Acetylcholinesterase Inhibitory, Antioxidant and Phytochemical Properties of Selected Medicinal Plants of the Lamiaceae Family
Abstract
:1. Introduction
2. Results and Discussion
2.1. Acetylcholinesterase (AChE) Inhibitory Activity
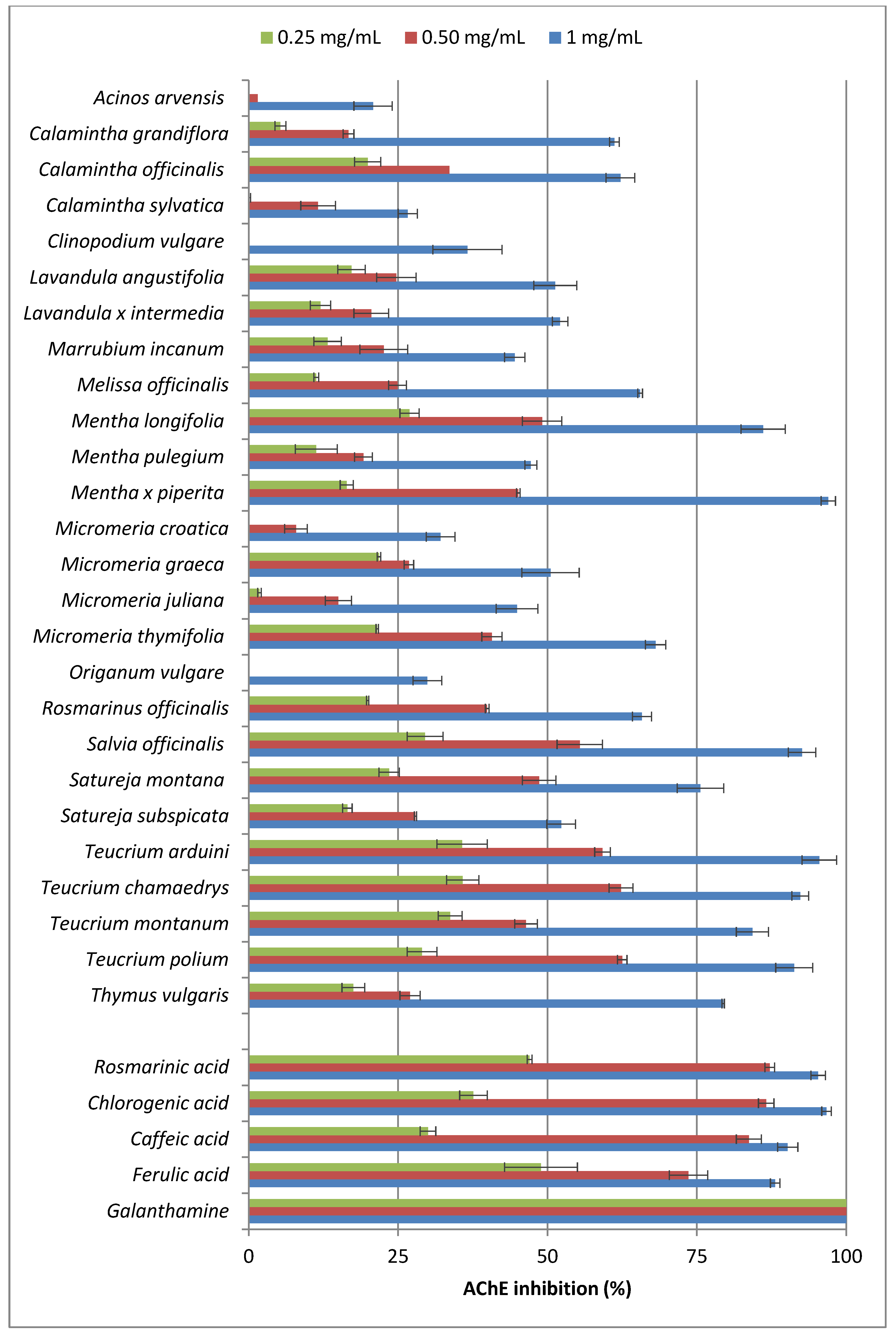
2.2. Antioxidant Activity
| Sample | Voucher number (FGHR) | Plant part used | Extract yield a | DPPH radical scavenging activity | Total antioxidant capacity (mg TE/g) |
|---|---|---|---|---|---|
| IC50 (μg/mL) | |||||
| Medicinal plant | |||||
| Acinos arvensis (Lam.) Dandy | L0111 | Aerial part | 15.47 | 17.50 ± 0.42 | 254.97 ± 2.68 |
| Calamintha grandiflora (L.) Moench | L0141 | Aerial part | 5.80 | 10.75 ± 0.22 | 609.03 ± 0.38 |
| Calamintha officinalis Moench | L0142 | Aerial part | 9.02 | 16.45 ± 0.32 | 650.65 ± 3.44 |
| Calamintha sylvatica Bromf. | L0143 | Aerial part | 11.62 | 18.61 ± 0.50 | 449.84 ± 3.82 |
| Clinopodium vulgare L. | L0151 | Aerial part | 7.98 | 17.39 ± 0.13 | 473.62 ± 1.53 |
| Lavandula angustifolia Mill. | L1101 | Flower | 11.05 | 31.62 ± 0.84 | 577.95 ± 13.76 |
| Lavandula x intermedia Emeric ex Loisel. | L1102 | Flower | 16.86 | 43.97 ± 0.47 | 439.03 ± 15.29 |
| Marrubium incanum Desr. | L1141 | Aerial part | 4.31 | 11.82 ± 0.33 | 502.54 ± 1.15 |
| Melissa officinalis L. | L1151 | Leaf | 5.79 | 7.74 ± 0.01 | 855.78 ± 6.12 |
| Mentha longifolia (L.) Huds. | L1173 | Aerial part | 7.34 | 8.43 ± 0.28 | 830.38 ± 6.12 |
| Mentha pulegium L. | L1176 | Aerial part | 7.52 | 24.27 ± 0.21 | 518.49 ± 22.17 |
| Mentha x piperita L. | L1175 | Leaf | 5.62 | 8.88 ± 0.13 | 790.38 ± 19.11 |
| Micromeria croatica (Pers.) Schott | L1181 | Aerial part | 7.24 | 29.48 ± 0.51 | 530.11 ± 26.37 |
| Micromeria graeca (L.) Benth. ex Reich. | L1184 | Aerial part | 7.42 | 8.79 ± 0.01 | 580.11 ± 3.06 |
| Micromeria juliana (L.) Benth. ex Rchb. | L1185 | Aerial part | 4.69 | 17.52 ± 0.33 | 403.62 ± 21.79 |
| Micromeria thymifolia (Scop.) Fritsch | L1189 | Aerial part | 6.67 | 6.53 ± 0.13 | 478.49 ± 7.64 |
| Origanum vulgare L. | L1202 | Aerial part | 13.47 | 4.19 ± 0.04 | 803.89 ± 0.76 |
| Rosmarinus officinalis L. | L1241 | Leaf | 19.32 | 5.06 ± 0.01 | 602.00 ± 3.44 |
| Salvia officinalis L. | L1259 | Leaf | 12.40 | 4.81 ± 0.30 | 838.22 ± 12.61 |
| Satureja montana L. | L1263 | Aerial part | 7.39 | 9.95 ± 0.06 | 717.41 ± 1.53 |
| Satureja subspicata Bartl. ex Vis. | L1264 | Aerial part | 17.60 | 3.08 ± 0.04 | 406.59 ± 19.11 |
| Teucrium arduini L. | L1301 | Aerial part | 12.48 | 18.06 ± 0.68 | 530.11 ± 30.96 |
| Teucrium chamaedrys L. | L1304 | Aerial part | 11.34 | 5.97 ± 0.11 | 792.81 ± 11.85 |
| Teucrium montanum L. | L1308 | Aerial part | 14.44 | 7.78 ± 0.13 | 771.19 ± 8.03 |
| Teucrium polium L. | L1309 | Aerial part | 15.67 | 5.90 ± 0.12 | 589.30 ± 14.52 |
| Thymus vulgaris L. | L1318 | Aerial part | 7.25 | 12.27 ± 0.07 | 679.83 ± 12.96 |
| Phenolic acid constituent | |||||
| Rosmarinic acid | 1.01 ± 0.07 | 1894.84 ± 10.51 | |||
| Chlorogenic acid | 1.69 ± 0.02 | 1240.65 ± 59.24 | |||
| Caffeic acid | 0.36 ± 0.03 | 1940.38 ± 5.35 | |||
| Ferulic acid | 3.56 ± 0.36 | 1923.62 ± 0.20 | |||
| Trolox (referent antioxidant) | 1.99 ± 0.03 | - |
2.3. Content and Composition of Hydroxycinnamic Acids
| Medicinal plant | Plant part used | Total hydroxycinnamic derivatives a | Rosmarinic acid b | Chlorogenic acid b | Caffeic acid b | Ferulic acid b |
|---|---|---|---|---|---|---|
| Acinos arvensis | Aerial part | 36.58 ± 0.42 | 2.21 | 26.06 | 3.53 | nd |
| Calamintha grandiflora | Aerial part | 75.42 ± 0.74 | 24.08 | 2.01 | 0.37 | nd |
| Calamintha officinalis | Aerial part | 30.17 ± 0.44 | 3.82 | 6.88 | 0.57 | nd |
| Calamintha sylvatica | Aerial part | 31.65 ± 0.19 | 0.70 | 11.18 | 0.74 | nd |
| Clinopodium vulgare | Aerial part | 27.12 ± 0.71 | 12.27 | 2.25 | 0.56 | nd |
| Lavandula angustifolia | Flower | 42.64 ± 0.19 | 3.31 | 3.22 | nd | 1.31 |
| Lavandula x intermedia | Flower | 26.00 ± 0.38 | 3.04 | 1.00 | 0.33 | 0.11 |
| Marrubium incanum | Aerial part | 57.78 ± 1.25 | 5.01 | 1.14 | 0.47 | nd |
| Melissa officinalis | Leaf | 77.70 ± 0. 94 | 39.48 | 1.16 | 1.05 | nd |
| Mentha longifolia | Aerial part | 80.06 ± 1.76 | 22.33 | 1.50 | 1.18 | nd |
| Mentha pulegium | Aerial part | 34.83 ± 0.38 | 12.72 | 1.64 | 0.72 | nd |
| Mentha x piperita | Leaf | 157.45 ± 2.71 | 61.05 | 1.78 | 2.15 | 0.50 |
| Micromeria croatica | Aerial part | 30.13 ± 0.33 | 9.95 | 1.26 | 0.70 | nd |
| Micromeria graeca | Aerial part | 81.76 ± 0.76 | 18.41 | 1.26 | 0.79 | 0.27 |
| Micromeria juliana | Aerial part | 41.50 ± 0.25 | 21.62 | 1.29 | 0.91 | 0.28 |
| Micromeria thymifolia | Aerial part | 79.86 ± 1.51 | 26.50 | 13.22 | 2.98 | 0.93 |
| Origanum vulgare | Aerial part | 129.16 ± 0.76 | 37.73 | nd | 1.12 | nd |
| Rosmarinus officinalis | Leaf | 55.57 ± 0.47 | 18.94 | 1.18 | 0.63 | nd |
| Salvia officinalis | Leaf | 86.86 ± 0.59 | 18.72 | 1.13 | 0.80 | 0.77 |
| Satureja montana | Aerial part | 95.29 ± 0.66 | 31.11 | 1.19 | 0.65 | nd |
| Satureja subspicata | Aerial part | 173.10 ± 1.68 | 46.11 | 1.62 | 1.78 | nd |
| Teucrium arduini | Aerial part | 10.75 ± 0.72 | nd | 1.75 | nd | 0.97 |
| Teucrium chamaedrys | Aerial part | 18.58 ± 0.97 | 1.03 | 4.06 | nd | 1.37 |
| Teucrium montanum | Aerial part | 15.11 ± 1.67 | nd | 2.25 | 2.36 | 1.70 |
| Teucrium polium | Aerial part | 13.91 ± 0.42 | nd | 2.19 | 2.36 | 1.38 |
| Thymus vulgaris | Aerial part | 72.98 ± 0.18 | 32.80 | 1.18 | 2.07 | 0.49 |

3. Experimental
3.1. Plant Material and Extraction Procedure
3.2. Chemicals
3.3. Acetylcholinesterase (AChE) Inhibition Assay
3.4. DPPH Free Radical Scavenging Assay
3.5. Total Antioxidant Capacity Assay
3.6. Determination of Total Hydroxycinnamic Derivatives
3.7. HPLC Analysis
3.8. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mattson, M.P. Pathways towards and away from Alzheimer’s disease. Nature 2004, 430, 631–639. [Google Scholar] [CrossRef]
- Zhao, Y.; Dou, J.; Wu, T.; Aisa, H.A. Investigating the antioxidant and acetylcholinesterase inhibition activities of Gossypium herbaceam. Molecules 2013, 18, 951–962. [Google Scholar] [CrossRef]
- Martorana, A.; Esposito, Z.; Koch, G. Beyond the cholinergic hypothesis: Do current drugs work in Alzheimer’s disease? CNS Neurosci. Ther. 2010, 16, 235–245. [Google Scholar]
- Biran, Y.; Masters, C.L.; Barnham, K.J.; Bush, A.I.; Adlard, P.A. Pharmacotherapeutic targets in Alzheimer’s disease. J. Cell Mol. Med. 2009, 13, 61–86. [Google Scholar]
- Carpinella, M.C.; Andrione, D.G.; Ruiz, G.; Palacios, S.M. Screening for acetylcholinesterase inhibitory activity in plant extracts from Argentina. Phytother. Res. 2010, 24, 259–263. [Google Scholar]
- Lee, H.P.; Zhu, X.; Casadesus, G.; Castellani, R.J.; Nunomura, A.; Smith, M.A.; Lee, H.; Perry, G. Antioxidant approaches for the treatment of Alzheimer’s disease. Expert Rev. Neurother. 2010, 10, 1201–1208. [Google Scholar] [CrossRef]
- Dastmalchi, K.; Dorman, H.J.D.; Viorela, H.; Hiltunen, R. Plants as potential source for drug development against Alzheimer’s disease. Int. J. Biomed. Pharmaceut. Sci. 2007, 1, 83–104. [Google Scholar]
- Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J. Acetylcholinesterase inhibitors from plants. Phytomedicine 2007, 14, 289–300. [Google Scholar] [CrossRef]
- Yoo, K.Y.; Park, S.Y. Terpenoids as potential anti-Alzheimer’s disease therapeutics. Molecules 2012, 17, 3524–3538. [Google Scholar] [CrossRef]
- Nikolić, T.; Rešetnik, I. Plant uses in Croatia. Phytol. Balcan. 2007, 13, 229–238. [Google Scholar]
- Bival Štefan, M.; Vuković Rodríguez, J.; Blažeković, B.; Kindl, M.; Vladimir-Knežević, S. Total hydroxycinnamic acids assay: Prevalidation and application on Lamiaceae species. Food Anal. Methods 2013, 6, 1–11. [Google Scholar] [CrossRef]
- Thompson, J.D.; Chalchat, J.C.; Michet, A.; Linhart, Y.B.; Ehlers, B. Qualitative and quantitative variation in monoterpene co-occurrence and composition in the essential oil of Thymus vulgaris chemotypes. J. Chem. Ecol. 2003, 29, 859–880. [Google Scholar] [CrossRef]
- Wang, M.; Li, J.; Ho, G.S.; Peng, X.; Ho, C.T. Isolation and identification of antioxidative flavonoid glycosides from thyme (Thymus vulgaris L.). J. Food Lipids 1998, 5, 313–321. [Google Scholar] [CrossRef]
- Fernández, L.F.; Palomino, O.M.; Frutos, G. Effectiveness of Rosmarinus officinalis essential oil as antihypotensive agent in primary hypotensive patients and its influence on health-related quality of life. J. Ethnopharmacol. 2014, 151, 509–516. [Google Scholar] [CrossRef]
- Bai, N.; He, K.; Roller, M.; Lai, C.S.; Shao, X.; Pan, M.H.; Ho, C.T. Flavonoids and phenolic compounds from Rosmarinus officinalis. J. Agric. Food Chem. 2010, 58, 5363–5367. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef]
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem. Toxicol. 2013, 55, 42–47. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Flavonoid and phenolic glycosides from Salvia officinalis. Phytochemistry 2000, 55, 263–267. [Google Scholar] [CrossRef]
- Arceusz, A.; Wesolowski, M. Quality consistency evaluation of Melissa officinalis L. commercial herbs by HPLC fingerprint and quantitation of selected phenolic acids. J. Pharm. Biomed. Anal. 2013, 83, 215–220. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.; Saraiva, J.A.; Nunes, M.L. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. J. Sci. Food Agric. 2013, 93, 2707–2714. [Google Scholar] [CrossRef]
- Jurišić Grubešić, R.; Kremer, D.; Vladimir-Knežević, S.; Vuković Rodríguez, J. Analysis of polyphenols, phytosterols, and bitter principles in Teucrium L. species. Central Europ. J. Biol. 2012, 7, 542–550. [Google Scholar] [CrossRef]
- Başer, K.H.C.; Demirçakmak, B.; Duman, H. Composition of the essential oils of three Teucrium species from Turkey. J. Essent. Oil Res. 1997, 9, 545–549. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Phytochemistry of the genus Lavandula. In Lavender: The genus Lavandula; Lis-Balchin, M., Ed.; CRC Press: London, UK, 2002; pp. 86–99. [Google Scholar]
- Vladimir-Knežević, S.; Kalođera, Z.; Blažević, N. Composition of the essential oil of Micromeria thymifolia (Scop.) Fritsch and its chemical variation. Pharmazie 2000, 55, 156–157. [Google Scholar]
- Karousou, R.; Hanlidou, E.; Lazari, D. Essential-oil diversity of three Calamintha species from Greece. Chem. Biodivers. 2012, 9, 1364–1372. [Google Scholar] [CrossRef]
- Marin, P.D.; Grayer, R.J.; Veitch, N.C.; Kite, G.C.; Harborne, J.B. Acacetin glycosides as taxonomic markers in Calamintha and Micromeria. Phytochemistry 2001, 58, 943–947. [Google Scholar] [CrossRef]
- Vrgoč, A. Uputa u farmakognoziju; Tiskara Dragutina Spullera u Samoboru: Zagreb, Croatia, 1931; pp. 331–344. [Google Scholar]
- Kušan, F. Ljekovito i Drugo Korisno Bilje; Poljoprivredni nakladni zavod: Zagreb, Croatia, 1956; pp. 439–465. [Google Scholar]
- Redžić, S. The ecological aspect of ethnobotany and ethnopharmacology of population in Bosnia and Herzegovina. Coll. Antropol. 2007, 31, 869–890. [Google Scholar]
- Orhan, I.; Aslan, S.; Kartal, M.; Şener, B.; Başer, K.H.C. Inhibitory effect of Turkish Rosmarinus officinalis L. on acetylcholinesterase and butyrylcholinesterase enzymes. Food Chem. 2008, 108, 663–668. [Google Scholar] [CrossRef]
- Dastmalchi, K.; Ollilainen, V.; Lackman, P.; Boije af Gennäs, G.; Dorman, H.J.; Järvinen, P.P.; Yli-Kauhaluoma, J.; Hiltunen, R. Acetylcholinesterase inhibitory guided fractionation of Melissa officinalis L. Bioorgan. Med. Chem. 2009, 17, 867–871. [Google Scholar] [CrossRef]
- Falé, P.L.; Borges, C.; Madeira, P.J.A.; Ascensão, L.; Araújo, M.E.M.; Florêncio, M.H.; Serralheiro, M.L. Rosmarinic acid, scutellarein 4'-methyl ether 7-O-glucuronide and (16S)-coleon E are the main compounds responsible for the antiacetylcholinesterase and antioxidant activity in herbal tea of Plectranthus barbatus (“falso boldo”). Food Chem. 2009, 114, 798–805. [Google Scholar] [CrossRef]
- Kovatcheva-Apostolova, E.G.; Georgiev, M.I.; Ilieva, M.P.; Skibsted, L.H.; Rødtjer, A.; Andersen, M.L. Extracts of plant cell cultures of Lavandula vera and Rosa damascena as sources of phenolic antioxidants for use in foods. Eur. Food Res. Technol. 2008, 227, 1243–1249. [Google Scholar] [CrossRef]
- Petersen, M. Rosmarinic acid: New aspects. Phytochem. Rev. 2013, 12, 207–227. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Inyushkina, Y.V.; Fedoreyev, S.A. Rosmarinic acid and its derivatives: Biotechnology and applications. Crit. Rev. Biotechnol. 2012, 32, 203–217. [Google Scholar] [CrossRef]
- Gholamhoseinian, A.; Moradi, M.N.; Sharifi-Far, F. Screening the methanol extracts of some Iranian plants for acetylcholinesterase inhibitory activity. Res. Pharm. Sci. 2009, 4, 105–112. [Google Scholar]
- Adsersen, A.; Gauguin, B.; Gudiksen, L.; Jäger, A.K. Screening of plants used in Danish folk medicine to treat memory dysfunction for acetylcholinesterase inhibitory activity. J. Ethnopharmacol. 2006, 104, 418–422. [Google Scholar] [CrossRef]
- Salah, S.M.; Jäger, A.K. Screening of traditionally used Lebanese herbs for neurological activities. J. Ethnopharmacol. 2005, 97, 145–149. [Google Scholar] [CrossRef]
- Mata, A.T.; Proença, C.; Ferreira, A.R.; Serralheiro, M.L.M.; Nogueira, J.M.F.; Araújo, M.E.M. Antioxidant and antiacetylcholinesterase activities of five plants used as Portuguese food spices. Food Chem. 2007, 103, 778–786. [Google Scholar] [CrossRef]
- Ferreira, A.; Proença, C.; Serralheiro, M.L.; Araújo, M.E. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J. Ethnopharmacol. 2006, 108, 31–37. [Google Scholar] [CrossRef]
- Dinis, P.C.; Falé, P.L.; Madeira, P.J.A.; Florêncio, M.H.; Serralheiro, M.L. Acetylcholinesterase inhibitory activity after in vitro gastrointestinal digestion of infusions of Mentha species. Eur. J. Med. Plants 2013, 3, 381–393. [Google Scholar]
- Orhan, I.; Aslan, M. Appraisal of scopolamine-induced antiamnesic effect in mice and in vitro antiacetylcholinesterase and antioxidant activities of some traditionally used Lamiaceae plants. J. Ethnopharmacol. 2009, 122, 327–332. [Google Scholar] [CrossRef]
- Silva, F.V.; Martins, A.; Salta, J.; Neng, N.R.; Nogueira, J.M.; Mira, D.; Gaspar, N.; Justino, J.; Grosso, C.; Urieta, J.S.; et al. Phytochemical profile and anticholinesterase and antimicrobial activities of supercritical versus conventional extracts of Satureja montana. J. Agric. Food Chem. 2009, 57, 11557–11563. [Google Scholar] [CrossRef]
- Oztürk, M.; Kolak, U.; Duru, M.E.; Harmandar, M. GC-MS analysis of the antioxidant active fractions of Micromeria juliana with anticholinesterase activity. Nat. Prod. Commun. 2009, 4, 1271–1276. [Google Scholar]
- Vladimir-Knežević, S.; Blažeković, B.; Bival Štefan, M.; Babac, M. Plant Polyphenols as Antioxidants Influencing the Human Health. In Phytochemicals as Nutraceuticals–Global Approaches to Their Role in Nutrition and Health; Rao, V., Ed.; InTech: Rijeka, Croatia, 2012; pp. 155–180. [Google Scholar]
- Teixeira, J.; Silva, T.; Andrade, P.B.; Borges, F. Alzheimer’s disease and antioxidant therapy: How long how far? Curr. Med. Chem. 2013, 20, 2939–2952. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef]
- Vladimir-Knežević, S.; Blažeković, B.; Bival Štefan, M.; Alegro, A.; Kőszegi, T.; Petrik, J. Antioxidant activities and polyphenolic contents of three selected Micromeria species from Croatia. Molecules 2011, 16, 1454–1470. [Google Scholar] [CrossRef]
- Muñoz-Muñoz, J.L.; Garcia-Molina, F.; Ros, E.; Tudela, J.; García-Canovas, F.; Rodriguez-Lopez, J.N. Prooxidant and antioxidant activities of rosmarinic acid. J. Food Biochem. 2013, 37, 396–408. [Google Scholar] [CrossRef]
- Maurya, D.K.; Devasagayam, T.P.A. Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food Chem. Toxicol. 2010, 48, 3369–3373. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Kivilompolo, M.; Hyötyläinen, T. Comprehensive two-dimensional liquid chromatography in analysis of Lamiaceae herbs: Characterisation and quantification of antioxidant phenolic acids. J. Chromatogr. A 2007, 1145, 155–164. [Google Scholar]
- Zgórka, G.; Głowniak, K. Variation of free phenolic acids in medicinal plants belonging to the Lamiaceae family. J. Pharm. Biomed. Anal. 2001, 26, 79–87. [Google Scholar] [CrossRef]
- Petersen, M.; Simmonds, M.S. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Sharififar, F.; Dehghn-Nudeh, G.; Mirtajaldini, M. Major flavonoids with antioxidant activity from Teucrium polium L. Food Chem. 2009, 112, 885–888. [Google Scholar] [CrossRef]
- Conforti, F.; Statti, G.A.; Tundis, R.; Loizzo, M.R.; Menichini, F. In vitro activities of Citrus medica L. cv. Diamante (Diamante citron) relevant to treatment of diabetes and Alzheimer’s disease. Phytother. Res. 2007, 21, 427–433. [Google Scholar] [CrossRef]
- Blažeković, B.; Vladimir-Knežević, S.; Brantner, A.; Bival Štefan, M. Evaluation of antioxidant potential of Lavandula x intermedia Emeric ex Loisel. ‘Budrovka’: A comparative study with L. angustifolia Mill. Molecules 2010, 15, 5971–5987. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines and Health Care (EDQM). European Pharmacopoeia, 4th ed.; Council of Europe: Strasbourg, France, 2002; pp. 1866–1867. [Google Scholar]
- Fecka, I.; Turek, S. Determination of water-soluble polyphenolic compounds in commercial herbal teas from Lamiaceae: Peppermint, melissa, and sage. J. Agric. Food Chem. 2007, 55, 10908–10917. [Google Scholar] [CrossRef]
- Sample Availability: Samples of investigated Lamiaceae species are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vladimir-Knežević, S.; Blažeković, B.; Kindl, M.; Vladić, J.; Lower-Nedza, A.D.; Brantner, A.H. Acetylcholinesterase Inhibitory, Antioxidant and Phytochemical Properties of Selected Medicinal Plants of the Lamiaceae Family. Molecules 2014, 19, 767-782. https://doi.org/10.3390/molecules19010767
Vladimir-Knežević S, Blažeković B, Kindl M, Vladić J, Lower-Nedza AD, Brantner AH. Acetylcholinesterase Inhibitory, Antioxidant and Phytochemical Properties of Selected Medicinal Plants of the Lamiaceae Family. Molecules. 2014; 19(1):767-782. https://doi.org/10.3390/molecules19010767
Chicago/Turabian StyleVladimir-Knežević, Sanda, Biljana Blažeković, Marija Kindl, Jelena Vladić, Agnieszka D. Lower-Nedza, and Adelheid H. Brantner. 2014. "Acetylcholinesterase Inhibitory, Antioxidant and Phytochemical Properties of Selected Medicinal Plants of the Lamiaceae Family" Molecules 19, no. 1: 767-782. https://doi.org/10.3390/molecules19010767
APA StyleVladimir-Knežević, S., Blažeković, B., Kindl, M., Vladić, J., Lower-Nedza, A. D., & Brantner, A. H. (2014). Acetylcholinesterase Inhibitory, Antioxidant and Phytochemical Properties of Selected Medicinal Plants of the Lamiaceae Family. Molecules, 19(1), 767-782. https://doi.org/10.3390/molecules19010767






