The Inhibitory Effects of Phenolic and Terpenoid Compounds from Baccharis trimera in Siha Cells: Differences in Their Activity and Mechanism of Action
Abstract
:1. Introduction
2. Results and Discussion
2.1. Reduction of SiHa Cell Viability
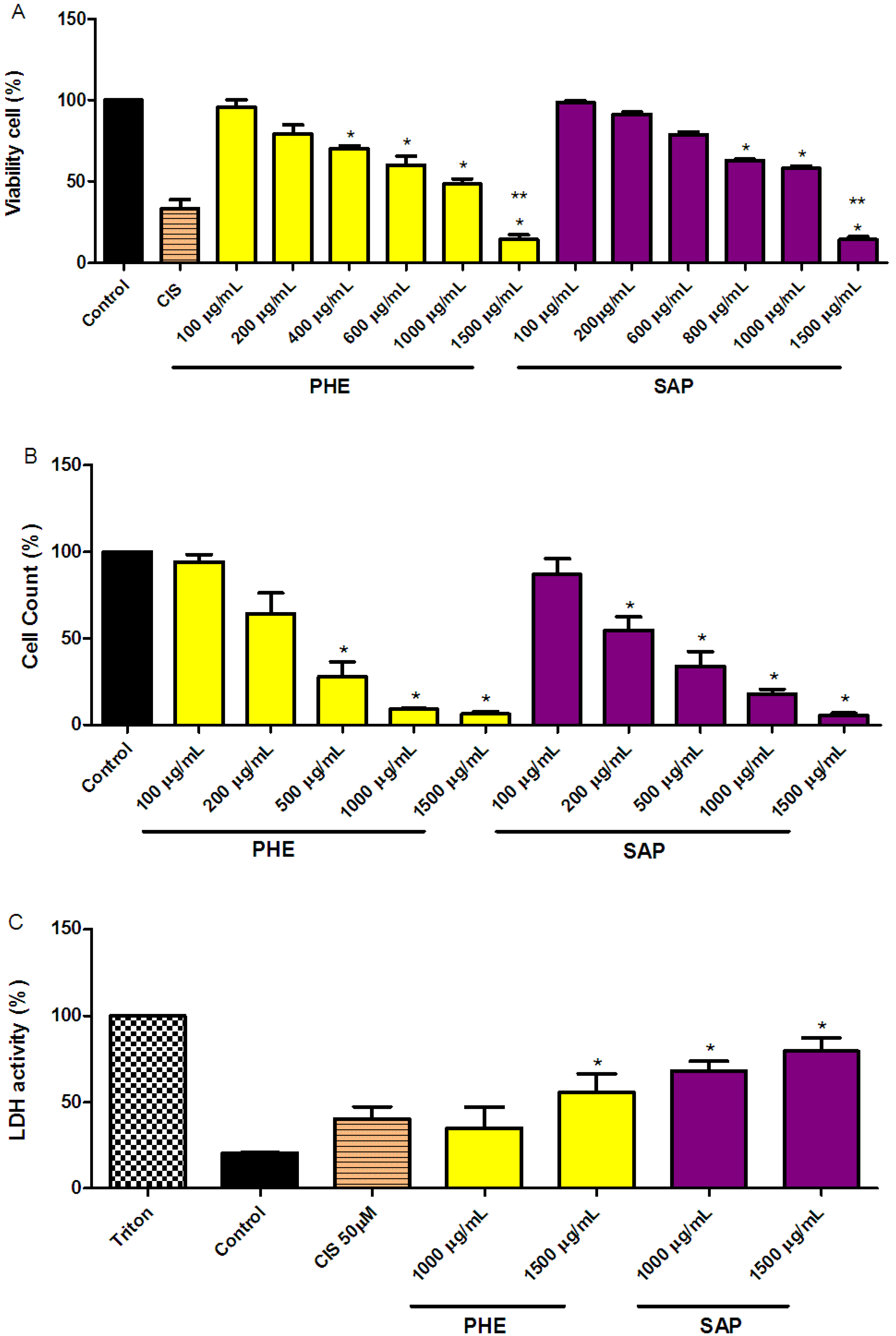
2.2. LDH Measurements in SiHa Cervical Cancer Cells
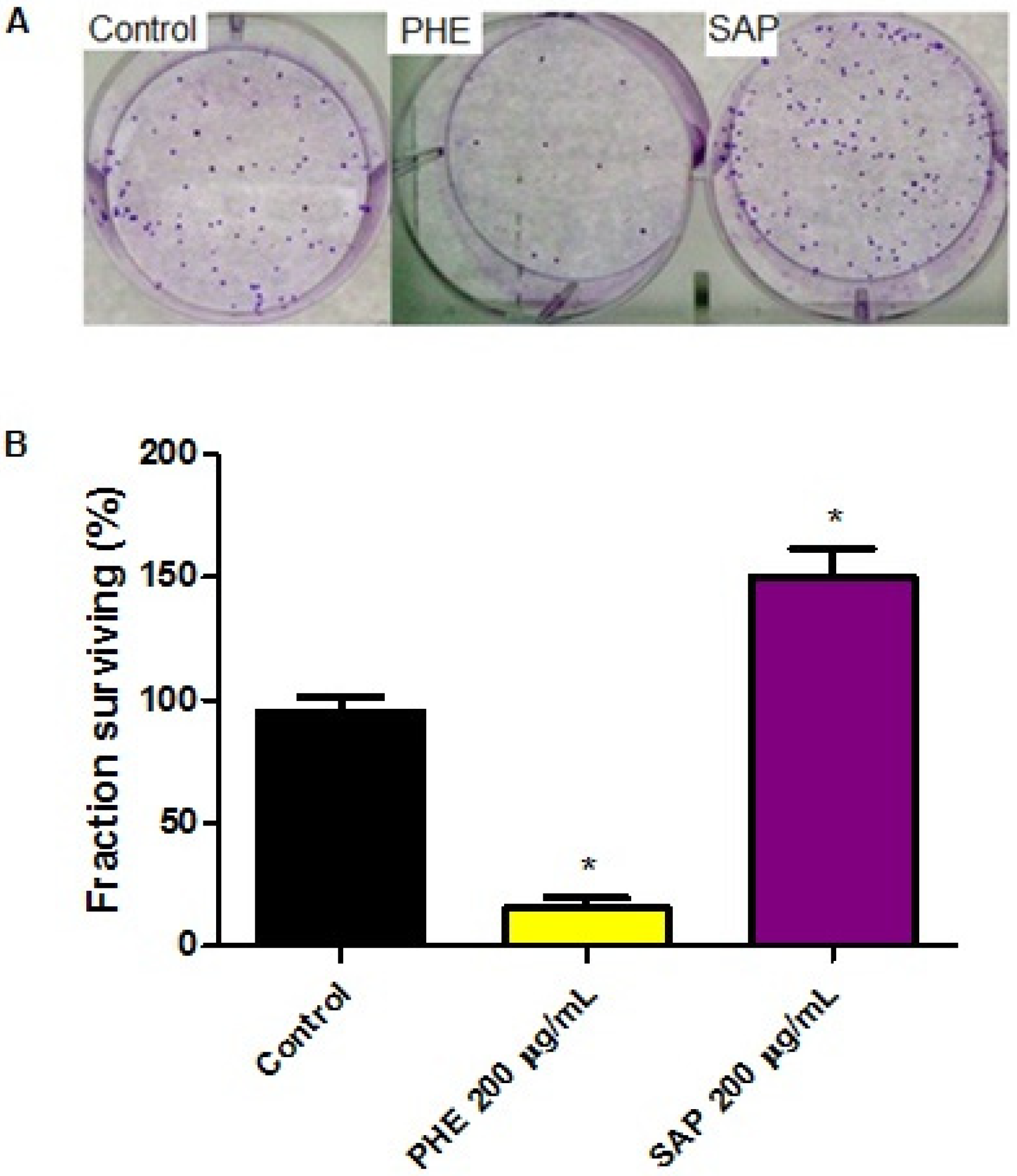
2.3. Effects on Clonogenic Survival
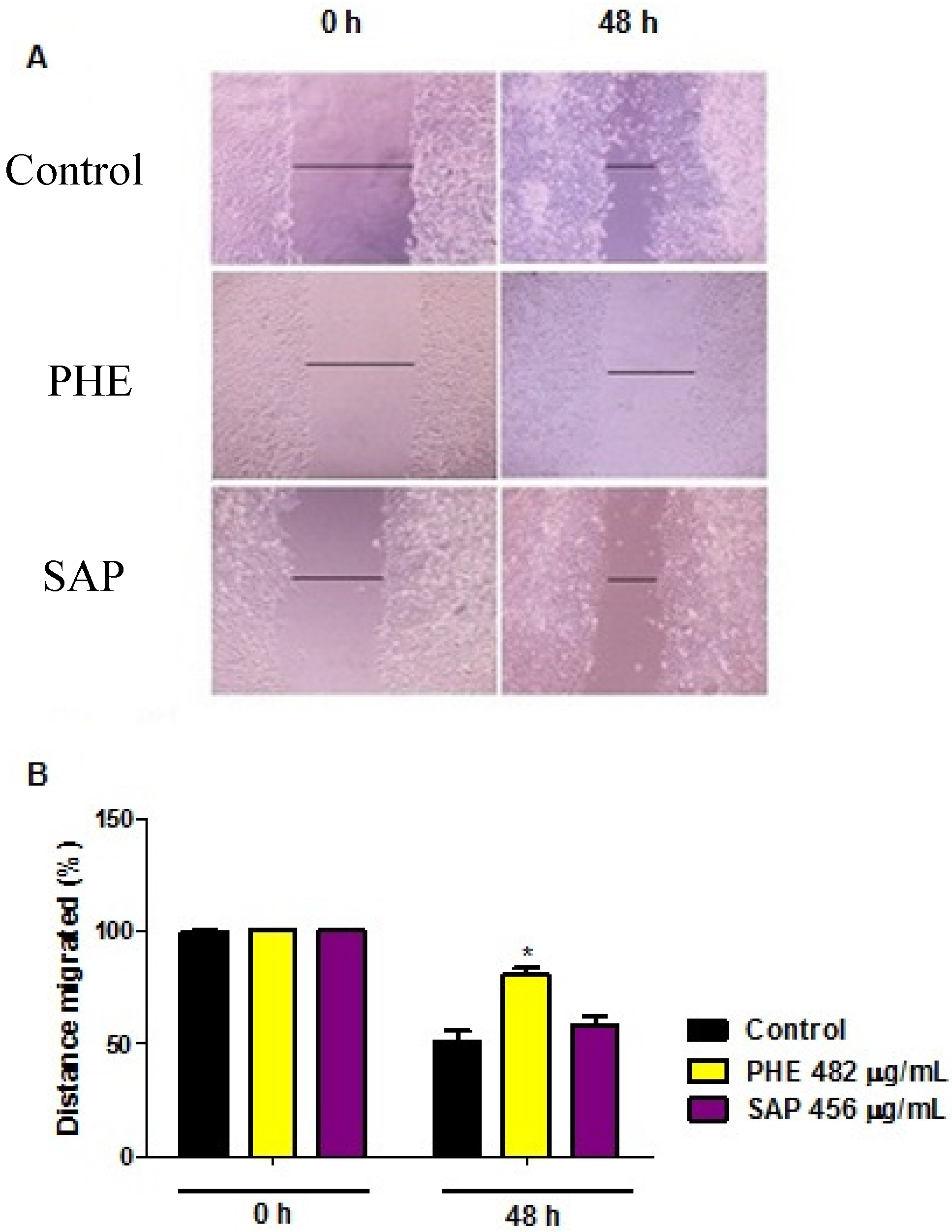
2.4. Wound Healing Migration Assay
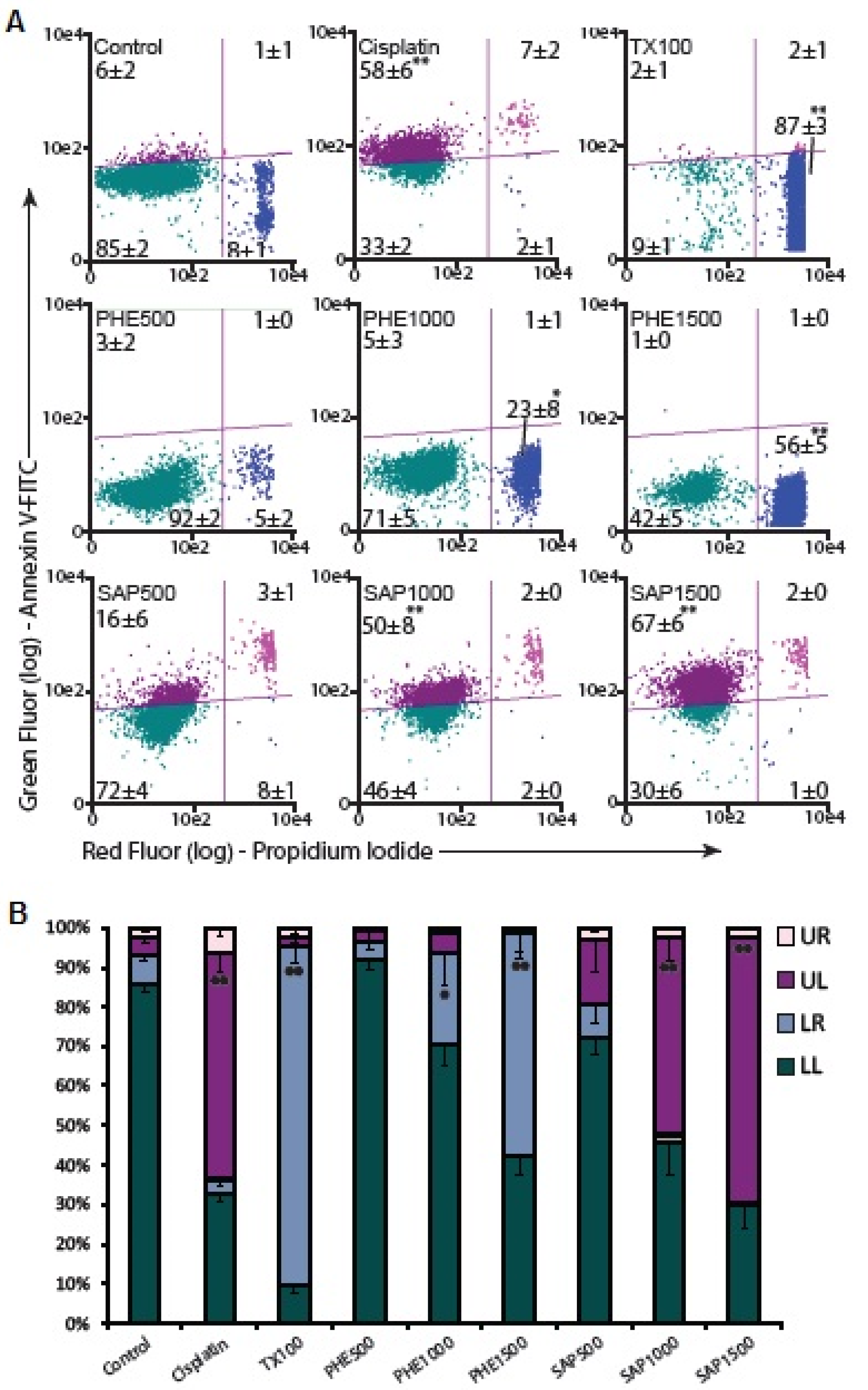
2.5. Apoptosis Assay
3. Experimental
3.1. Plant Material
3.2. Chemical
3.3. Extraction
3.4. Cell Culture
3.5. Treatments
3.6. Cell Proliferation Assay
3.7. Cell Counting
3.8. LDH Measurement
3.9. Clonogenic Assay
3.10. Wound Healing Migration Assay
3.11. Apoptosis Assay
3.12. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- INCA, National Cancer Institute José Alencar Gomes da Silva. 2011 General Coordination of Strategic Actions, Coordination of Prevention and Surveillance. Estimate 2012: Cancer Incidence in Brazil; INCA: Rio de Janeiro, Brazil. Available online: http://www.inca.gov.br/estimativa/ 2012/estimativa20122111.pdf (accessed on 12 July 2013).
- Lim, C.B.; Ky, N.; Ng, H.M.; Hamza, M.S.; Zhao, Y. Curcuma wenyujin extracts induces apoptosis and inhibits proliferation of human cervical cancer cells in vitro and in vivo. Integr. Cancer Ther. 2010, 9, 36–49. [Google Scholar] [CrossRef]
- Abad, M.J.; Barmejo, M. Baccharis (Compositae): A review update. Arkivoc 2007, vii, 76–96. [Google Scholar]
- Herz, W.; Pilotti, A.; Söderholm, A.C.; Shuhama, I.K.; Vichnewski, W. New ent-clerodane-type diterpenoids from Baccharis trimera. J. Org. Chem. 1977, 42, 3913–3917. [Google Scholar] [CrossRef]
- Gosmann, G.; de Oliveira, C.B.; Comunello, L.N. Baccharis trimera (Less.) DC. Carqueja. In Recent Progress in Medicinal Plants: Drug Plants II; Awaad, A.S., Singh, V.K., Govil, J.N., Eds.; Studium Press LLC: Houston, TX, USA, 2010; pp. 107–120. [Google Scholar]
- Soicke, H.; Leng-Peschlow, E. Characterization of flavonoids from Baccharis trimera and their antihepatotoxic properties. Planta Med. 1987, 53, 37–39. [Google Scholar] [CrossRef]
- Gené, R.M.; Cartañá, C.; Adzet, T.; Marin, E.; Parella, T.; Cañigueral, S. Anti-inflammatory and analgesic activity of Baccharis trimera: Identification of active constituents. Planta Med. 1996, 62, 232–235. [Google Scholar] [CrossRef]
- Simões-Pires, C.A.; Queiroz, E.F.; Henriques, A.T.; Hostettmann, K. Isolation and on-line identification of antioxidant compounds from three Baccharis species by HPLC-UV-MS/MS with post-column derivatisation. Phytochem. Anal. 2005, 16, 307–314. [Google Scholar] [CrossRef]
- Paul, E.L.; Lunardelli, A.; Caberlon, E.; de Oliveira, C.B.; Santos, R.C.V.; Biolchi, V.; Bastos, C.M.A.; Moreira, K.B.; Nunes, F.B.; Gosmann, G.; et al. Anti-inflammatory and immunomodulatory effects of Baccharis trimera aqueous extract on induced pleurisy in rats and lymphoproliferation in vitro. Inflammation 2009, 32, 419–425. [Google Scholar] [CrossRef]
- De Oliveira, S.Q.; Dal-Pizzol, F.; Moreira, J.C.F.; Schenkel, E.P.; Gosmann, G. Antioxidant activity of Baccharis spicata, Baccharis trimera and Baccharis usterii. Acta Farm. Bonaer. 2004, 23, 265–268. [Google Scholar]
- De Oliveira, C.B.; Comunello, L.N.; Lunardelli, A.; Amaral, R.H.; Pires, M.G.S.; Silva, G.L.; Manfredini, V.; Vargas, C.R.; Gnoatto, S.C.B.; de Oliveira, J.R.; et al. Phenolic enriched extract of Baccharis trimera presents anti-inflammatory and antioxidant activities. Molecules 2012, 17, 1113–1123. [Google Scholar] [CrossRef]
- Gerhäuser, C.; Klimo, K.; Heiss, E.; Neumann, I.; Eldeen, A.G.; Knauft, J.; Liu, G.Y.; Sitthimonchai, S.; Frank, N. Mechanism-based in vitro screening of potential cancer chemopreventive agents. Mutat. Res. 2003, 523–524, 163–172. [Google Scholar] [CrossRef]
- Haghiac, M.; Walle, T. Quercetin induces necrosis and apoptosis in SCC-9 oral cancer cells. Nutr. Cancer 2005, 53, 220–231. [Google Scholar] [CrossRef]
- Priyadarsini, R.V.; Murugan, R.S.; Maitreyi, S.; Ramalingam, K.; Karunagaran, D.; Nagini, S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur. J. Pharmacol. 2010, 649, 84–91. [Google Scholar] [CrossRef]
- Bishayee, K.; Ghosh, S.; Mukherjee, A.; Sadhukhan, R.; Mondal, J.; Khuda-Bukhsh, A.R. Quercetin induces cytochrome-c release and ROS accumulation to promote apoptosis and arrest the cell cycle in G2/M, in cervical carcinoma: Signal cascade and drug-DNA interaction. Cell Prolif. 2013, 46, 153–163. [Google Scholar] [CrossRef]
- Hernandez-Flores, G.; Ortiz-Lazareno, P.C.; Lerma-Diaz, J.M.; Dominguez-Rodriguez, J.R.; Jave-Suarez, L.F.; Aguilar-Lemarroy, A.C.; de Celis-Carrillo, R.; del Toro-Arreola, S.; Castellanos-Esparza, Y.C.; Bravo-Cuellar, A. Pentoxifilline sensitizes human cervical tumor cells to cislplatin-induced apoptosis by suppressing NF-kappa B and decrease cell senescence. BMC Cancer 2011, 11, 483–497. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Abrams, J.M.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; Dawson, T.M.; Dawson, V.L.; El-Deiry, W.S.; Fulda, S.; et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012, 19, 107–120. [Google Scholar] [CrossRef]
- Bras, M.; Queenan, B.; Susin, S.A. Programmed cell death via mitochondria: Different modes of dying. Biochemistry 2005, 70, 231–239. [Google Scholar]
- Huang, X.; Lee, S.; Chen, X. Design of “smart” probes for optical imaging of apoptosis. Am. J. Nucl. Med. Mol. Imaging 2011, 1, 3–17. [Google Scholar]
- Yan, Y.Y.; Bai, J.P.; Xie, Y.; Yu, J.Z.; Ma, C.G. The triterpenoid pristimerin induces U87 glioma cell apoptosis through reactive oxygen species- mediated mitochondrial dysfunction. Oncol. Lett. 2013, 5, 242–248. [Google Scholar]
- Uto, T.; Sakamoto, A.; Tung, N.H.; Fujiki, T.; Kishihara, K.; Oiso, S.; Kariyazono, H.; Morinaga, O.; Shoyama, Y. Anti-proliferative activities and apoptosis induction by triterpenes derived from Eriobotrya japonica in human leukemia cell lines. Int. J. Mol. Sci. 2013, 14, 4106–4120. [Google Scholar] [CrossRef]
- Wu-Yang, H.; Yi-Zhong, C.; Yanbo, Z. Natural phenolic compounds from medicinal herbs and dietary plants: Potential use for cancer prevention. Nutr. Cancer 2009, 62, 1–20. [Google Scholar] [CrossRef]
- Singh, M.; Tyagi, S.; Bhui, K.; Prasad, S.; Shukla, Y. Regulation of cell growth through cell cycle arrest and apoptosis in HPV 16 positive human cervical cancer cells by tea polyphenols. Invest. New Drugs 2010, 28, 216–224. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, K.; Zhang, Q.; Mei, J.; Chen, C.J.; Feng, Z.Z.; Yu, D.H. Effects of quercetin on the apoptosis of the human gastric carcinoma cells. Toxicol. In Vitro 2012, 26, 221–228. [Google Scholar] [CrossRef]
- Sreelatha, S.; Jeyachitra, A.; Padma, P.R. Antiproliferation and induction of apoptosis by Moringa oleifera leaf extract on human cancer cell. Food Chem. Toxicol. 2011, 49, 1270–1275. [Google Scholar] [CrossRef]
- Buffon, A.; Wink, M.R.; Ribeiro, B.V.; Casali, E.A.; Libermann, T.A.; Zerbini, L.F.; Robson, S.C.; Sarkis, J.J.F. NTPDase and 5′-ecto-nucleotidase expression profiles and the pattern of extracellular ATP metabolism in the Walker 256 tumor. Biochim. Biophys. Acta 2007, 1770, 1259–1265. [Google Scholar]
- Franken, N.A.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Mileo, A.M.; Venere, D.D.; Linsalata, V.; Fraioli, R.; Miccadei, S. Artichoke polyphenols induce apoptosis and decrease the invasive potential of the human breast cancer cell line MDA-MB231. J. Cell Physiol. 2012, 227, 3301–3309. [Google Scholar] [CrossRef]
- Sample Availability: Samples of PHE and SAP are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
De Oliveira, C.B.; Comunello, L.N.; Maciel, É.S.; Giubel, S.R.; Bruno, A.N.; Chiela, E.C.F.; Lenz, G.; Gnoatto, S.C.B.; Buffon, A.; Gosmann, G. The Inhibitory Effects of Phenolic and Terpenoid Compounds from Baccharis trimera in Siha Cells: Differences in Their Activity and Mechanism of Action. Molecules 2013, 18, 11022-11032. https://doi.org/10.3390/molecules180911022
De Oliveira CB, Comunello LN, Maciel ÉS, Giubel SR, Bruno AN, Chiela ECF, Lenz G, Gnoatto SCB, Buffon A, Gosmann G. The Inhibitory Effects of Phenolic and Terpenoid Compounds from Baccharis trimera in Siha Cells: Differences in Their Activity and Mechanism of Action. Molecules. 2013; 18(9):11022-11032. https://doi.org/10.3390/molecules180911022
Chicago/Turabian StyleDe Oliveira, Cristiane B., Lucimara N. Comunello, Érica S. Maciel, Scheron R. Giubel, Alessandra N. Bruno, Eduardo C. F. Chiela, Guido Lenz, Simone C. B. Gnoatto, Andréia Buffon, and Grace Gosmann. 2013. "The Inhibitory Effects of Phenolic and Terpenoid Compounds from Baccharis trimera in Siha Cells: Differences in Their Activity and Mechanism of Action" Molecules 18, no. 9: 11022-11032. https://doi.org/10.3390/molecules180911022
APA StyleDe Oliveira, C. B., Comunello, L. N., Maciel, É. S., Giubel, S. R., Bruno, A. N., Chiela, E. C. F., Lenz, G., Gnoatto, S. C. B., Buffon, A., & Gosmann, G. (2013). The Inhibitory Effects of Phenolic and Terpenoid Compounds from Baccharis trimera in Siha Cells: Differences in Their Activity and Mechanism of Action. Molecules, 18(9), 11022-11032. https://doi.org/10.3390/molecules180911022



