Eckol Enhances Heme Oxygenase-1 Expression through Activation of Nrf2/JNK Pathway in HepG2 Cells
Abstract
:1. Introduction
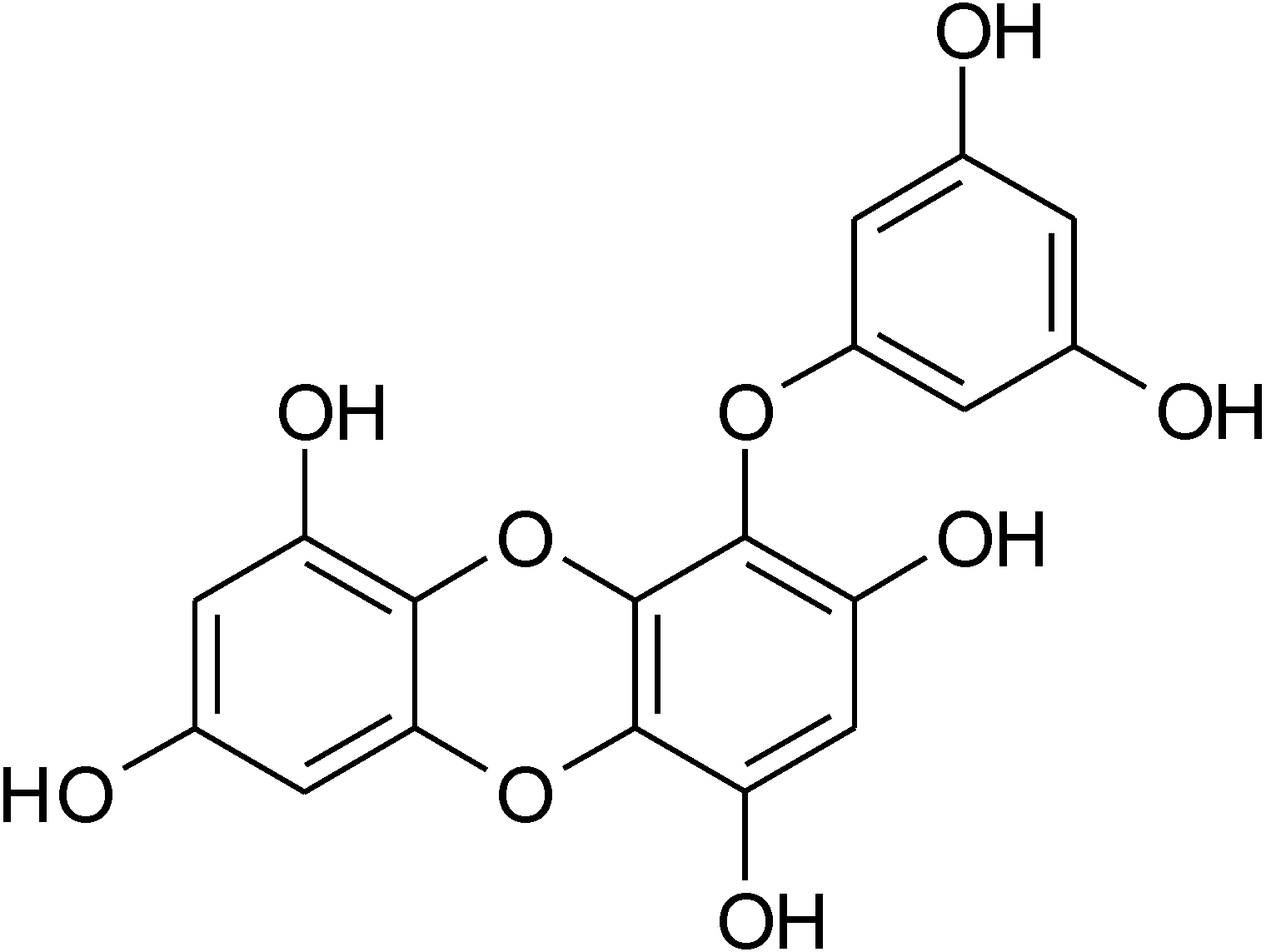
2. Results and Discussion
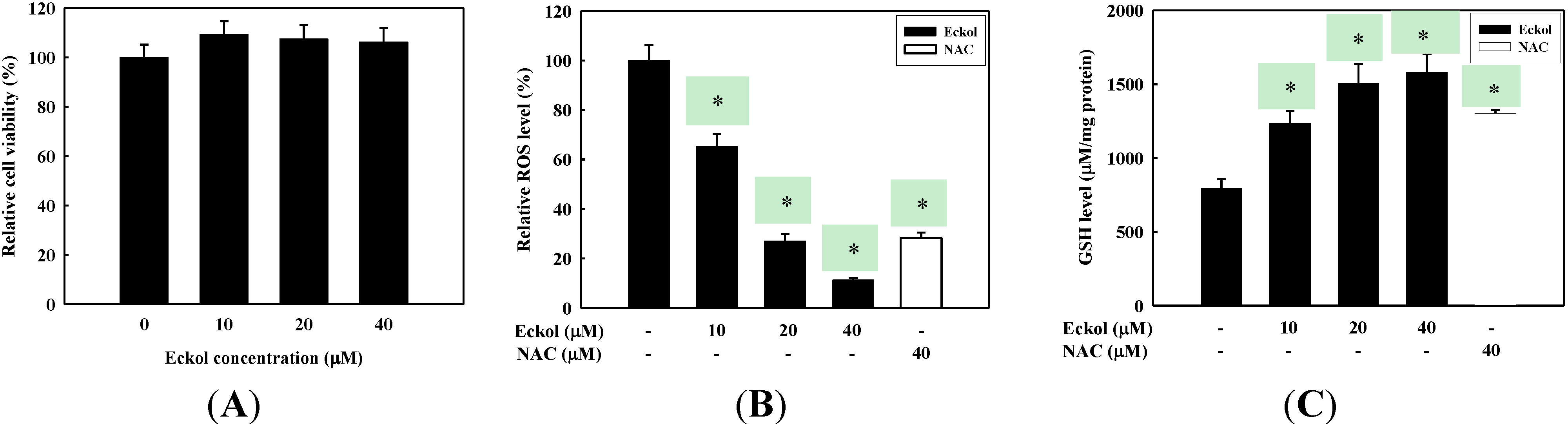
2.1. Antioxidant Activity of Eckol
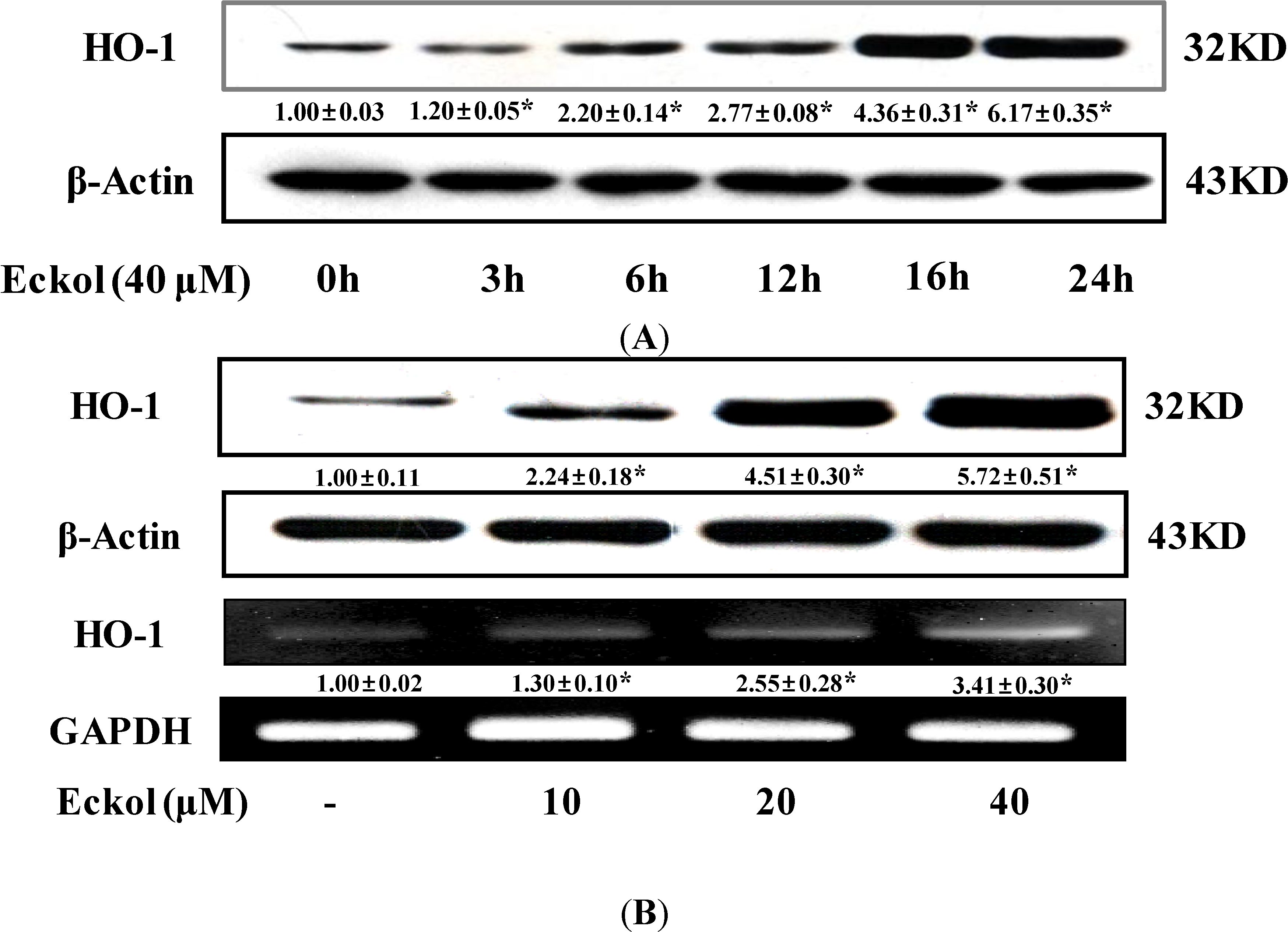
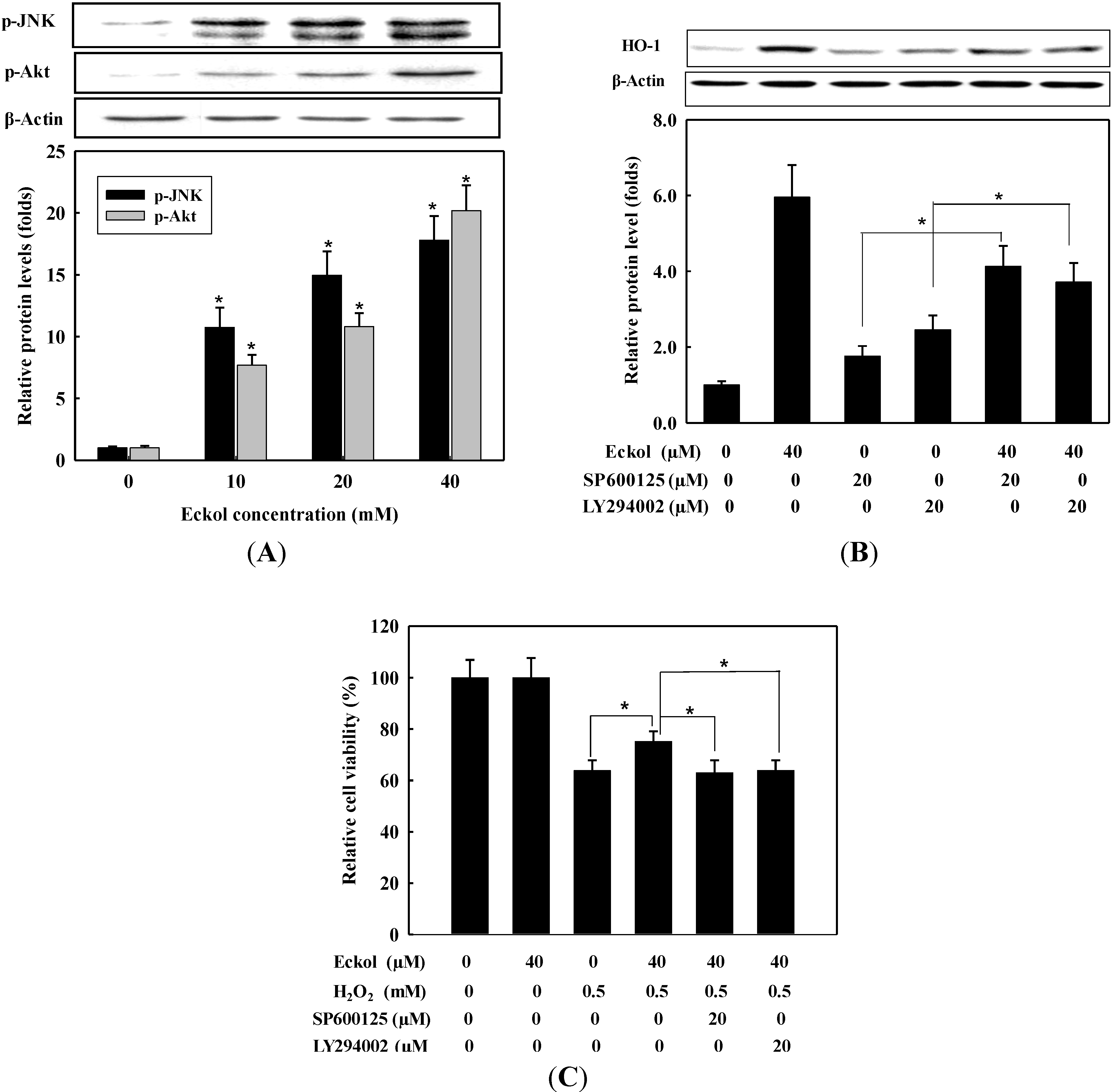
2.2. Effect of Eckol on HO-1 Expression
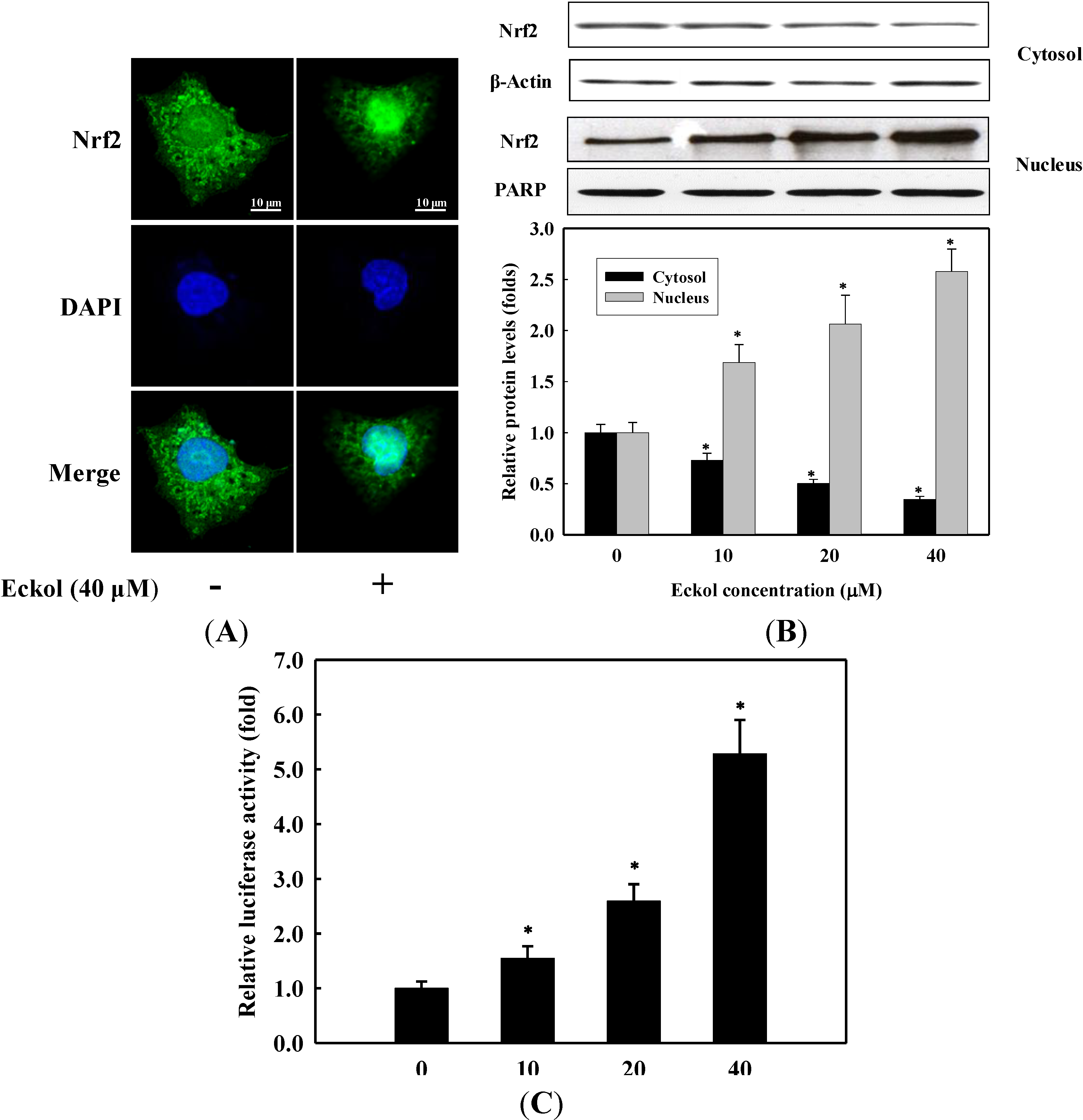
2.3. Effect of Eckol on Nrf2 Nuclear Translocation and Activation
2.4. Effect of Eckol on Phosphorylation of MAPKs and PI3K/Akt
3. Experimental Section
3.1. Chemicals
3.2. Isolation of Eckol
3.3. Cell Culture and Cell Viability Assay
3.4. Measurement of Intracellular ROS
3.5. Measurement of Reduced Glutathione
3.6. Preparation of Cell Lysates and Western Blotting
3.7. Transfection and Luciferase Assay
3.8. Isolation of Nuclear and Cytosolic Fraction
3.9. Immunofluoresence Analysis
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar]
- Migliore, L.; Coppede, F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutat. Res. 2009, 674, 73–84. [Google Scholar] [PubMed]
- Ha, H.L.; Shin, H.J.; Feitelson, M.A.; Yu, D.Y. Oxidative stress and antioxidants in hepatic pathogenesis. World J. Gastroenterol. 2010, 16, 6035–6043. [Google Scholar] [PubMed]
- Martin, G.M.; Austad, S.N.; Johnson, T.E. Genetic analysis of ageing: Role of oxidative damage and environmental stresses. Nat. Genet. 1996, 13, 25–34. [Google Scholar] [PubMed]
- Hu, R.; Saw, C.L.; Yu, R.; Kong, A.N. Regulation of NF-E2-related factor 2 signaling for cancer chemoprevention: Antioxidant coupled with antiinflammatory. Antioxid. Redox Signal. 2010, 13, 1679–1698. [Google Scholar] [PubMed]
- Hu, R.; Kong, A.N. Activation of MAP kinases, apoptosis and nutrigenomics of gene expression elicited by dietary cancer-prevention compounds. Nutrition 2004, 20, 83–88. [Google Scholar] [PubMed]
- Kim, K.C.; Kang, K.A.; Zhang, R.; Piao, M.J.; Kim, G.Y.; Kang, M.Y.; Lee, S.J.; Lee, N.H.; Surh, Y.J.; Hyun, J.W. Up-regulation of Nrf2-mediated heme oxygenase-1 expression by eckol, a phlorotannin compound, through activation of Erk and PI3K/Akt. Int. J. Biochem. Cell Biol. 2010, 42, 297–305. [Google Scholar] [PubMed]
- Maghzal, G.J.; Leck, M.C.; Collinson, E.; Li, C.; Stocker, R. Limited role for the bilirubin-biliverdin redox amplification cycle in the cellular antioxidant protection by biliverdin reductase. J. Biol. Chem. 2009, 284, 29251–29259. [Google Scholar] [PubMed]
- Stocker, R.; Glazer, A.N.; Ames, B.N. Antioxidant activity of albumin-bound bilirubin. Proc. Natl. Acad. Sci. USA 1987, 84, 5918–5922. [Google Scholar] [PubMed]
- Surh, Y.J.; Kundu, J.K.; Na, H.K. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008, 74, 1526–1539. [Google Scholar] [PubMed]
- Surh, Y.J.; Kundu, J.K.; Li, M.H.; Na, H.K.; Cha, Y.N. Role of Nrf2-mediated heme oxygenase-1 upregulation in adaptive survival response to nitrosative stress. Arch. Pharm. Res. 2009, 32, 1163–1176. [Google Scholar] [PubMed]
- Ungvari, Z.; Bagi, Z.; Feher, A.; Recchia, F.A.; Sonntag, W.E.; Pearson, K.; de Cabo, R.; Csiszar, A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H18–H24. [Google Scholar] [PubMed]
- Garg, R.; Gupta, S.; Maru, G.B. Dietary curcumin modulates transcriptional regulators of phase I and phase II enzymes in benzo[α]pyrene-treated mice: Mechanism of its anti-initiating action. Carcinogenesis 2008, 29, 1022–1032. [Google Scholar] [PubMed]
- Na, H.K.; Kim, E.H.; Jung, J.H.; Lee, H.H.; Hyun, J.W.; Surh, Y.J. (−)-Epigallocatechin gallate induces Nrf2-mediated antioxidant enzyme expression via activation of PI3K and ERK in human mammary epithelial cells. Arch. Biochem. Biophys. 2008, 476, 171–177. [Google Scholar] [PubMed]
- Kim, A.R.; Shin, T.S.; Lee, M.S.; Park, J.Y.; Park, K.E.; Yoon, N.Y.; Kim, J.S.; Choi, J.S.; Jang, B.C.; Byun, D.S.; et al. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. J. Agric. Food Chem. 2009, 57, 3483–3489. [Google Scholar]
- Kim, S.M.; Kang, K.; Jeon, J.S.; Jho, E.H.; Kim, C.Y.; Nho, C.W.; Um, B.H. Isolation of phlorotannins from Eisenia bicyclis and their hepatoprotective effect against oxidative stress induced by tert-butyl hyperoxide. Appl. Biochem. Biotechnol. 2011, 165, 1296–1307. [Google Scholar] [PubMed]
- Myung, C.S.; Shin, H.C.; Bao, H.Y.; Yeo, S.J.; Lee, B.H.; Kang, J.S. Improvement of memory by dieckol and phlorofucofuroeckol in ethanol-treated mice: Possible involvement of the inhibition of acetylcholinesterase. Arch. Pharm. Res. 2005, 28, 691–698. [Google Scholar] [PubMed]
- Joung, E.J.; Lee, M.S.; Choi, J.W.; Kim, J.S.; Shin, T.; Jung, B.M.; Kim, J.I.; Kim, H.R. Anti-inflammatory effects of phlorofucofuroeckol B-rich ethyl acetate fraction obtained from Myagropsis myagroides on lipopolysaccharide-stimulated RAW 264.7 cells and mouse edema. Int. Immunopharmacol. 2012, 14, 471–480. [Google Scholar]
- Salih, S.A.; Kim, H.R. Anti-inflammatory effect of an ethanolic extract of Myagropsis yendoi in lipopolysaccharide-stimulated BV-2 microglia cells. Fish. Aquat. Sci. 2014, 17, 27–35. [Google Scholar]
- Lee, S.H.; Park, M.H.; Heo, S.J.; Kang, S.M.; Ko, S.C.; Han, J.S.; Jeon, Y.J. Dieckol isolated from Ecklonia cava inhibits α-glucosidase and alpha-amylase in vitro and alleviates postprandial hyperglycemia in streptozotocin-induced diabetic mice. Food Chem. Toxicol. 2010, 48, 2633–2637. [Google Scholar] [PubMed]
- Lee, M.S.; Kim, J.I.; Utsuki, T.; Park, N.G.; Kim, H.R. Cytoprotective effects of phlorofucofuroeckol A isolated from Ecklonia stolonifera against tacrine-treated HepG2 cells. Fitoterapia 2012, 83, 1060–1067. [Google Scholar] [PubMed]
- Lee, M.S.; Shin, T.; Utsuki, T.; Choi, J.S.; Byun, D.S.; Kim, H.R. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and hepatoprotective properties in tacrine-treated HepG2 cells. J. Agric. Food Chem. 2012, 60, 5340–5349. [Google Scholar] [PubMed]
- Choi, A.M.; Alam, J. Heme oxygenase-1: Function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am. J. Respir. Cell Mol. Biol. 1996, 15, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Ryter, S.W.; Otterbein, L.E.; Morse, D.; Choi, A.M. Heme oxygenase/carbon monoxide signaling pathways: Regulation and functional significance. Mol. Cell. Biochem. 2002, 7, 249–263. [Google Scholar] [CrossRef]
- Song, Y.S.; Park, C.M. Luteolin and luteolin-7-O-glucoside strengthen antioxidative potential through the modulation of Nrf2/MAPK mediated HO-1 signaling cascade in RAW 264.7 cells. Food Chem. Toxicol. 2014, 65, 70–75. [Google Scholar] [PubMed]
- Qaisiya, M.; Coda Zabetta, C.D.; Bellarosa, C.; Tiribelli, C. Bilirubin mediated oxidative stress involves antioxidant response activation via Nrf2 pathway. Cell. Signal. 2014, 26, 512–520. [Google Scholar] [PubMed]
- Chen, X.L.; Kunsch, C. Induction of cytoprotective genes through Nrf2/antioxidant response element pathway: A new therapeutic approach for the treatment of inflammatory diseases. Curr. Pharm. Des. 2004, 10, 879–891. [Google Scholar] [PubMed]
- Itoh, K.; Tong, K.I.; Yamamoto, M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic. Biol. Med. 2004, 36, 1208–1213. [Google Scholar] [PubMed]
- Nguyen, T.; Sherratt, P.J.; Pickett, C.B. Regulatory mechanisms controlling gene expression mediated by the antioxidant response element. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 233–260. [Google Scholar] [PubMed]
- Yuan, X.; Xu, C.; Pan, Z.; Keum, Y.S.; Kim, J.H.; Shen, G.; Yu, S.; Oo, K.T.; Ma, J.; Kong, A.N. Butylated hydroxyanisole regulates ARE-mediated gene expression via Nrf2 coupled with ERK and JNK signaling pathway in HepG2 cells. Mol. Carcinog. 2006, 45, 841–850. [Google Scholar]
- Zipper, L.M.; Mulcahy, R.T. Erk activation is required for Nrf2 nuclear localization during pyrrolidine dithiocarbamate induction of glutamate cysteine ligase modulatory gene expression in HepG2 cells. Toxicol. Sci. 2003, 73, 124–134. [Google Scholar] [PubMed]
- Lee, S.E.; Yang, H.; Jeong, S.I.; Jin, Y.H.; Park, C.S.; Park, Y.S. Induction of heme oxygenase-1 inhibits cell death in crotonaldehyde-stimulated HepG2 cells via the PKC-δ-p38-Nrf2 pathway. PLoS One 2012, 7, e41676. [Google Scholar] [PubMed]
- Yu, R.; Chen, C.; Mo, Y.Y.; Hebbar, V.; Owuor, E.D.; Tan, T.H.; Kong, A.N. Activation of mitogen-activated protein kinase pathways induces antioxidant response element-mediated gene expression via a Nrf2-dependent mechanism. J. Biol. Chem. 2000, 275, 39907–39913. [Google Scholar] [PubMed]
- Yang, C.M.; Huang, S.M.; Liu, C.L.; Hu, M.L. Apo-8'-lycopenal induces expression of HO-1 and NQO-1 via the ERK/p38-Nrf2-ARE pathway in human HepG2 cells. J. Agric. Food Chem. 2012, 60, 1576–1585. [Google Scholar] [PubMed]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [PubMed]
- Sample Availability: Sample of the compound is available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jun, Y.-J.; Lee, M.; Shin, T.; Yoon, N.; Kim, J.-H.; Kim, H.-R. Eckol Enhances Heme Oxygenase-1 Expression through Activation of Nrf2/JNK Pathway in HepG2 Cells. Molecules 2014, 19, 15638-15652. https://doi.org/10.3390/molecules191015638
Jun Y-J, Lee M, Shin T, Yoon N, Kim J-H, Kim H-R. Eckol Enhances Heme Oxygenase-1 Expression through Activation of Nrf2/JNK Pathway in HepG2 Cells. Molecules. 2014; 19(10):15638-15652. https://doi.org/10.3390/molecules191015638
Chicago/Turabian StyleJun, Young-Jin, Minsup Lee, Taisun Shin, Nayoung Yoon, Ji-Hoe Kim, and Hyeung-Rak Kim. 2014. "Eckol Enhances Heme Oxygenase-1 Expression through Activation of Nrf2/JNK Pathway in HepG2 Cells" Molecules 19, no. 10: 15638-15652. https://doi.org/10.3390/molecules191015638
APA StyleJun, Y.-J., Lee, M., Shin, T., Yoon, N., Kim, J.-H., & Kim, H.-R. (2014). Eckol Enhances Heme Oxygenase-1 Expression through Activation of Nrf2/JNK Pathway in HepG2 Cells. Molecules, 19(10), 15638-15652. https://doi.org/10.3390/molecules191015638




