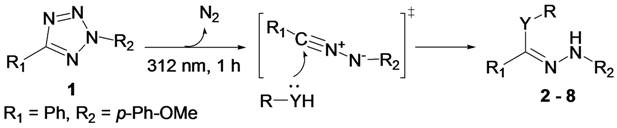Nucleophilic Trapping Nitrilimine Generated by Photolysis of Diaryltetrazole in Aqueous Phase
Abstract
:1. Introduction

2. Results and Discussion
2.1. Photochemical Reaction between Diaryltetrazole and Nucleophiles
| Entry | Nucleophile | Product | Isolated Yield (%) |
|---|---|---|---|
| 1 | Imidazole | 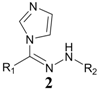 | 34.2% |
| 2 | HOCH2CH2SH | 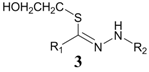 | 23.3% |
| 3 | n-C4H9NH2 | 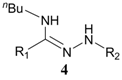 | >15% b |
| 4 | Glycine | 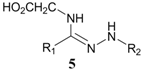 | 10% b |
| 5 | Histidine | 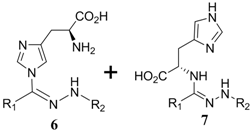 | 10% b |
| 6 | Phenol |  | <5% b |
2.2. Photochemical Reaction between Diaryltetrazole and Amino Acids
| Entry | Amino Acid | Coupling Product [M + H+] | Entry | Amino Acid | Coupling Product [M + H+] | ||
|---|---|---|---|---|---|---|---|
| Expected | Found | Expected | Found | ||||
| 1 | 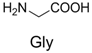 | 300.0 | 322.1 (+Na+) | 11 | 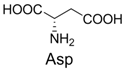 | 358.1 | 358.2 |
| 2 | 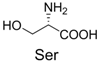 | 330.0 | 330.0 | 12 |  | 374.2 | 374.1 |
| 3 | 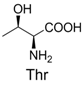 | 344.1 | 344.1 | 13 |  | 390.1 | 390.1 |
| 4 | 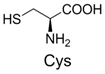 | 346.1 | 346.0 | 14 | 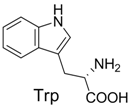 | 429.2 | 429.1 |
| 5 | 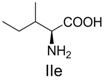 | 356.1 | 356.1 | 15 | 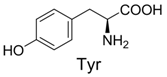 | 406.1 | 406.1 |
| 6 |  | 380.1 | 380.1 | 16 | 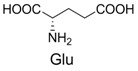 | 372.1 | 372.1 |
| 7 | 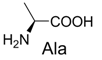 | 314.1 | 336.1 (+Na+) | 17 | 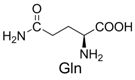 | 370.1 | 370.1 |
| 8 |  | 356.1 | 356.1 | 18 | 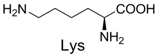 | 371.1 | 371.1 |
| 9 |  | 340.1 | 340.1 | 19 | 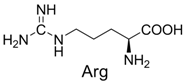 | 399.2 | 399.1 |
| 10 | 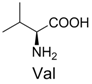 | 342.1 | 342.1 | 20 | 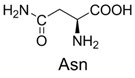 | 357.1 | 357.1 |
2.3. Photochemical Reaction between Diaryltetrazole and a Peptide
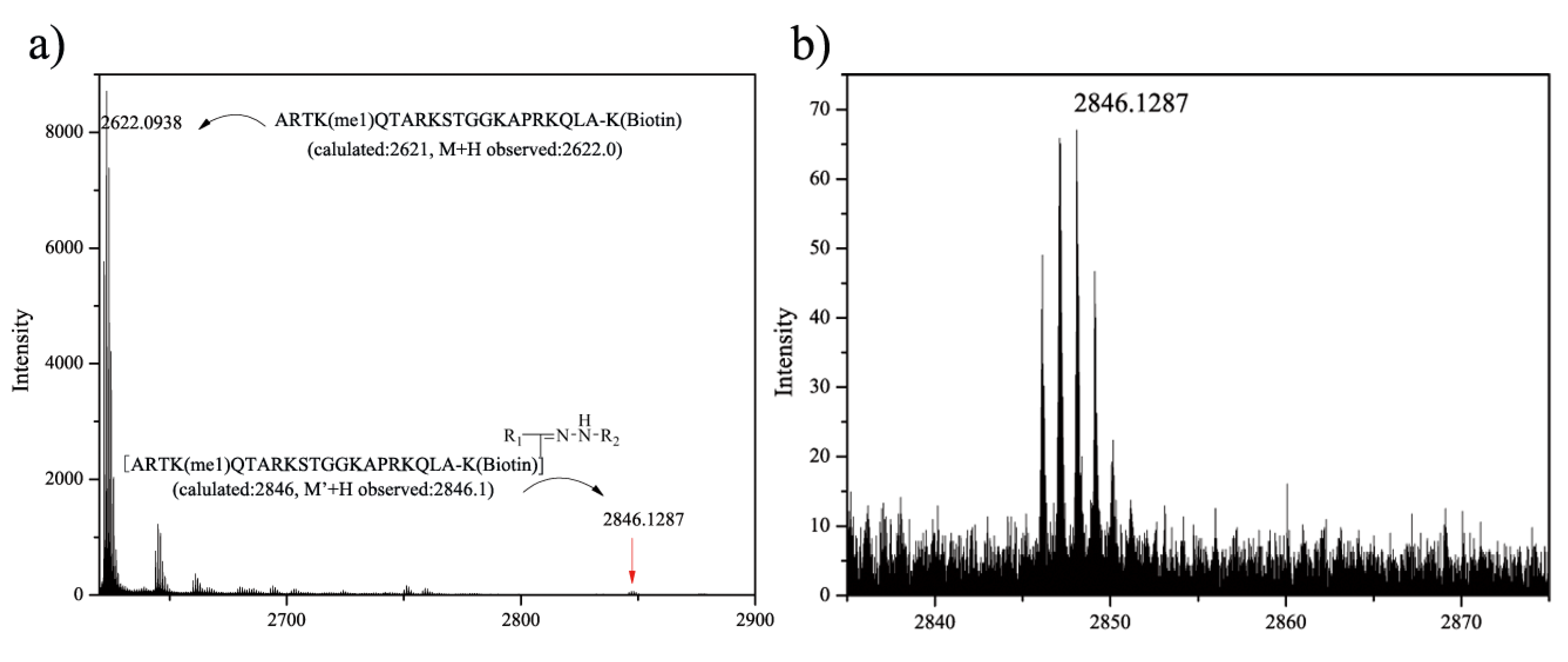
2.4. Competitive Study
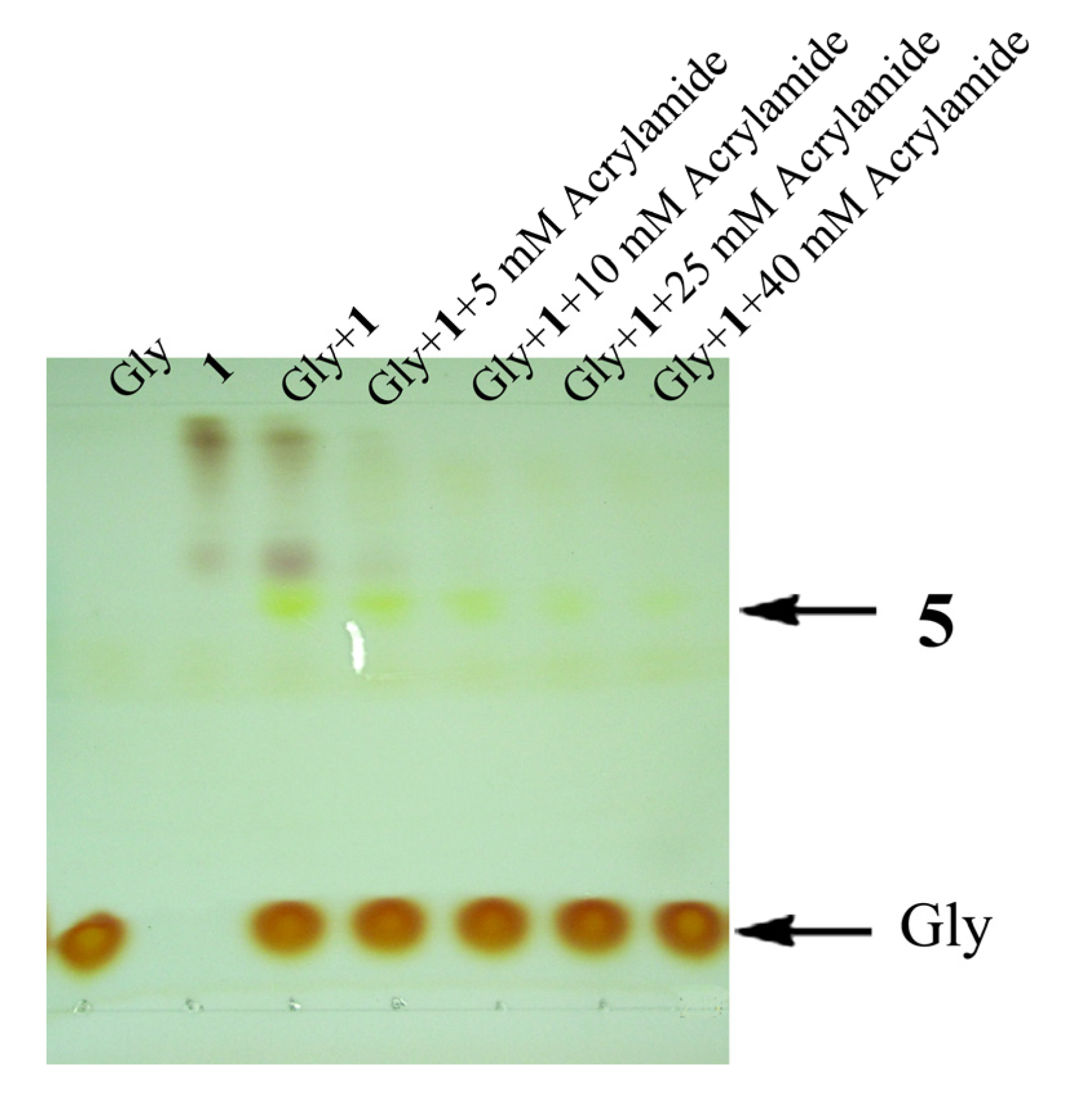
2.5. Discussion
3. Experimental
3.1. General Information
3.2. Experimental Procedures and Characterization Data
3.2.1. General Procedure for the Photochemical Reaction between Diaryltetrazole and Nucleophiles
3.2.2. General Procedure for the Photochemical Reaction between Diaryltetrazole and Amino Acid
3.2.3. General Procedure for the Photochemical Reaction between Diaryltetrazole and Polypeptide
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References and Notes
- Huisgen, R.; Seidel, M.; Sauer, J.; McFarland, J.W.; Wallbillich, G. The formation of nitrile imines in the thermal breakdown of 2,5-disubstituted tetrazoles. J. Org. Chem. 1956, 24, 892–893. [Google Scholar]
- Garofalo, A.W.; Jagodzinski, J.J.; Konradi, A.W.; Ng, R.A.; Semko, C.M.; Sham, H.L.; Sun, M.; Ye, X.M. Synthesis of novel tetrahydro-1H-pyrozolo [4,3-c] pyridines via intramolecular nitrilimine cycloaddition. Chem. Pharm. Bull. 2012, 60, 1063–1066. [Google Scholar]
- Sibi, M.P.; Stanley, L.M.; Jasperse, C.P. An entry to a chiral dihydropyrazole scaffold: Enantioselective [3 + 2] cycloaddition of nitrile imines. J. Am. Chem. Soc. 2005, 127, 8276–8277. [Google Scholar]
- Frohberg, P.; Schulze, I.; Donner, C.; Krauth, F. Remarkable stereoselectivity switch in synthesis of carbonyl substituted N2-arylamidrazones with low lipophilicity. Tetrahedron Lett. 2012, 53, 4507–4509. [Google Scholar]
- Dalloul, H.M.M. Synthesis of spiroheterocycles containing thiadiazole thiadiazine and triazine moieties from nitrilimines. Phosphorus Sulfur Silicon 2011, 186, 1876–1884. [Google Scholar]
- Wolkoff, P.; Hammerum, S. Reaction of hydrazonyl halides with primary thioamides; formation of thiohydrazides and hydrazonyl sulfides. Acta Chem. Scand. B 1976, 30, 831–836. [Google Scholar]
- Csongár, C.; Grubert, L.; Tomaschewski, G. Photochemie arylsubstituierter Sydnone und 2H-TetrazoleReaktion von Nitriliminen mit Phenolen. Z. Chem. 1998, 28, 24–25. [Google Scholar]
- Paulvannan, K.; Chen, T.; Hale, R. An improved synthesis of 1,2,4-Triazoles using Ag2CO3. Tetrahedron 2000, 56, 8071–8076. [Google Scholar]
- Dalloul, H.M.M. Heterocyclic synthesis using nitrilimines. Part 13: Synthesis of new 1,2,3,4-tetrahydra-s-tetrazine derivatives. Tetrahedron 2009, 65, 8722–8726. [Google Scholar]
- Thaher, B.A.; Otto, H.H. On the synthesis of 2-acetyl-4-aryl-6H-1,3,4-thiadiazin-5-ones by reaction of nitrilimines with α-mercapto alkanoic acids. Monatsh. Chem. 2002, 133, 1011–1016. [Google Scholar]
- Thaher, B.; Abu; Zahra, J.A.; El-Abadelah, M.M.; Otto, H.H. On the synthesis of 3-acetyl-1-aryl-1,4,5,6-tetrahydro-1H-1,2,4-triazepin-7-ones by reaction of nitrilimines with 3-aminopropanoic acid. Monatsh. Chem. 2004, 135, 435–439. [Google Scholar]
- Darkow, R.; Yoshikawa, M.; Kitao, T.; Tomaschewski, G.; Schellenberg, J. Photomodification of a poly (acrylonitrile-co-butadiene-co-styrene) containing diaryltetrazolyl groups. J. Polym. Sci. Part B-Polym. Chem. 1994, 32, 1657–1664. [Google Scholar]
- Sayed, A.R.; Wiggins, J.S. 1,3-dipolar cycloaddition polymerization reactions of novel macromolecules containing sym-tetrazine rings. Polymer 2008, 49, 2253–2259. [Google Scholar]
- Lim, R.K.V.; Lin, Q. Photoinducible bioorthogonal chemistry: A spatiotemporally controllable tool to visualize and perturb proteins in live cells. Acc. Chem. Res. 2011, 44, 828–839. [Google Scholar]
- Meier, H.; Heimgartner, H. Intramolecular 1,3-dipolar cyclo-additions of diaryl-nitrile-imines generated from 2,5-diaryl-tetrazoles. Helv. Chim. Acta 1985, 68, 1283–1300. [Google Scholar]
- Xia, M.; Pan, X. Microwave-assisted synthesis of pyrazoline derivatives on soluble polymer. Synth. Commun. 2004, 34, 3521–3528. [Google Scholar]
- Shawali, A.S.; Farghaly, T.A.; Hussein, S.M.; Abdalla, M.M. Site-selective reactions of hydrazonoyl chlorides with cyanoacetic hydrazide and its N-arylidene derivatives and anti-aggressive activity of prepared products. Arch. Pharm. Res. 2013, 36, 694–701. [Google Scholar]
- Al-Noaimi, M.Z.A.; Abdel-Jalil, J.; El-Abadelah, M.M.; Haddad, S.F.; Baqi, Y.N.H.; Voelter, W. Metal-assisted oxidative cyclization of arylamidrazones I. Synthesis of 3-acetyl-1,4-dihydro-1-phenyl-1,2,4-benzotriazine. Monatsh. Chem. 2006, 137, 745–750. [Google Scholar]
- Lucero, P.L.; Peláez, W.J.; Riedl, Z.; Hajós, G.; Moyano, E.L.; Yranzo, G.I. Flash vacuum pyrolysis of azolylacroleins and azolylbutadienes. Tetrahedron 2012, 68, 1299–1305. [Google Scholar]
- Pagacz-Kostrzewa, M.; Mucha, M.; Weselski, M.; Wierzejewska, M. Conformational properties and photochemistry of new allyl tetrazoles: Matrix isolation FTIR and computational approach. J. Photochem. Photobiol. A 2013, 251, 118–127. [Google Scholar]
- Frija, L.M.T.; Reva, I.D.; Gomez-Zavaglia, A.; Cristiano, M.L.S.; Fausto, R. UV-induced photochemistry of matrix-isolated 1-phenyl-4-allyl-tetrazolone. Photochem. Photobiol. Sci. 2007, 6, 1170–1176. [Google Scholar]
- Wang, Y.C.; Vera, I.R.; Lin, Q. Convenient synthesis of highly functionalized pyrazolines via mild, photoactivated 1,3-dipolar cycloaddition. Org. Lett. 2007, 9, 4155–4158. [Google Scholar]
- Wang, Y.; Hu, W.J.; Song, W.; Lint, R.K.V.; Lin, Q. Discovery of long-wavelength photoactivatable diaryltetrazoles for bioorthogonal 1,3-dipolar cycloaddition reactions. Org. Lett. 2008, 10, 3725–3728. [Google Scholar]
- Ito, S.; Tanaka, Y.; Kakehi, A.; Kondo, K. Facile synthesis of 2,5-disubstituted tetrazoles by reaction of phenylsulfonylhydrazones with arenediazonium salts. Bull. Chem. Soc. Jpn. 1976, 49, 1920–1923. [Google Scholar]
- Molteni, G.; Orlandi, M.; Broggini, G. Nitrilimine cycloadditions in aqueous media. J. Chem. Soc. Perkin Trans. I 2000, 1, 3742–3745. [Google Scholar]
- Song, W.; Wang, Y.; Qu, J.; Madden, M.M.; Lin, Q. A photoinducible 1,3-dipolar cycloaddition reaction for rapid, selective modification of tetrazole-containing proteins. Angew. Chem. Int. Ed. 2008, 47, 2832–2835. [Google Scholar]
- Zheng, S.-L.; Wang, Y.; Yu, Z.; Lin, Q.; Coppens, P. Direct observation of a photoinduced nonstabilized nitrile imine structure in the solid state. J. Am. Chem. Soc. 2009, 131, 18036–18037. [Google Scholar]
- Song, W.; Wang, Y.; Yu, Z.; Vera, C.I.R.; Qu, J.; Lin, Q. A metabolic alkene reporter for spatiotemporally controlled imaging of newly synthesized proteins in mammalian cells. ACS Chem. Biol. 2010, 5, 875–885. [Google Scholar]
- Wang, Y.; Song, W.; Hu, W.J.; Lin, Q. Fast alkene functionalization in vivo by photoclick chemistry: HOMO lifting of nitrile imine dipoles. Angew. Chem. Int. Ed. 2009, 48, 5330–5333. [Google Scholar]
- Zhang, Y.; Pan, Y.; Yang, W.; Liu, W.; Zhao, Z.K. Protein arginine allylation and subsequent fluorophore targeting. ChemBioChem 2013, 14, 1438–1443. [Google Scholar]
- Zhang, Y.; Zhao, Z.K.; Dalian Institute of Chemical Physics, Dalian, China. Methylproteome analysis based on protein allylation. unpublished results. 2013. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, Y.; Liu, W.; Zhao, Z.K. Nucleophilic Trapping Nitrilimine Generated by Photolysis of Diaryltetrazole in Aqueous Phase. Molecules 2014, 19, 306-315. https://doi.org/10.3390/molecules19010306
Zhang Y, Liu W, Zhao ZK. Nucleophilic Trapping Nitrilimine Generated by Photolysis of Diaryltetrazole in Aqueous Phase. Molecules. 2014; 19(1):306-315. https://doi.org/10.3390/molecules19010306
Chicago/Turabian StyleZhang, Yixin, Wujun Liu, and Zongbao K. Zhao. 2014. "Nucleophilic Trapping Nitrilimine Generated by Photolysis of Diaryltetrazole in Aqueous Phase" Molecules 19, no. 1: 306-315. https://doi.org/10.3390/molecules19010306
APA StyleZhang, Y., Liu, W., & Zhao, Z. K. (2014). Nucleophilic Trapping Nitrilimine Generated by Photolysis of Diaryltetrazole in Aqueous Phase. Molecules, 19(1), 306-315. https://doi.org/10.3390/molecules19010306