Anti-Fibrosis Effect of Scutellarin via Inhibition of Endothelial–Mesenchymal Transition on Isoprenaline-Induced Myocardial Fibrosis in Rats
Abstract
:1. Introduction
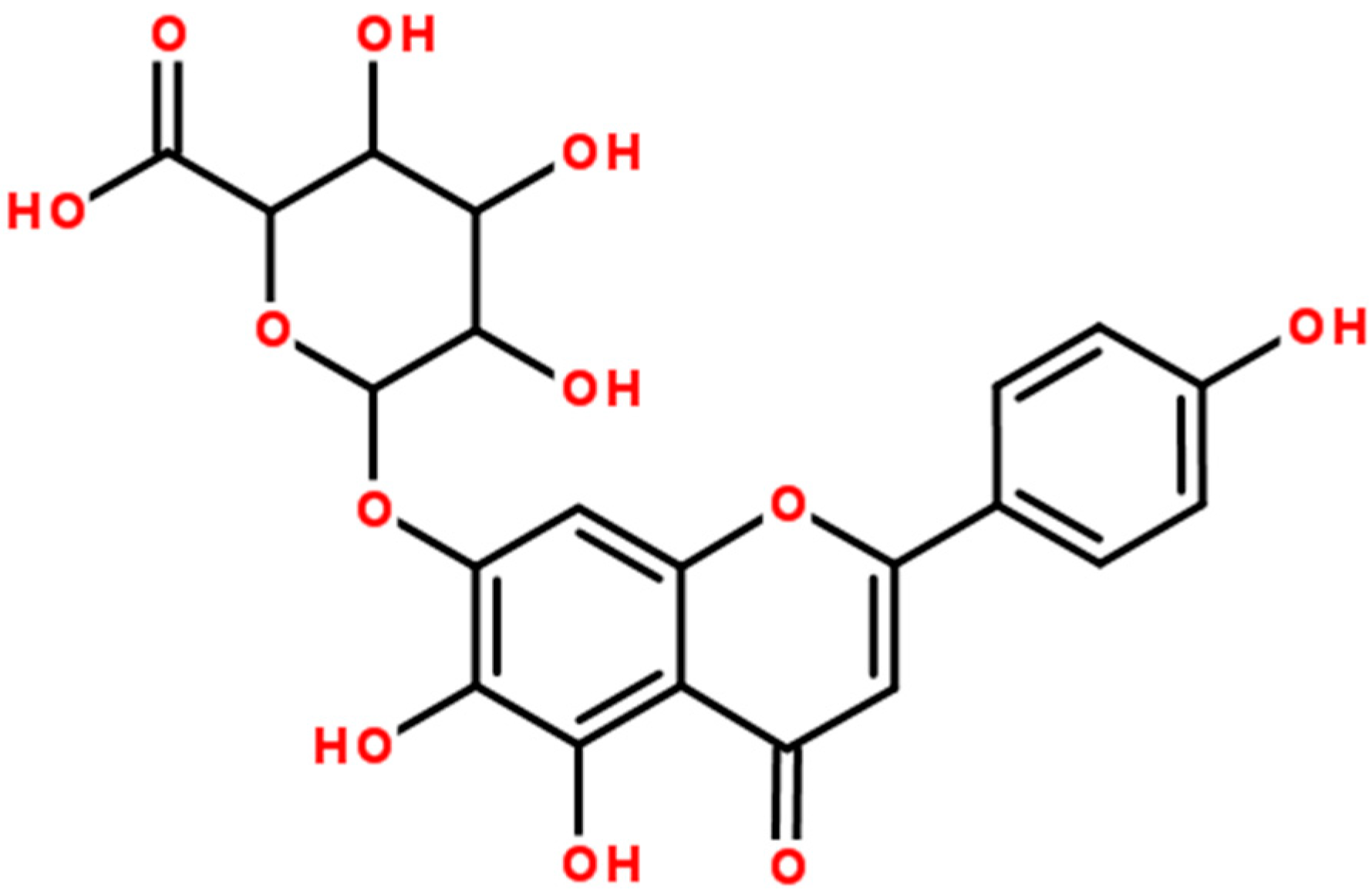
2. Results and Discussion
2.1. SCU Improves Cardiac Function
| Group | LVSP/mmHg | LVEDP/mmHg | +dp/dtmax/mmHg/s | −dp/dtmax/mmHg/s |
|---|---|---|---|---|
| NS control | 139.4 ± 12.6 | −7.70 ± 8.35 | 10257.0 ± 2500.7 | −9082.2 ± 2239.7 |
| Iso | 108.8 ± 11.6 ** | 20.34 ± 8.21 ** | 3547.3 ± 1651.0 ** | −3095.3 ± 1249.2 ** |
| Iso + low dose SCU | 124.5 ± 14.7 # | 1.55 ± 3.07 ## | 6015.5 ± 1995.7 # | −5241.2 ± 1492.9 # |
| Iso + high dose SCU | 136.9 ± 11.7 ## | −3.67 ± 4.09 ## | 9323.6 ± 2409.7 # | −7974.8 ± 2683.7 ## |
2.2. SCU Treatment Results in Decreased LVWI and RVWI
| Group | LVWI/mg·g−1 | RVWI/mg·g−1 |
|---|---|---|
| NS control | 2.46 ± 0.20 | 0.63 ± 0.08 |
| Iso | 3.10 ± 0.30 ** | 0.93 ± 0.15 ** |
| Iso + low dose SCU | 2.65 ± 0.34 ## | 0.74 ± 0.13 ## |
| Iso + high dose SCU | 2.51 ± 0.28 ## | 0.64 ± 0.08 ## |
2.3. Histopathological Observations of the Myocardium
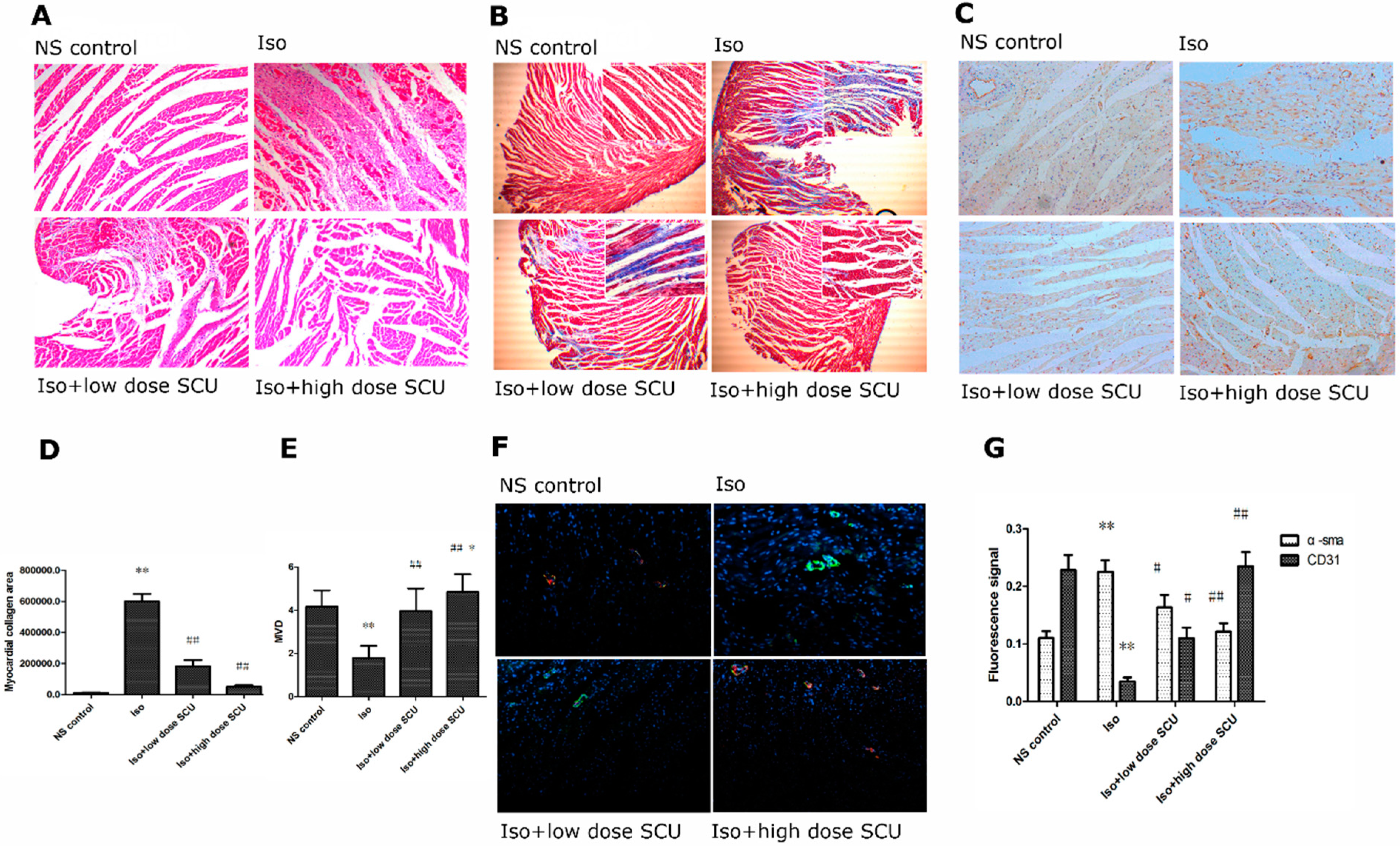
2.4. SCU Treatment Results in Decreased Myocardial Fibrosis
| Group | n | Type I collagen/ng·mL−1 | Type III collagen/ng·mL−1 |
|---|---|---|---|
| NS control | 10 | 2.37 ± 0.86 | 2.36 ± 1.04 |
| Iso | 9 | 7.54 ± 1.67 ** | 5.68 ± 1.31 ** |
| Iso + low dose SCU | 10 | 5.73 ± 1.86 ## | 4.12 ± 0.94 ## |
| Iso + high dose SCU | 10 | 2.79 ± 0.76 ## | 3.00 ± 0.86 ## |
2.5. SCU Treatment Increases the Microvascular Density (MVD)
2.6. Immunofluorescence Observations of the Myocardium
2.7. SCU Treatment Leads to an Increase in the Expression of CD31 Protein and a Decrease in the Expression of α-sma Protein in Isoprenaline-Induced Myocardial Fibrosis in Rats
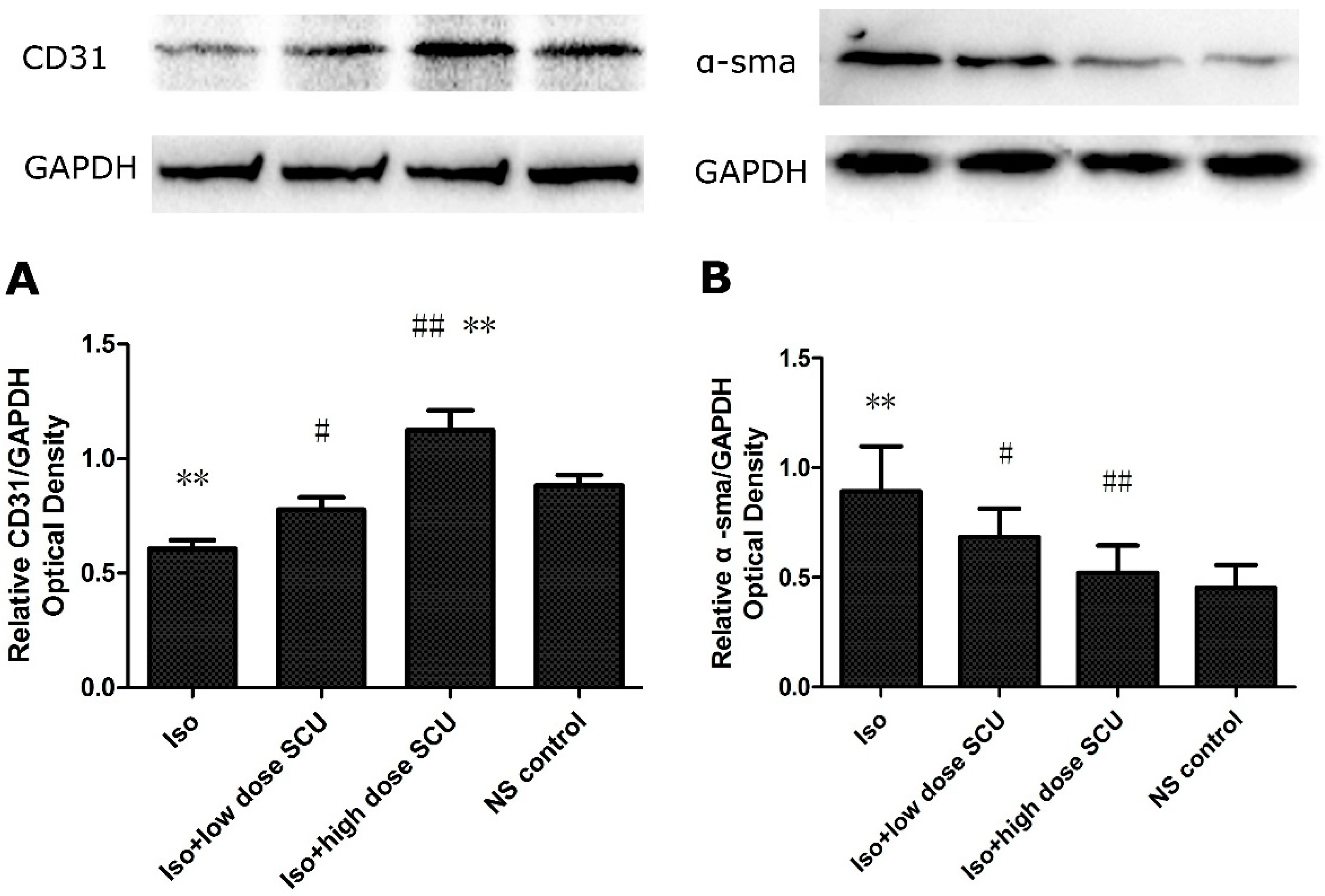
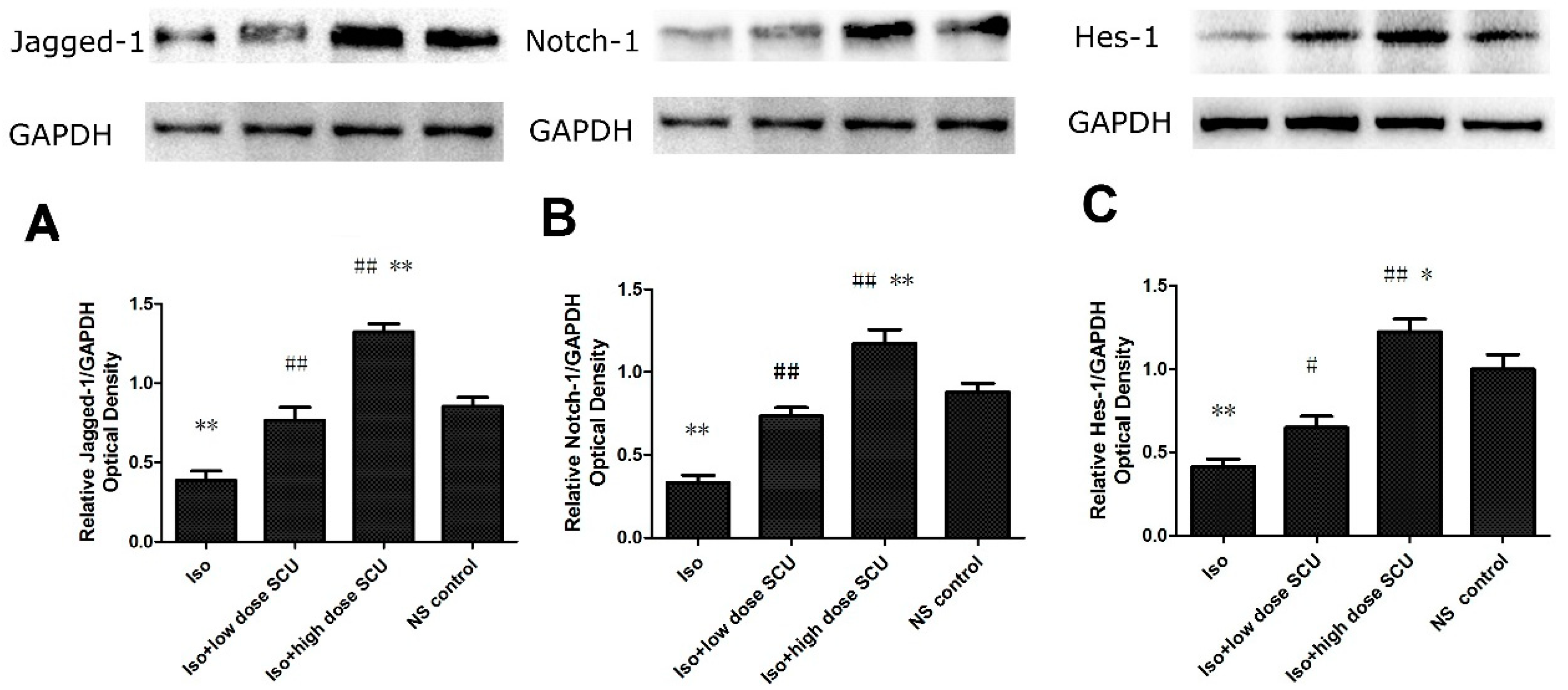
2.8. SCU Increases the Expression of Jagged1, Notch 1, and Hes1 Proteins
2.9. Discussion
3. Experimental Section
3.1. SCU Preparation Method
3.2. Animals and Treatments
3.3. Methods
3.3.1. Cardiac Functional Measurements
3.3.2. Left Ventricular Weight Index (LVWI) and Right Ventricular Weight Index (RVWI)
3.3.3. Haematoxylin and Eosin Staining of the Left Ventricular Myocardium
3.3.4. Masson Trichrome Staining of the Left Ventricular Myocardium
3.3.5. Enzyme-Linked Immunoassay (ELISA) for Type I and III Collagen
3.3.6. Immunohistochemistry
3.3.7. Immunofluorescence Methods (IFA)
3.3.8. Western Blot
3.3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Piera-Velazquez, S.; Li, Z.; Jimenez, S.A. Role of endothelial-mesenchymal transition (EndoMT) in the pathogenesis of fibrotic disorders. Am. J. Pathol. 2011, 179, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Nemir, M.; Metrich, M.; Plaisance, I.; Lepore, M.; Cruchet, S.; Berthonneche, C.; Sarre, A.; Radtke, F.; Pedrazzini, T. The Notch pathway controls fibrotic and regenerativerepair in the adult heart. Eur. Heart J. 2012, 35, 2174–85. [Google Scholar]
- Cao, F.; Guo, J.X.; Ping, Q.N.; Shao, Y.; Liang, J. Ester prodrug of scutellarin: Synthesis, physicochemical property and degradation. Yao Xue Xue Bao 2006, 41, 595–602. [Google Scholar] [PubMed]
- Zhu, L.W.; Liu, X.Q.; Feng, J.; Gao, H.M.; Yi, H.; Wang, Z.M.; Meng, Q.J. Pharmacokinetics of scutellarin and its derivant scutellarin ethyl ester in rats. Zhongguo Zhong Yao Za Zhi 2013, 38, 3373–3377. [Google Scholar] [PubMed]
- Pan, Z.; Feng, T.; Shan, L.; Cai, B.; Chu, W.; Niu, H.; Lu, Y.; Yang, B. Scutellarin-induced endothelium-independent relaxation in rat aorta. Phytother. Res. 2008, 22, 1428–1433. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Tan, Z.H.; Zhang, Z.F.; Zhang, H.; Liu, X.F.; Mo, Z.J. Scutellarin isolated fromErigeron multiradiatus inhibits high glucose-mediated vascular inflammation. Yakugaku Zasshi 2008, 128, 1293–1299. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Y.; Jiang, W.W.; Zhou, Z.T.; Li, J.M.; Wang, H.Y. The promoting angiogenesis andanti-inflammation effect of scutellarin on polyglycolic acid scaffold of balb/c mice model. J. Asian Nat. Prod. Res. 2008, 10, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Zhao, W.; Zhang, X.; Wang, B.; Wang, J.; Sun, X.; Liu, X.; Feng, S.; Yang, B.; Lu, Y. Scutellarin alleviates interstitial fibrosis and cardiac dysfunction of rats by inhibiting TGFβ1 expression and activation of p38-MAPK and ERK1/2. Br. J. Pharmacol. 2011, 162, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Willis, B.C.; Borok, Z. TGF-beta-induced EMT: Mechanisms and implications for fibrotic lung disease. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2007, 293, L525–L534. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymaltransition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Goumans, M.J.; van Zonneveld, A.J.; ten Dijke, P. Transforming Growth Factor-β–Induced Endothelial-to-Mesenchymal Transition Is Partly Mediated by MicroRNA-21? Trends Cardiovasc. Med. 2008, 18, 293–298. [Google Scholar]
- Kang, D.H.; Kanellis, J.; Hugo, C.; Truong, L.; Anderson, S.; Kerjaschki, D.; Schreiner, G.F.; Johnson, R.J. Role of the microvascular endothelium in progressive renal disease. J. Am. Soc. Nephrol. 2002, 13, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Sassoli, C.; Chellini, F.; Pini, A.; Tani, A.; Nistri, S.; Nosi, D.; Zecchi-Orlandini, S.; Bani, D.; Formigli, L. Relaxin prevents cardiac fibroblast-myofibroblast transition via notch-1-mediated inhibition of TGF-β/Smad3 signaling. PLoS One 2013, 8, e63896. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.H.; Dong, H.; Pan, Q.; Cao, Y.J.; Li, H.; Wang, H.C. Notch signaling may negativelyregulate neonatal rat cardiac fibroblast-myofibroblast transformation. Physiol. Res. 2011, 60, 739–748. [Google Scholar] [PubMed]
- Stanton, H.C.; Brenner, G.; Mayfield, E.D., Jr. Studies on isoproterenol-induced cardiomegaly in rats. Am. Heart J. 1969, 77, 72–80. [Google Scholar] [CrossRef]
- Lin, L.L.; Liu, A.J.; Liu, J.G.; Yu, X.H.; Qin, L.P.; Su, D.F. Protective effects of scutellarin and breviscapine on brain and heart ischemia in rats. J. Cardiovasc. Pharmacol. 2007, 50, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Chen, X.; Chen, L.; Zhou, X.; Zheng, G.; Zhang, H.; Huang, W.; Cai, J. Anti-Fibrosis Effect of Scutellarin via Inhibition of Endothelial–Mesenchymal Transition on Isoprenaline-Induced Myocardial Fibrosis in Rats. Molecules 2014, 19, 15611-15623. https://doi.org/10.3390/molecules191015611
Zhou H, Chen X, Chen L, Zhou X, Zheng G, Zhang H, Huang W, Cai J. Anti-Fibrosis Effect of Scutellarin via Inhibition of Endothelial–Mesenchymal Transition on Isoprenaline-Induced Myocardial Fibrosis in Rats. Molecules. 2014; 19(10):15611-15623. https://doi.org/10.3390/molecules191015611
Chicago/Turabian StyleZhou, Hao, Xiao Chen, Lingzhi Chen, Xi Zhou, Gaoshu Zheng, Huaiqin Zhang, Weijian Huang, and Jiejie Cai. 2014. "Anti-Fibrosis Effect of Scutellarin via Inhibition of Endothelial–Mesenchymal Transition on Isoprenaline-Induced Myocardial Fibrosis in Rats" Molecules 19, no. 10: 15611-15623. https://doi.org/10.3390/molecules191015611
APA StyleZhou, H., Chen, X., Chen, L., Zhou, X., Zheng, G., Zhang, H., Huang, W., & Cai, J. (2014). Anti-Fibrosis Effect of Scutellarin via Inhibition of Endothelial–Mesenchymal Transition on Isoprenaline-Induced Myocardial Fibrosis in Rats. Molecules, 19(10), 15611-15623. https://doi.org/10.3390/molecules191015611




