Epoxidized Vegetable Oils Plasticized Poly(lactic acid) Biocomposites: Mechanical, Thermal and Morphology Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fourier Transform Infrared (FTIR) Spectroscopy

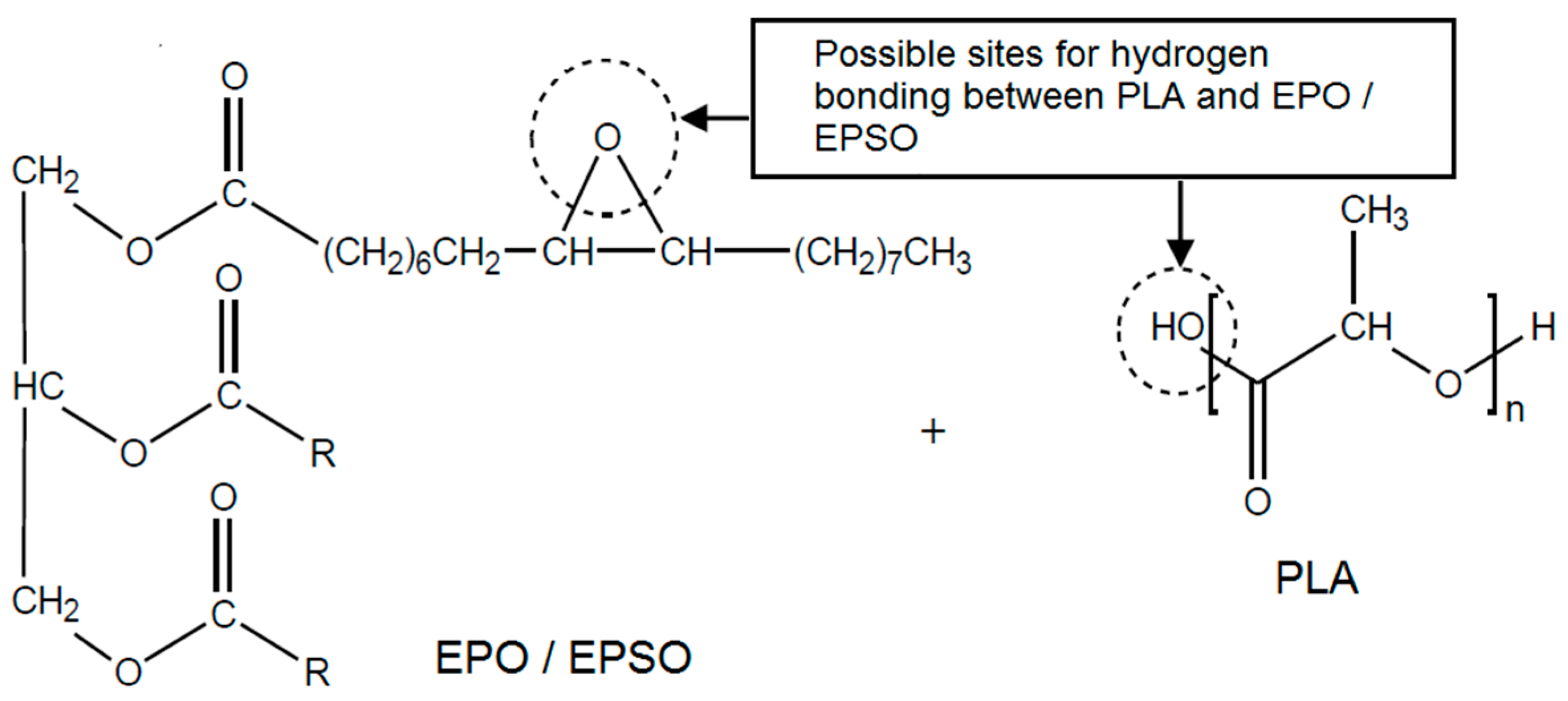
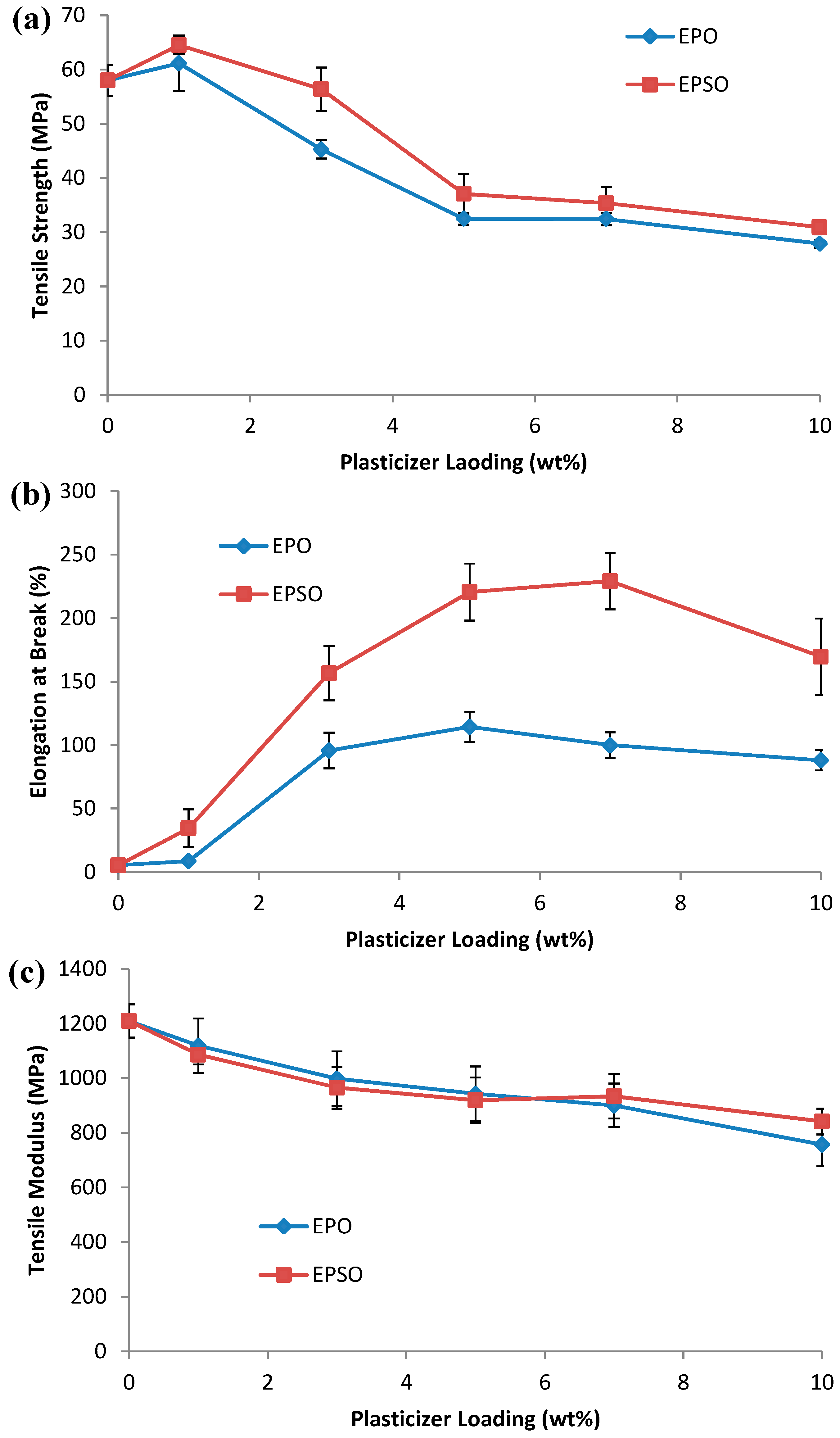
2.2. Mechanical Properties
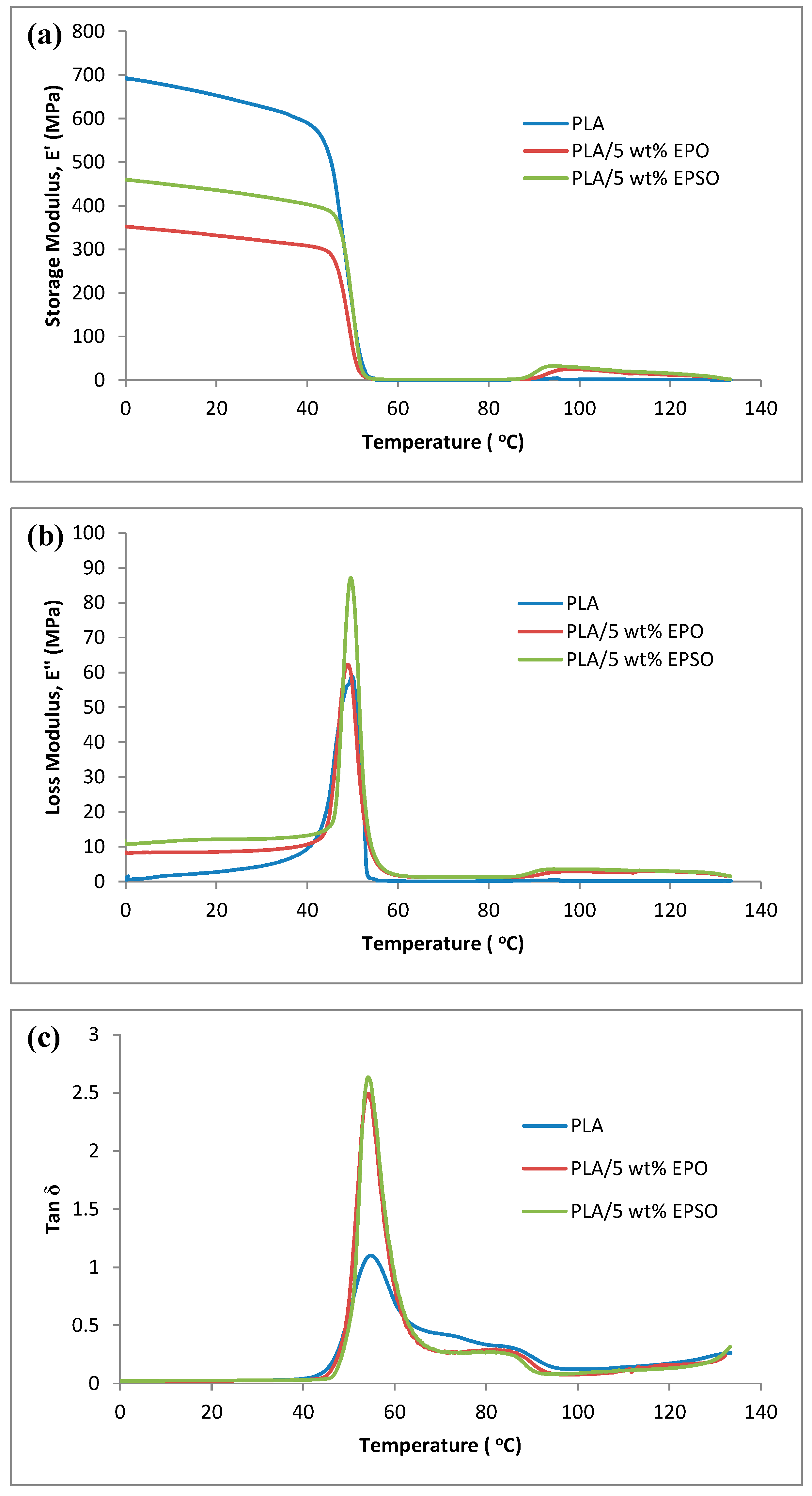
2.3. Dynamic Mechanical Analysis (DMA)
2.4. Thermal Properties
2.4.1. Thermogravimetry Analysis (TGA)
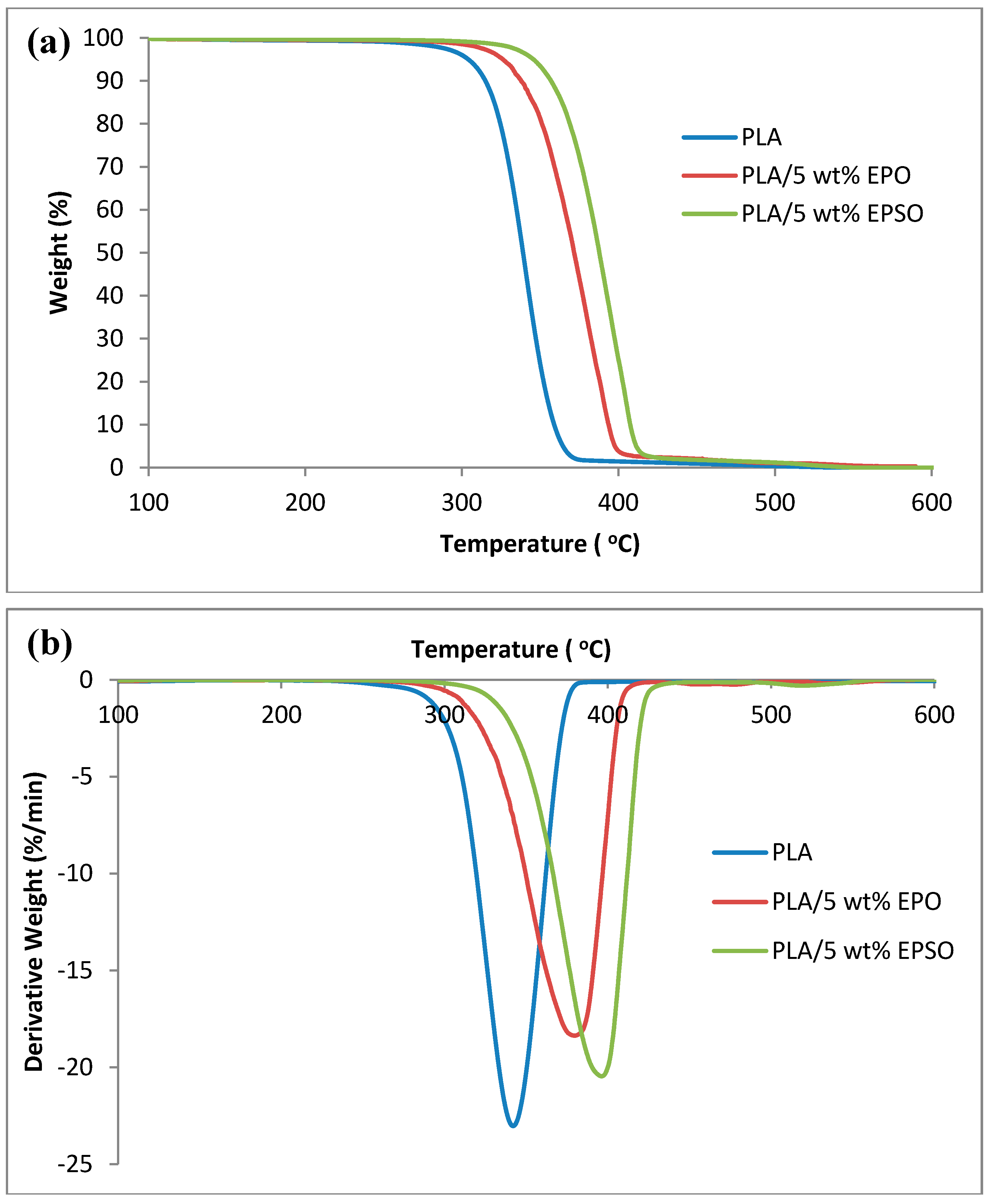
| Tonset (°C) | Tmax (°C) | T50 (°C) | |
|---|---|---|---|
| PLA | 274.26 | 345.12 | 339.16 |
| PLA/5 wt % EPO | 313.54 | 379.79 | 371.67 |
| PLA/5 wt % EPSO | 330.40 | 396.34 | 387.77 |
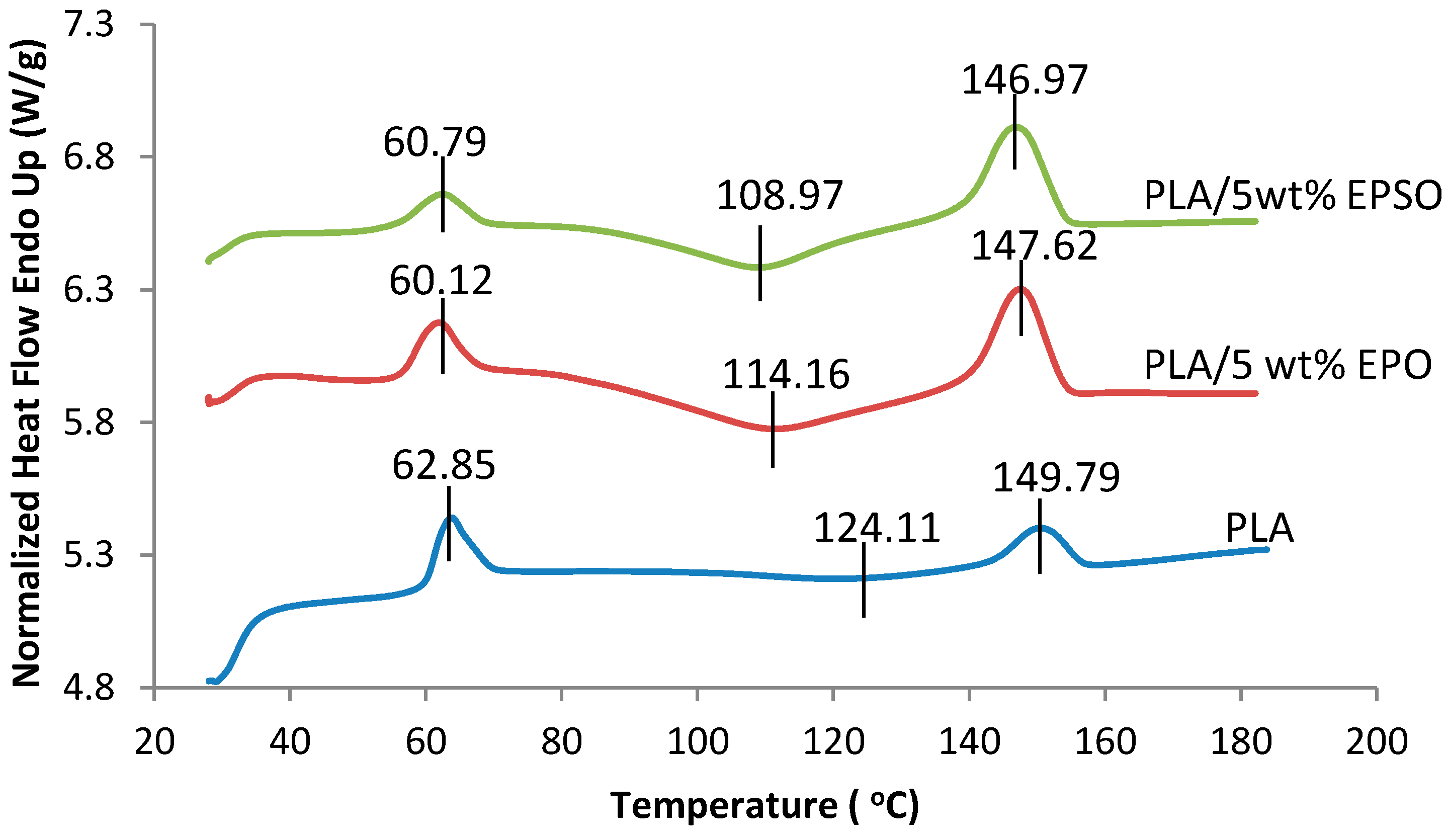
2.4.2. Differential Scanning Calorimetry (DSC)
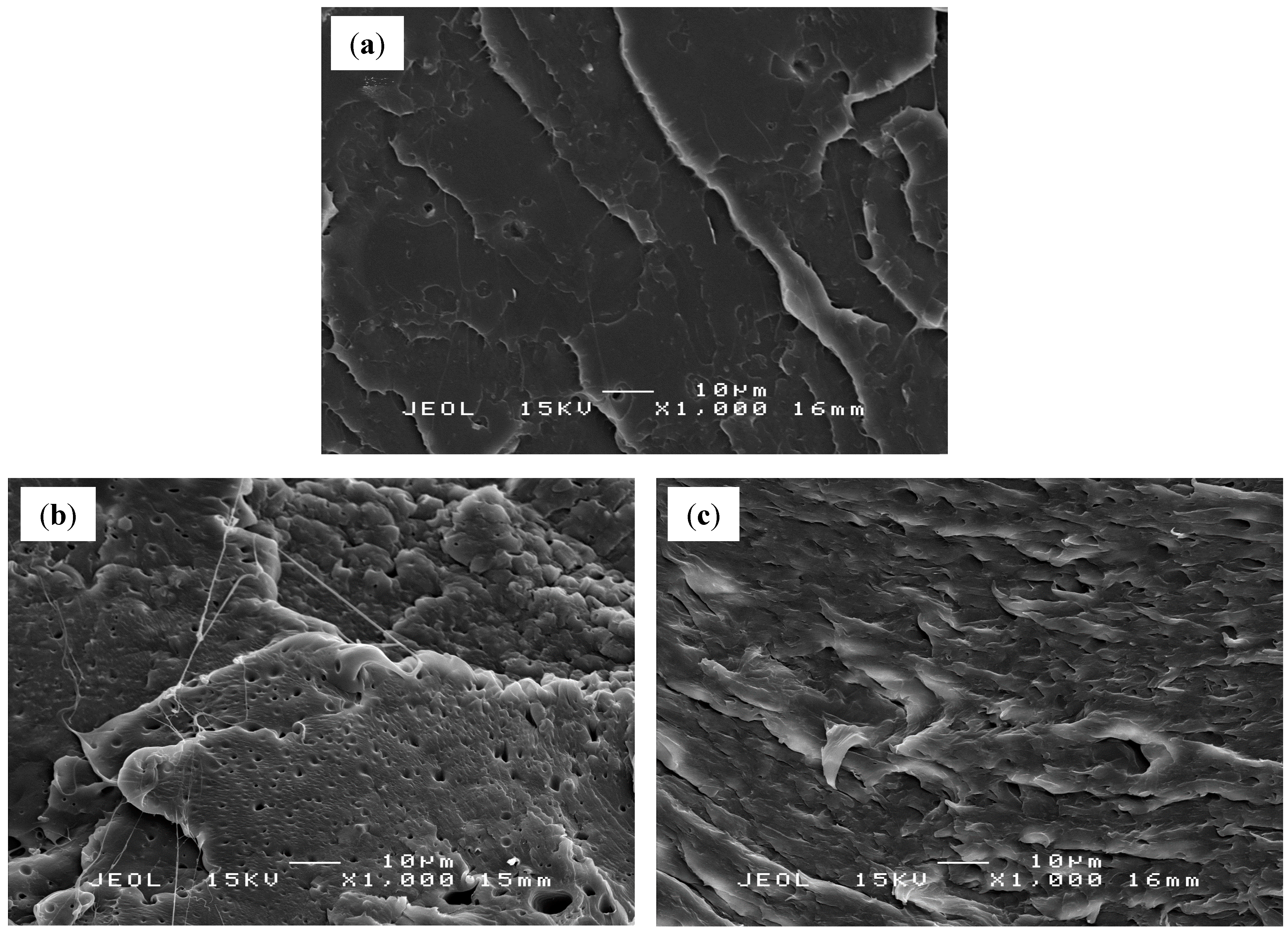
2.5. Scanning Electron Microscopy (SEM)
3. Experimental Section
3.1. Materials
| Sample Composition | Epoxidized Palm Oil (EPO) | Mixture of Epoxidized Palm Oil and Soybean Oil (EPSO) |
|---|---|---|
| Oxygen Oxirane Content (%) | 3.2309 | 3.5803 |
| Acid Value (mg KOH/g sample) | 0.4287 | 0.5990 |
| Iodine Value (g I2/100 g sample) | 0.6371 | 0.4999 |
| Moisture Content | 0.08 | 0.06 |
| pH | 5–6 | 5–6 |
3.2. Preparation of PLA/EVO Biocomposites
3.3. Characterizations
3.3.1. Fourier Transform Infrared (FTIR) Spectra
3.3.2. Tensile Properties Measurement
3.3.3. Dynamic Mechanical Analysis
3.3.4. Thermal Properties
3.3.5. Morphology
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chieng, B.W.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hussein, M.Z. Plasticized poly(lactic acid) with low molecular weight poly(ethylene glycol): Mechanical, thermal, and morphology properties. J. Appl. Polym. Sci. 2013, 130, 4576–4580. [Google Scholar]
- Rahman, M.A.; de Santis, D.; Spagnoli, G.; Ramorino, G.; Penco, M.; Phuong, V.T.; Lazzeri, A. Biocomposites based on lignin and plasticized poly(L-lactic acid). J. Appl. Polym. Sci. 2013, 129, 202–214. [Google Scholar] [CrossRef]
- Hu, Y.; Rogunova, M.; Topolkaraev, V.; Hiltner, A.; Baer, E. Aging of poly(lactide)/poly(ethylene glycol) blends. Part 1. Poly(lactide) with low stereoregularity. Polymer 2003, 44, 5701–5710. [Google Scholar] [CrossRef]
- Cataldo, F.; Ursini, O.; Angelini, G. Biodiesel as a Plasticizer of a SBR-Based Tire Tread Formulation. ISRN Polym. Sci. 2013, 2013, 340426:1–340426:9. [Google Scholar]
- Tan, C.P.; Man, Y.B.C. Comparative differential scanning calorimetric analysis of vegetable oils: I. Effects of heating rate variation. Phytochem. Anal. 2002, 13, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Renewable polymeric materials from vegetable oils: A perspective. Mater. Today 2013, 16, 337–343. [Google Scholar] [CrossRef]
- Tu, Y.C.; Kiatsimkul, P.; Suppes, G.; Hsieh, F.H. Physical properties of water-blown rigid polyurethane foams from vegetable oil-based polyols. J. Appl. Polym. Sci. 2007, 105, 453–459. [Google Scholar] [CrossRef]
- Al-Mulla, E.; Ibrahim, N.A.; Shameli, K.; Ahmad, M.; Yunus, W.M.Z.W. Effect of epoxidized palm oil on the mechanical and morphological properties of a PLA–PCL blend. Res. Chem. Intermed. 2013, 40, 689–698. [Google Scholar]
- Wu, X.; Zhang, X.; Yang, S.; Chen, H.; Wang, D. The study of epoxidized rapeseed oil used as a potential biodegradable lubricant. J. Am. Oil Chem. Soc. 2000, 77, 561–563. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Erhan, S.Z. Epoxidized soybean oil as a potential source of high-temperature lubricants. Ind. Crops Prod. 2002, 15, 247–254. [Google Scholar] [CrossRef]
- Lathi, P.S.; Mattiasson, B. Green approach for the preparation of biodegradable lubricant base stock from epoxidized vegetable oil. Appl. Catal. B Environ. 2007, 69, 207–212. [Google Scholar] [CrossRef]
- Campanella, A.; Rustoy, E.; Baldessari, A.; Baltanás, M.A. Lubricants from chemically modified vegetable oils. Bioresour. Technol. 2010, 101, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Suppes, G.J.; Dasari, M.A. Synthesis and Evaluation of Alkyl Nitrates from Triglycerides as Cetane Improvers. Ind. Eng. Chem. Res. 2003, 42, 5042–5053. [Google Scholar] [CrossRef]
- Zlatanić, A.; Lava, C.; Zhang, W.; Petrović, Z.S. Effect of structure on properties of polyols and polyurethanes based on different vegetable oils. J. Polym. Sci. Part B Polym. Phys. 2004, 42, 809–819. [Google Scholar] [CrossRef]
- Caillol, S.; Desroches, M.; Boutevin, G.; Loubat, C.; Auvergne, R.; Boutevin, B. Synthesis of new polyester polyols from epoxidized vegetable oils and biobased acids. Eur. J. Lipid Sci. Technol. 2012, 114, 1447–1459. [Google Scholar]
- Derawi, D.; Salimon, J. Optimization on Epoxidation of Palm Olein by Using Performic Acid. E-J. Chem. 2010, 7, 1440–1448. [Google Scholar] [CrossRef]
- Xu, Y.-Q.; Qu, J.-P. Mechanical and rheological properties of epoxidized soybean oil plasticized poly(lactic acid). J. Appl. Polym. Sci. 2009, 112, 3185–3191. [Google Scholar] [CrossRef]
- George, W. Handbook of Plasticizers; ChemTech Publishing: Toronto, ON, Canada, 2004; pp. 112–117. [Google Scholar]
- Silverajah, V.S.G.; Ibrahim, N.A.; Yunus, W.M.Z.W.; Hassan, H.A.; Chieng, B.W. A Comparative Study on the Mechanical, Thermal and Morphological Characterization of Poly(lactic acid)/Epoxidized Palm Oil Blend. Int. J. Mol. Sci. 2012, 13, 5878–5898. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dever, M.; Fair, N.; Benson, R.S. Thermal and mechanical properties of poly(lactic acid) and poly(ethylene/butylene succinate) blends. J. Environ. Polym. Degrad. 1997, 5, 225–235. [Google Scholar]
- Marcilla, A.; Beltran, M. Mechanisms of Plasticizers Action. In Handbook of Plasticizers, 2nd ed.; William Andrew Publishing: Boston, MA, USA, 2012; pp. 119–133. [Google Scholar]
- Ibrahim, N.A.; Chieng, B.W.; Yunus, W.M.Z.W. Morphology, Thermal and Mechanical Properties of Biodegradable Poly(butylene succinate)/Poly(butylene adipate-co-terephthalate)/Clay Nanocomposites. Polym. Plast. Technol. Eng. 2010, 49, 1571–1580. [Google Scholar] [CrossRef]
- Pluta, M.; Galeski, A. Crystalline and supermolecular structure of polylactide in relation to the crystallization method. J. Appl. Polym. Sci. 2002, 86, 1386–1395. [Google Scholar] [CrossRef]
- Kulinski, Z.; Piorkowska, E. Crystallization, structure and properties of plasticized poly(l-lactide). Polymer 2005, 46, 10290–10300. [Google Scholar] [CrossRef]
- Fenollar, O.; García, D.; Sánchez, L.; López, J.; Balart, R. Optimization of the curing conditions of PVC plastisols based on the use of an epoxidized fatty acid ester plasticizer. Eur. Polym. J. 2009, 45, 2674–2684. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the epoxidized palm oil plasticized poly(lactic acid) biocomposites are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chieng, B.W.; Ibrahim, N.A.; Then, Y.Y.; Loo, Y.Y. Epoxidized Vegetable Oils Plasticized Poly(lactic acid) Biocomposites: Mechanical, Thermal and Morphology Properties. Molecules 2014, 19, 16024-16038. https://doi.org/10.3390/molecules191016024
Chieng BW, Ibrahim NA, Then YY, Loo YY. Epoxidized Vegetable Oils Plasticized Poly(lactic acid) Biocomposites: Mechanical, Thermal and Morphology Properties. Molecules. 2014; 19(10):16024-16038. https://doi.org/10.3390/molecules191016024
Chicago/Turabian StyleChieng, Buong Woei, Nor Azowa Ibrahim, Yoon Yee Then, and Yuet Ying Loo. 2014. "Epoxidized Vegetable Oils Plasticized Poly(lactic acid) Biocomposites: Mechanical, Thermal and Morphology Properties" Molecules 19, no. 10: 16024-16038. https://doi.org/10.3390/molecules191016024
APA StyleChieng, B. W., Ibrahim, N. A., Then, Y. Y., & Loo, Y. Y. (2014). Epoxidized Vegetable Oils Plasticized Poly(lactic acid) Biocomposites: Mechanical, Thermal and Morphology Properties. Molecules, 19(10), 16024-16038. https://doi.org/10.3390/molecules191016024





