Gastroprotective Mechanisms of Action of Semisynthetic Carnosic Acid Derivatives in Human Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Cytotoxicity
2.2. Sodium Taurocholate-Induced Damage to AGS Cells
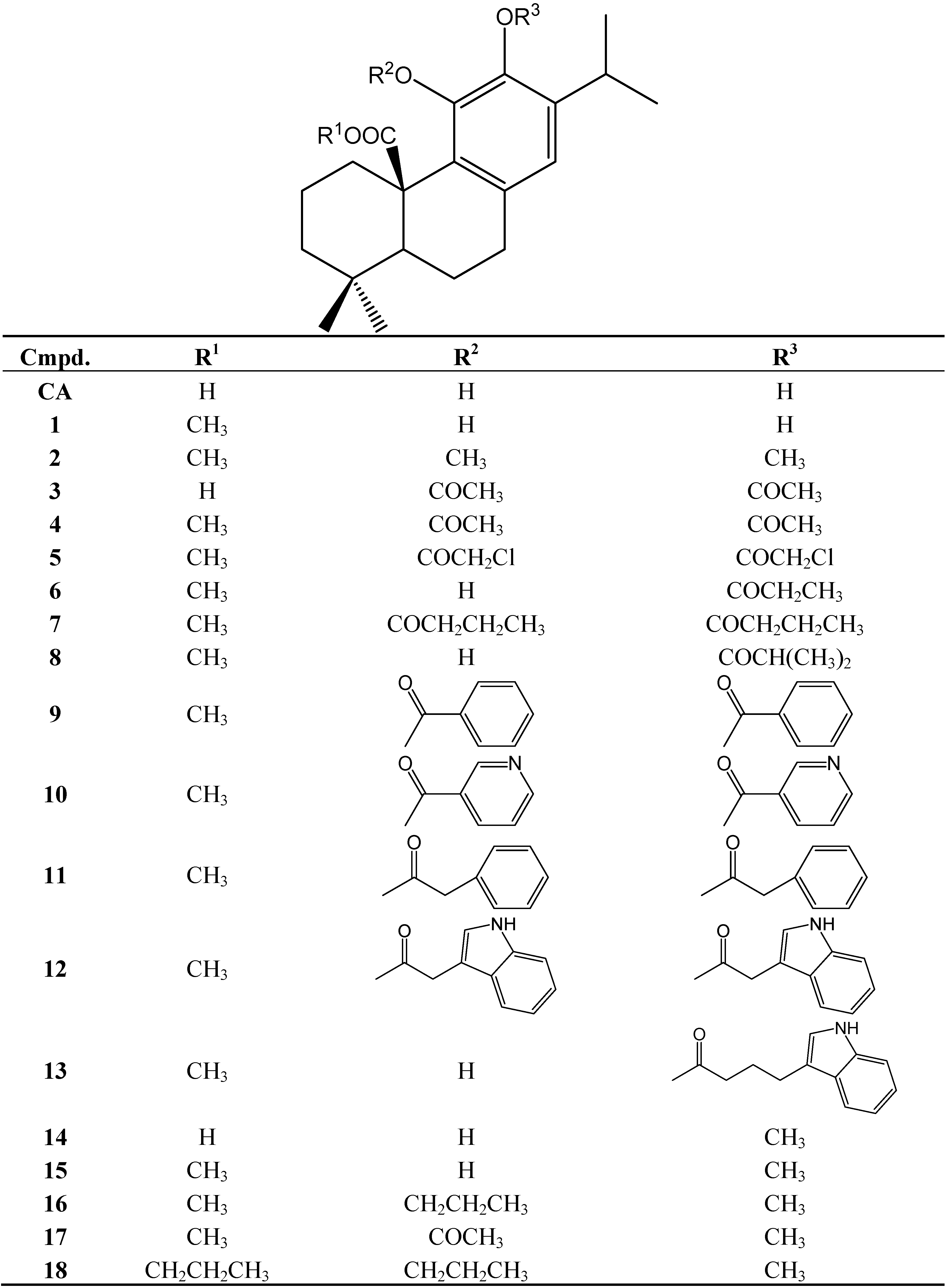
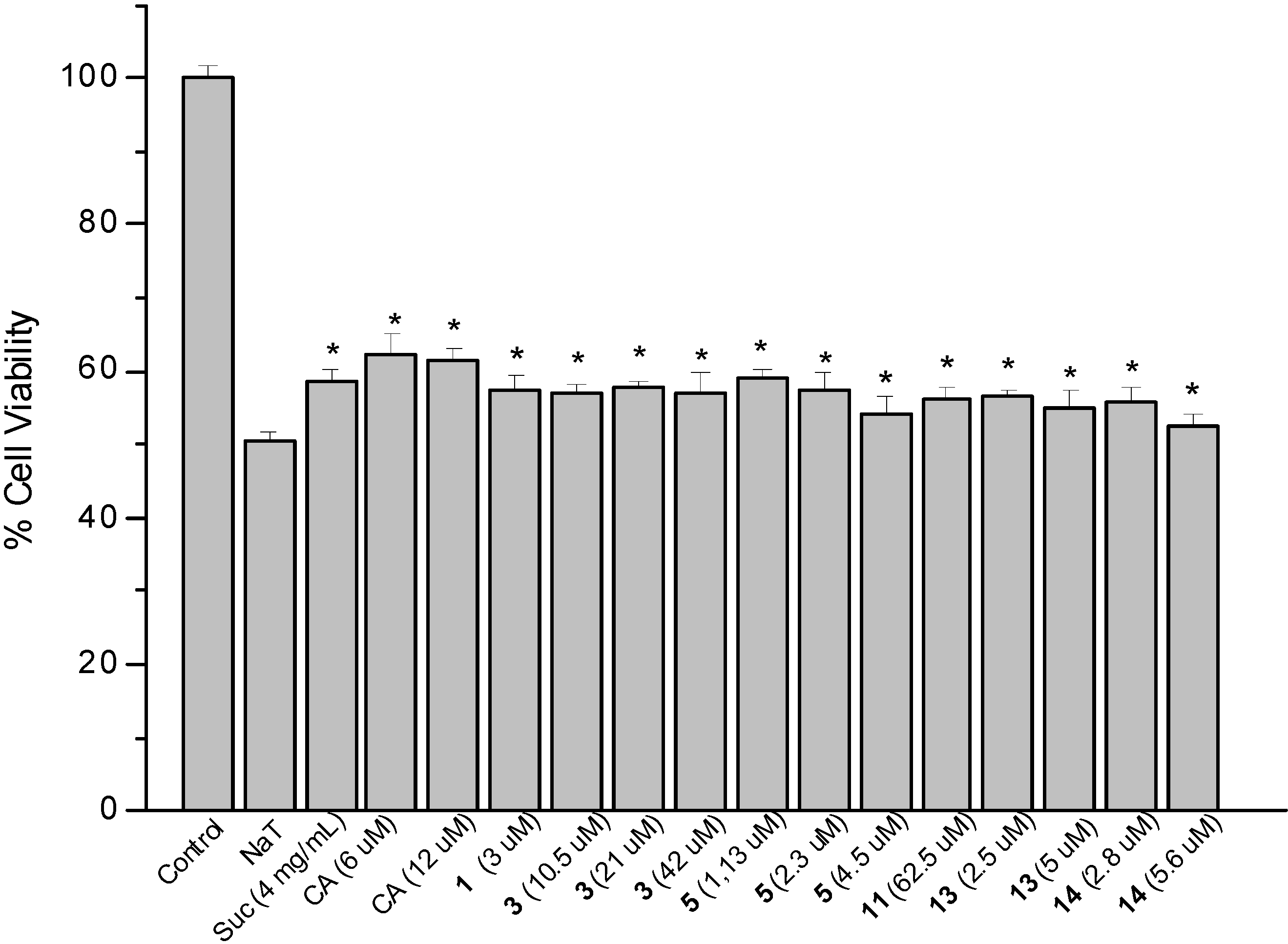
2.3. Determination of Cellular Reduced Glutathione (GSH) Content
| Compound | Concentration (µM) | GSH (nmol/106 cells) |
|---|---|---|
| Control | - | 3.6 ± 0.2 |
| NAC a | 750 | 4.0 ± 0.2 * |
| CA | 4.5 | 4.3 ± 0.2 * |
| 1 | 2.5 | 3.6 ± 0.1 |
| 2 | 4.0 | 3.7 ± 0.2 |
| 3 | 8.5 | 3.5 ± 0.2 |
| 4 | 50.0 | 3.4 ± 0.1 |
| 5 | 0.9 | 3.4 ± 0.1 |
| 6 | 1.2 | 3.7 ± 0.2 |
| 7 | 50.0 | 3.6 ± 0.2 |
| 8 | 2.4 | 3.7 ± 0.1 |
| 9 | 50.0 | 3.5 ± 0.1 |
| 10 | 1.4 | 4.1 ± 0.2 * |
| 11 | 50.0 | 4.2 ± 0.2 * |
| 12 | 32.5 | 4.3 ± 0.3 * |
| 13 | 2.0 | 4.0 ± 0.2 * |
| 14 | 2.3 | 3.9 ± 0.3 * |
| 15 | 4.5 | 4.0 ± 0.3* |
| 16 | 25.0 | 4.1 ± 0.2 * |
| 17 | 26.0 | 4.0 ± 0.1 * |
| 18 | 43.5 | 4.0 ± 0.2 * |
2.4. Determination of Prostaglandin E2 (PGE2) Content
| Compound | Concentration (µM) | PGE2 (pg/mL) |
|---|---|---|
| Control | - | 24.9 ± 2.1 |
| Indomethacin a | 100 | 8.5 ± 1.3 * |
| CA | 45 | Bdl b |
| 22.5 | Bdl b | |
| 1 | 25 | 8.1 ± 0.8 * |
| 12.5 | 10.0 ± 1.2 * | |
| 2 | 40 | Bdl b |
| 20 | 29.1 ± 2.7 * | |
| 3 | 84 | 29.2 ± 2.9 * |
| 42 | 25.1 ± 1.7 | |
| 4 | 500 | 35.5 ± 3.2 * |
| 250 | 29.2 ± 2.6 * | |
| 5 | 9 | 28.1 ± 2.7 * |
| 4.5 | 29.2 ± 2.4 * | |
| 6 | 12 | 30.3 ± 3.7 * |
| 6 | 24.1 ± 1.5 | |
| 7 | 500 | 42.1 ± 4.4 * |
| 250 | 35.5 ± 3.1 * | |
| 8 | 24 | 27.1 ± 2.9 * |
| 12 | 15.5 ± 0.9 * | |
| 9 | 500 | 55.1 ± 4.7 * |
| 250 | 58.0 ± 4.5 * | |
| 10 | 14 | 24.1 ± 2.3 |
| 7 | 30.3 ± 2.9 * | |
| 11 | 500 | 29.2 ± 1.8 * |
| 250 | 34.2 ± 2.1 * | |
| 12 | 325 | 16.7 ± 0.8 * |
| 162.5 | 32.6 ± 3.3 * | |
| 13 | 20 | 68.0 ± 4.1 * |
| 10 | 9.3 ± 0.6 * | |
| 14 | 23 | 16.7 ± 1.1 * |
| 11.5 | 23.1 ± 2.5 | |
| 15 | 45 | 34.2 ± 3.9 * |
| 22.5 | 34.2 ± 4.0 * | |
| 16 | 246 | 38.2 ± 2.9 * |
| 123 | Bdl b | |
| 17 | 26 | 31.5 ± 2.6 * |
| 13 | 27.0 ± 2.4 * | |
| 18 | 437 | 34.2 ± 3.3 * |
| 219 | Nd c |
2.5. Proliferation Assay of MRC-5 Fibroblasts
2.6. Antioxidant Activity
| Compound | Inhibition of the lipoperoxidation a |
|---|---|
| CA | IC50 2.4 ± 0.18 |
| 1 | IC50 34.4 ± 4.1 |
| 2 | 53 |
| 3 | 11 |
| 4 | 17 |
| 5 | IC50 27.6 ± 3.0 |
| 6 | 42 |
| 7 | 34 |
| 8 | IC50 13.6 ± 1.48 |
| 9 | IC50 147.2 ± 16.6 |
| 10 | 22 |
| 11 | IC50 140.4 ± 12.6 |
| 12 | 38 |
| 13 | 49 |
| 14 | IC50 186.3 ± 16.9 |
| 15 | IC50 165.0 ± 14.9 |
| 16 | 46 |
| 17 | Nd |
| 18 | Nd |
| Catechin b | IC50 75.4 ± 6.0 |
3. Experimental
3.1. Compounds
3.2. MRC-5 Cell Culture
3.3. AGS Cell Culture
3.4. Cytotoxicity Assay
3.5. Sodium Taurocholate-induced Damage to AGS Cells
3.6. Determination of Cellular Reduced Glutathione (GSH) Content
3.7. Determination of Prostaglandin E2 (PGE2) Content
3.8. Proliferation Assay of MRC-5 Fibroblasts
3.9. Inhibition of Lipoperoxidation in Erythrocyte Membranes
3.10. Superoxide Anion Scavenging
3.11. DPPH Discoloration Assay
3.12. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Dias, P.C.; Foglio, M.A.; Possenti, A.; de Carvalho, J.E. Antiulcerogenic activity of crude hydroalcoholic extract of Rosmarinus officinalis L. J. Ethnopharmacol. 2000, 69, 57–62. [Google Scholar] [CrossRef]
- Amaral, G.P.; de Carvalho, N.R.; Barcelos, R.P.; Dobrachinski, F.; Portell, R.L.; da Silva, M.H.; Lugokenski, T.H.; Dias, G.R.M.; da Luz, S.C.A.; Boligon, A.A.; et al. Protective action of ethanolic extract of Rosmarinus officinalis L. in gastric ulcer prevention induced by ethanol in rats. Food. Chem. Toxicol. 2013, 55, 48–55. [Google Scholar] [CrossRef]
- Pertino, M.; Rodríguez, J.A.; Theoduloz, C.; Yáñez, T.; Lazo, V.; Schmeda-Hirschmann, G. Gastroprotective effect of carnosic acid γ-lactone derivatives. J. Nat. Prod. 2010, 73, 639–643. [Google Scholar] [CrossRef]
- Theoduloz, C.; Pertino, M.W.; Rodríguez, J.A.; Schmeda-Hirschmann, G. Gastroprotective effect and cytotoxicity of carnosic acid derivatives. Planta Med. 2011, 77, 882–887. [Google Scholar] [CrossRef]
- Mayer, B.; Baggio, C.H.; Freitas, C.S.; dos Santos, A.C.; Twardowschy, A.; Horst, H.; Pizzolatti, M.G.; Micke, G.A.; Heller, M.; dos Santos, E.P.; et al. Gastroprotective constituents of Salvia officinalis L. Fitoterapia 2009, 80, 421–426. [Google Scholar] [CrossRef]
- Poeckel, D.; Greiner, C.; Verhoff, M.; Rau, O.; Tausch, L.; Hörnig, C.; Steinhilber, D.; Schubert-Zsilavecz, M.; Werz, O. Carnosic acid and carnosol potently inhibit human 5-lipoxygenase and suppress pro-inflammatory responses of stimulated human polymorphonuclear leukocytes. Biochem. Pharmacol. 2008, 76, 91–97. [Google Scholar] [CrossRef]
- Fischedick, J.T.; Standiford, M.; Johnson, D.A.; Johnson, J.A. Structure activity relationship of phenolic diterpenes from Salvia officinalis as activators of the nuclear factor E2-related factor 2 pathway. Bioorg. Med. Chem. 2013, 21, 2618–2622. [Google Scholar] [CrossRef]
- Gaya, M.; Repetto, V.; Toneatto, J.; Anesini, C.; Piwien-Pilipuk, G.; Moreno, S. Antiadipogenic effect of carnosic acid, A natural compound present in Rosmarinus officinalis, is exerted through the C/EBPs and PPARγ pathways at the onset of the differentiation program. Biochim. Biophys. Acta 2013, 1830, 3796–3806. [Google Scholar] [CrossRef]
- Zheng, H.; Shah, P.K.; Audus, K.L. Evaluation of antiulcer agents with a human adenocarcinoma cell line (AGS). Int. J. Pharm. 1996, 129, 103–112. [Google Scholar] [CrossRef]
- Romano, M.; Razandi, M.; Ivey, K. Effect of sucralfate and its components on taurocholate-induced damage to rat gastric mucosal cells in tissue culture. Dig. Dis. Sci. 1990, 35, 467–476. [Google Scholar] [CrossRef]
- Rodríguez, J.A.; Theoduloz, C.; Sánchez, M.; Razmilic, I.; Schmeda-Hirschmann, G. Gastroprotective and ulcer-healing effect of new solidagenone derivatives in human cell cultures. Life Sci. 2005, 77, 2193–2205. [Google Scholar] [CrossRef]
- Sánchez, M.; Theoduloz, C.; Schmeda-Hirschmann, G.; Razmilic, I.; Yañez, T.; Rodríguez, J.A. Gastroprotective and ulcer-healing activity of oleanolic acid derivatives: In vitro-in vivo relationships. Life Sci. 2006, 79, 1349–1356. [Google Scholar] [CrossRef]
- Rodríguez, J.A.; Theoduloz, C.; Yáñez, T.; Becerra, J.; Schmeda-Hirschmann, G. Gastroprotective effect of the diterpene ferruginol in mice: Protection against membrane lipid peroxidation and involvement of prostaglandins. Life Sci. 2006, 78, 2503–2509. [Google Scholar] [CrossRef]
- Naito, Y.; Yoshikawa, T.; Boku, Y.; Fujii, T.; Mascui, Y.; Tanaka, Y.; Fujita, N.; Yoshida, N.; Kondo, M. Protective role of intracellular glutathione against nitric oxide-induced necrosis in rat gastric mucosal cells. Aliment. Pharmacol. Ther. 2000, 14, 145–152. [Google Scholar]
- Mutoh, H.; Hiraishi, H.; Ota, H.; Yoshida, H.; Ivey, K.; Terano, A.; Sugimoto, T. Protective role of extracellular glutathione against ethanol-induced damage in cultured rat gastric mucosal cells. Gastroenterology 1990, 98, 1452–1459. [Google Scholar]
- Arakawa, T.; Higuchi, K.; Takashi, F. Prostaglandins in the stomach: An update. J. Clin. Gastroenterol. 1998, 27, S1–S11. [Google Scholar] [CrossRef]
- Halter, F.; Tarnawski, S.; Schmassmann, A.; Peskar, B.M. Cyclooxygenase 2-implications on maintenance of gastric mucosal integrity and ulcer healing: Controversial issues and perspectives. Gut 2001, 49, 443–453. [Google Scholar] [CrossRef]
- Ye, Y.N.; Liu, E.S.; Koo, M.W.; Li, Y.; Matsui, H.; Ch, C.H. A mechanistic study of proliferation induced by Angelica sinensis in a normal gastric epithelial cell line. Biochem. Pharmacol. 2001, 61, 1439–1448. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Astudillo, L.; Rodríguez, J.A.; Theoduloz, C.; Yáñez, T. Gastroprotective effect of the Mapuche crude drug Araucaria araucana resin and its main constituents. J. Ethnopharmacol. 2005, 101, 271–276. [Google Scholar] [CrossRef]
- Moraes, T.M.; Kushima, H.; Moleiro, F.C.; Santos, R.C.; Rocha, L.R.M.; Marques, M.O.; Vilegas, W.; Hiruma-Lima, C.A. Effects of limonene and essential oil from Citrus aurantium on gastric mucosa: Role of prostaglandins and gastric mucus secretion. Chem.-Biol. Interact. 2009, 180, 499–505. [Google Scholar] [CrossRef]
- Graham, D.Y.; Sackman, J.W.; Giesing, D.H.; Runser, D.J. In vitro adsorption of bile salts and aspirin to sucralfate. Dig. Dis. Sci. 1984, 29, 402–406. [Google Scholar] [CrossRef]
- Güldütuna, S.; Zimmer, G.; Kurtz, W.; Leuschner, U. Prostaglandin E2 directly protects isolated rat gastric surface cell membranes against bile salts. Biochim. Biophys. Acta 1987, 902, 217–222. [Google Scholar] [CrossRef]
- Yu, B.P.; Sun, J.; Li, M.Q.; Luo, H.S.; Yu, J.P. Preventive effect of hydrotalcite on gastric mucosal injury in rats induced by taurocholate. World J. Gastroenterol. 2003, 9, 1427–1430. [Google Scholar]
- Areche, C.; Theoduloz, C.; Yañez, T.; Souza-Brito, A.R.M.; Barbastefano, V.; de Paula, D.; Ferreira, A.L.; Schmeda-Hirschmann, G.; Rodríguez, J.A. Gastroprotective activity of ferruginol in mice and rats: Effects on gastric secretion, endogenous prostaglandins and non-protein sulfhydryls. J. Pharm. Pharmacol. 2008, 60, 245–251. [Google Scholar] [CrossRef]
- Rios, E.R.V.; Rocha, N.F.M.; Venâncio, E.T.; Moura, B.A.; Feitosa, M.L.; Cerqueira, G.S.; Gomes Soares, M.P.; Woods, D.J.; de Sousa, F.C.F.; Leal, L.K.A.M.; et al. Mechanisms involved in the gastroprotective activity of esculin on acute gastric lesions in mice. Chem.-Biol. Interact. 2010, 188, 246–254. [Google Scholar] [CrossRef]
- Lewis, D.A.; Hanson, P.J. Anti-Ulcer Drugs of Plant Origin. In Progress in Medicinal Chemistry; Ellis, G.P., West, G.B., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1991; Volume 28, pp. 201–231. [Google Scholar]
- Robert, A.; Nezamis, J.E.; Lancaster, C.; Davis, J.P.; Field, S.O.; Hanchar, A.J. Mild irritants prevent gastric necrosis through “adaptive cytoprotection” mediated by prostaglandins. Am. J. Physiol. 1983, 245, G113–G121. [Google Scholar]
- Fu, H.-Y.; Yabe, Y.; Asahi, K.; Hayashi, Y.; Murata, H.; Eguchi, H.; Tsujii, M.; Tsuji, S.; Kawano, S. (2E,6Z,10E)-7-Hydroxymethyl-3,11,15-trimethyl-2,6,10,14-hexadecatetraen-1-ol (Plaunotol) increases cyclooxygenase-2 expression via nuclear factor κB and cyclic AMP response element in rat gastric epithelial cells. Eur. J. Pharmacol. 2005, 524, 38–43. [Google Scholar] [CrossRef]
- De Olinda, T.M.; Lemos, T.L.G.; Machado, L.L.; Rao, V.S.; Santos, F.A. Quebrachitol-induced gastroprotection against acute gastric lesions: Role of prostaglandins, nitric oxide and K+ATP channels. Phytomedicine 2008, 15, 327–333. [Google Scholar] [CrossRef]
- Hiruma-Lima, C.A.; Rodrigues, C.M.; Kushima, H.; Moraes, T.M.; de Lolis, S.F.; Feitosa, S.B.; Magri, L.P.; Soares, F.R.; Cola, M.M.; Andrade, F.D.P.; et al. The anti-ulcerogenic effects of Curatella americana L. J. Ehnopharmacol. 2009, 121, 425–432. [Google Scholar] [CrossRef]
- Heeba, G.H.; Hassan, M.K.A.; Amin, R.S. Gastroprotective effect of simvastatin against indomethacin-induced gastric ulcer in rats: Role of nitric oxide and prostaglandins. Eur. J. Pharmacol. 2009, 607, 188–193. [Google Scholar] [CrossRef]
- Tarnawsky, A.; Szabo, I.L.; Husain, S.S.; Soreghan, B. Regeneration of gastric mucosa during ulcer healing is triggered by growth factors and signal transduction pathways. J. Physiol. Paris 2001, 95, 337–344. [Google Scholar] [CrossRef]
- Rodriguez, J.A.; Haun, M. Cytotoxicity of trans-dehydrocrotonin from Croton cajucara (Euphorbiaceae) on V79 cells and rat hepatocytes. Planta Med. 1999, 65, 522–526. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Rodríguez, J.A.; Theoduloz, C.; Astudillo, L.; Feresin, G.E.; Tapia, A. Free radical scavengers and antioxidants from Peumus. boldus Mol. (“Boldo”). Free Radic. Res. 2003, 37, 447–452. [Google Scholar] [CrossRef]
- Sample Availability: Samples of carnosic acid are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Theoduloz, C.; Pertino, M.W.; Schmeda-Hirschmann, G. Gastroprotective Mechanisms of Action of Semisynthetic Carnosic Acid Derivatives in Human Cells. Molecules 2014, 19, 581-594. https://doi.org/10.3390/molecules19010581
Theoduloz C, Pertino MW, Schmeda-Hirschmann G. Gastroprotective Mechanisms of Action of Semisynthetic Carnosic Acid Derivatives in Human Cells. Molecules. 2014; 19(1):581-594. https://doi.org/10.3390/molecules19010581
Chicago/Turabian StyleTheoduloz, Cristina, Mariano Walter Pertino, and Guillermo Schmeda-Hirschmann. 2014. "Gastroprotective Mechanisms of Action of Semisynthetic Carnosic Acid Derivatives in Human Cells" Molecules 19, no. 1: 581-594. https://doi.org/10.3390/molecules19010581
APA StyleTheoduloz, C., Pertino, M. W., & Schmeda-Hirschmann, G. (2014). Gastroprotective Mechanisms of Action of Semisynthetic Carnosic Acid Derivatives in Human Cells. Molecules, 19(1), 581-594. https://doi.org/10.3390/molecules19010581






