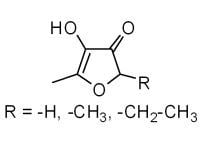
Natural 4-Hydroxy-2,5-dimethyl-3(2H)-furanone (Furaneol®)
Abstract
Share and Cite
Schwab, W. Natural 4-Hydroxy-2,5-dimethyl-3(2H)-furanone (Furaneol®). Molecules 2013, 18, 6936-6951. https://doi.org/10.3390/molecules18066936
Schwab W. Natural 4-Hydroxy-2,5-dimethyl-3(2H)-furanone (Furaneol®). Molecules. 2013; 18(6):6936-6951. https://doi.org/10.3390/molecules18066936
Chicago/Turabian StyleSchwab, Wilfried. 2013. "Natural 4-Hydroxy-2,5-dimethyl-3(2H)-furanone (Furaneol®)" Molecules 18, no. 6: 6936-6951. https://doi.org/10.3390/molecules18066936
APA StyleSchwab, W. (2013). Natural 4-Hydroxy-2,5-dimethyl-3(2H)-furanone (Furaneol®). Molecules, 18(6), 6936-6951. https://doi.org/10.3390/molecules18066936