. Chem. C 2013, 117, 19544–19551. [Google Scholar] [CrossRef]
- Yamashita, H.; Nose, H.; Kuwahara, Y.; Nishida, Y.; Yuan, S.; Mori, K. TiO2 Photocatalyst Loaded on Hydrophobic Si3N4 Support for Efficient Degradation of Organics Diluted in Water. Appl. Catal. A: Gen. 2008, 350, 164–168. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Aoyama, J.; Miyakubo, K.; Eguchi, T.; Kamegawa, T.; Mori, K.; Yamashita, H. TiO2 Photocatalyst for Degradation of Organic Compounds in Water and Air Supported on Highly Hydrophobic FAU Zeolite: Structural, Sorptive, and Photocatalytic Studies. J. Catal. 2012, 285, 223–234. [Google Scholar] [CrossRef]
- Inumaru, K.; Kasahara, T.; Yasui, M.; Yamanaka, S. Direct Nanocomposite of Crystalline TiO2 Particles and Mesoporous Silica as a Molecular Selective and Highly Active Photocatalyst. Chem. Commun. 2005, 2131–2133. [Google Scholar]
- Torimoto, T.; Okawa, Y.; Takeda, N.; Yoneyama, H. Effect of Activated Carbon Content in TiO2-loaded Activated Carbon on Photodegradation Behaviors of Dichloromethane. J. Photochem. Photobiol. A: Chem. 1997, 103, 153–157. [Google Scholar] [CrossRef]
- Kamegawa, T.; Kido, R.; Yamahana, D.; Yamashita, H. Design of TiO2-zeolite Composites with Enhanced Photocatalytic Performances under Irradiation of UV and Visible Light. Microporous Mesoporous Mater. 2013, 165, 142–147. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Maki, K.; Matsumura, Y.; Kamegawa, T.; Mori, K.; Yamashita, H. Hydrophobic Modification of a Mesoporous Silica Surface Using a Fluorine-Containing Silylation Agent and Its Application as an Advantageous Host Material for the TiO2 Photocatalyst. J. Phys. Chem. C 2009, 113, 1552–1559. [Google Scholar] [CrossRef]
- Kamegawa, T.; Yamahana, D.; Yamashita, H. Graphene Coating of TiO2 Nanoparticles Loaded on Mesoporous Silica for Enhancement of Photocatalytic Activity. J. Phys. Chem. C 2011, 114, 15049–15053. [Google Scholar] [CrossRef]
- Lassaletta, G.; Fernandez, A.; Espinos, J.P.; Gonzalez-Elipe, A.R. Spectroscopic Characterization of Quantum-Sized TiO2 Supported on Silica: Influence of Size and TiOz-SiOz Interface Composition. J. Phys. Chem. 1995, 99, 1484–1490. [Google Scholar] [CrossRef]
- Yamashita, H.; Honda, M.; Harada, M.; Ichihashi, Y.; Anpo, M.; Hirao, T.; Itoh, N.; Iwamoto, N. Preparation of Titanium Oxide Photocatalysts Anchored on Porous Silica Glass by a Metal Ion-Implantation Method and Their Photocatalytic Reactivities for the Degradation of 2-Propanol Diluted in Water. J. Phys. Chem. B 1998, 102, 10707–10711. [Google Scholar] [CrossRef]
- Liu, Z.; Davis, R.J. Investigation of the Structure of Microporous Ti-Si Mixed Oxides by X-ray, UV Reflectance, FT-Raman, and FT-IR Spectroscopies. J. Phys. Chem. 1994, 98, 1253–1261. [Google Scholar] [CrossRef]
- Du, X.; He, J.; Zhao, Y. Facile Preparation of F and N Codoped Pinecone-Like Titania Hollow Microparticles with Visible Light Photocatalytic Activity. J. Phys. Chem. C 2009, 113, 14151–14158. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, J.; Ho, W.; Jiang, Z.; Zhang, L. Effects of F− Doping on the Photocatalytic Activity and Microstructures of Nanocrystalline TiO2 Powders. Chem. Mater. 2002, 14, 3808–3816. [Google Scholar] [CrossRef]
- Greegor, R.B.; Lytle, F.W.; Sandstrom, D.R.; Wong, J.; Schultz, P. Investigation of TiO2SiO2 Glasses by X-ray Absorption Spectroscopy. J. Non-Cryst. Solids 1983, 55, 27–43. [Google Scholar] [CrossRef]
- Chen, L.X.; Rajh, T.; Wang, Z.; Thurnauer, M.C. XAFS Studies of Surface Structures of TiO2 Nanoparticles and Photocatalytic Reduction of Metal Ions. J. Phys. Chem. B 1997, 101, 10688–10697. [Google Scholar] [CrossRef]
- Chen, D.; Jiang, Z.; Geng, J.; Zhu, J.; Yang, D. A Facile Method to Synthesize Nitrogen and Fluorine Co-doped TiO2 Nanoparticles by Pyrolysis of (NH4)2TiF6. J. Nanopart. Res. 2009, 11, 303–313. [Google Scholar] [CrossRef]
- Furube, A.; Asahi, T.; Masuhara, H.; Yamashita, H.; Anpo, M. Charge Carrier Dynamics of Standard TiO2 Catalysts Revealed by Femtosecond Diffuse Reflectance Spectroscopy. J. Phys. Chem. B 1999, 103, 3120–3127. [Google Scholar] [CrossRef]
- Kominami, H.; Murakami, S.; Kato, J.; Kera, Y.; Ohtani, B. Correlation between Some Physical Properties of Titanium Dioxide Particles and Their Photocatalytic Activity for Some Probe Reactions in Aqueous Systems. J. Phys. Chem. B 2002, 106, 10501–10507. [Google Scholar] [CrossRef]
- Sample Availability: Samples are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamegawa, T.; Ishiguro, Y.; Kido, R.; Yamashita, H. Design of Composite Photocatalyst of TiO2 and Y-Zeolite for Degradation of 2-Propanol in the Gas Phase under UV and Visible Light Irradiation. Molecules 2014, 19, 16477-16488. https://doi.org/10.3390/molecules191016477
Kamegawa T, Ishiguro Y, Kido R, Yamashita H. Design of Composite Photocatalyst of TiO2 and Y-Zeolite for Degradation of 2-Propanol in the Gas Phase under UV and Visible Light Irradiation. Molecules. 2014; 19(10):16477-16488. https://doi.org/10.3390/molecules191016477
Chicago/Turabian StyleKamegawa, Takashi, Yasushi Ishiguro, Ryota Kido, and Hiromi Yamashita. 2014. "Design of Composite Photocatalyst of TiO2 and Y-Zeolite for Degradation of 2-Propanol in the Gas Phase under UV and Visible Light Irradiation" Molecules 19, no. 10: 16477-16488. https://doi.org/10.3390/molecules191016477
APA StyleKamegawa, T., Ishiguro, Y., Kido, R., & Yamashita, H. (2014). Design of Composite Photocatalyst of TiO2 and Y-Zeolite for Degradation of 2-Propanol in the Gas Phase under UV and Visible Light Irradiation. Molecules, 19(10), 16477-16488. https://doi.org/10.3390/molecules191016477



