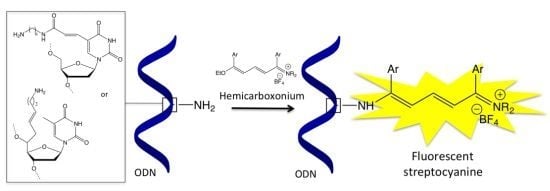
Oligonucleotide Labelling Using a Fluorogenic “Click” Reaction with a Hemicarboxonium Salt
Abstract
:1. Introduction
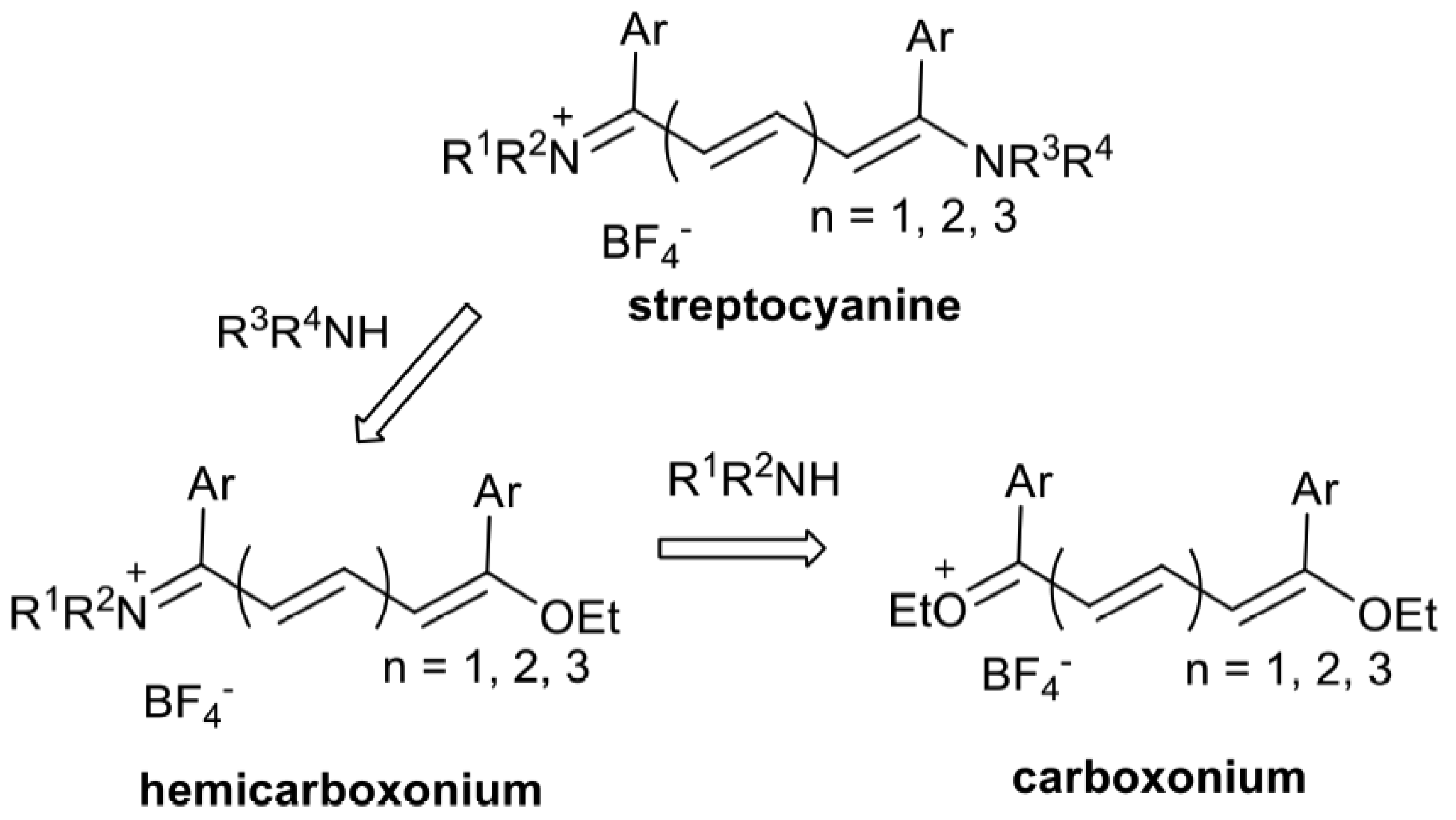
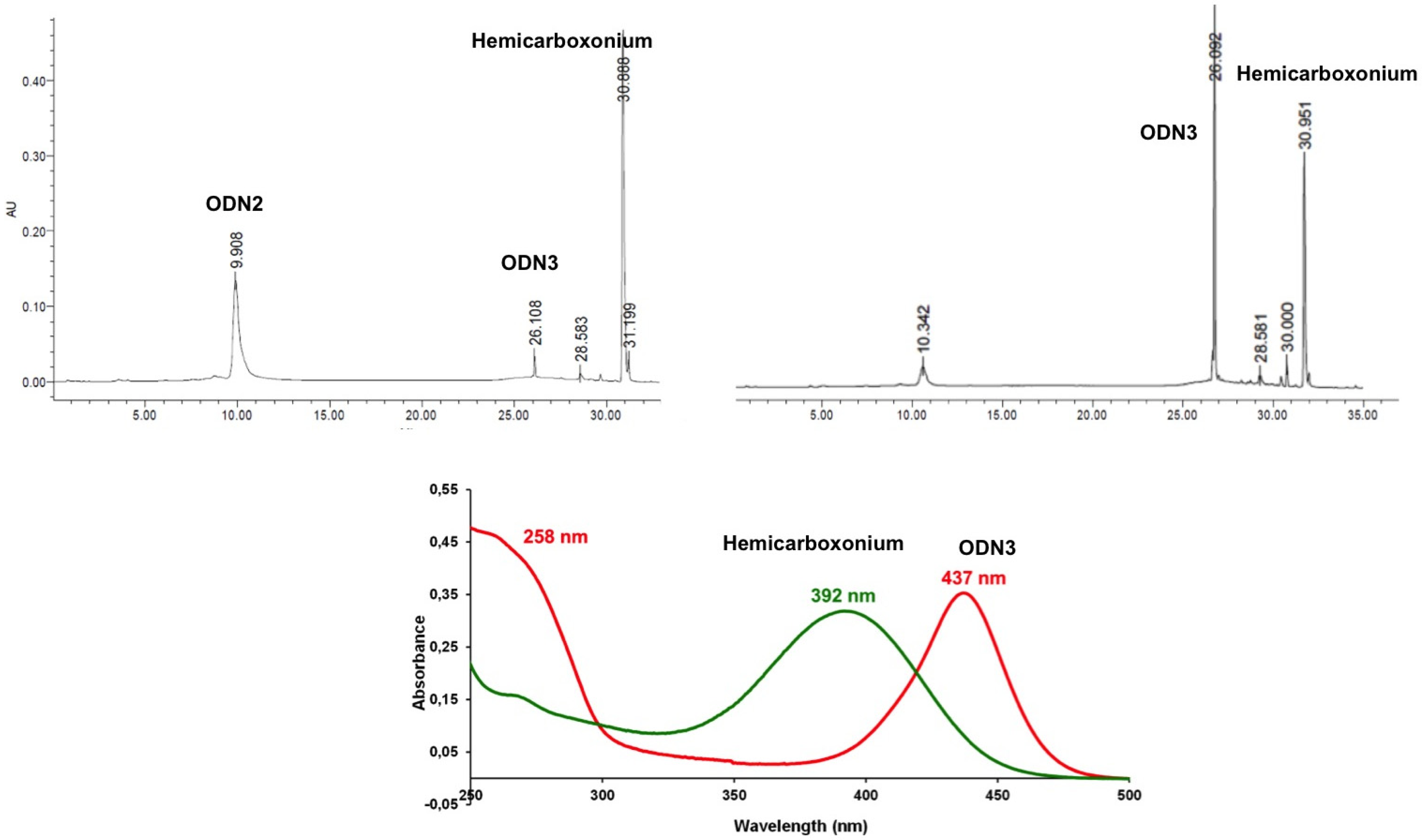
2. Results and Discussion

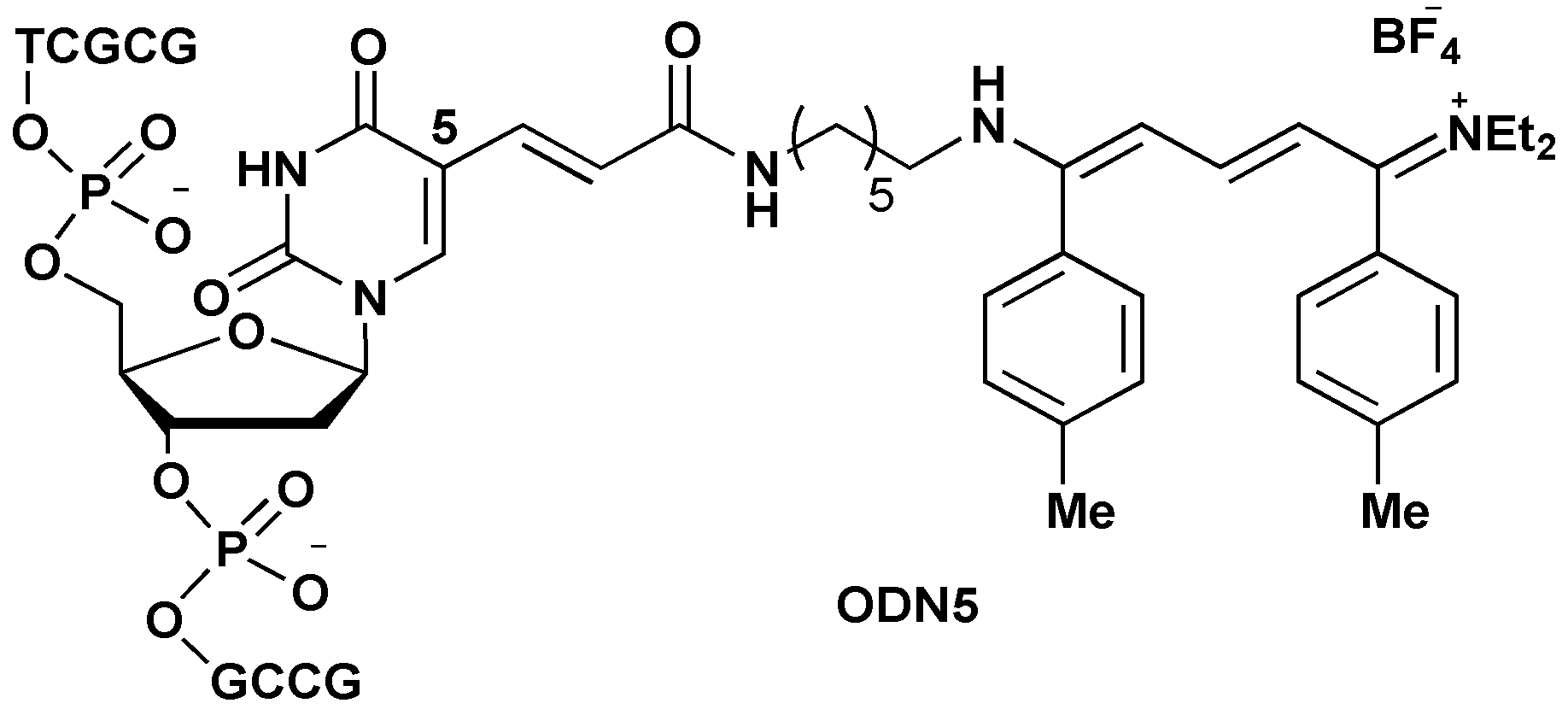
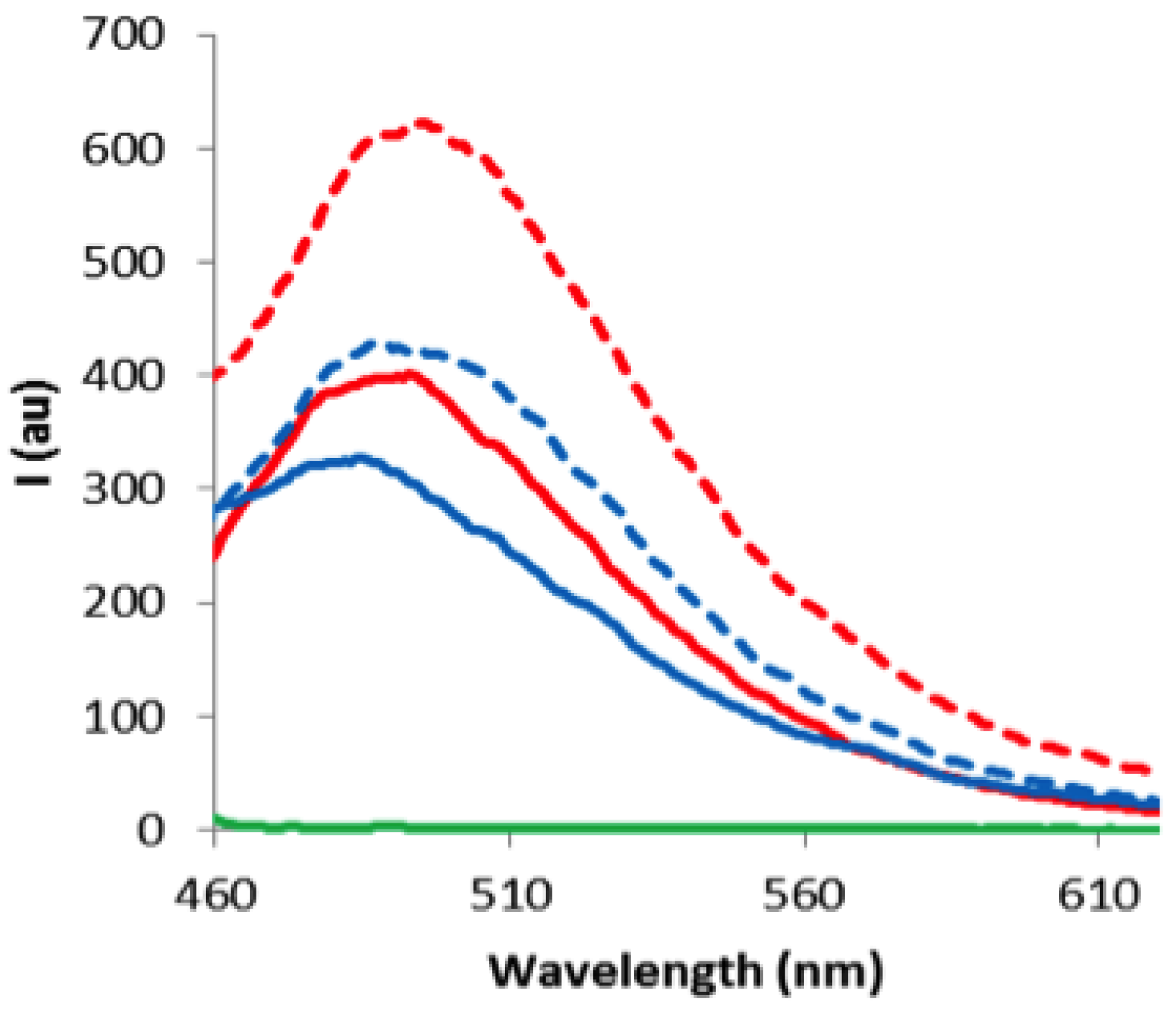
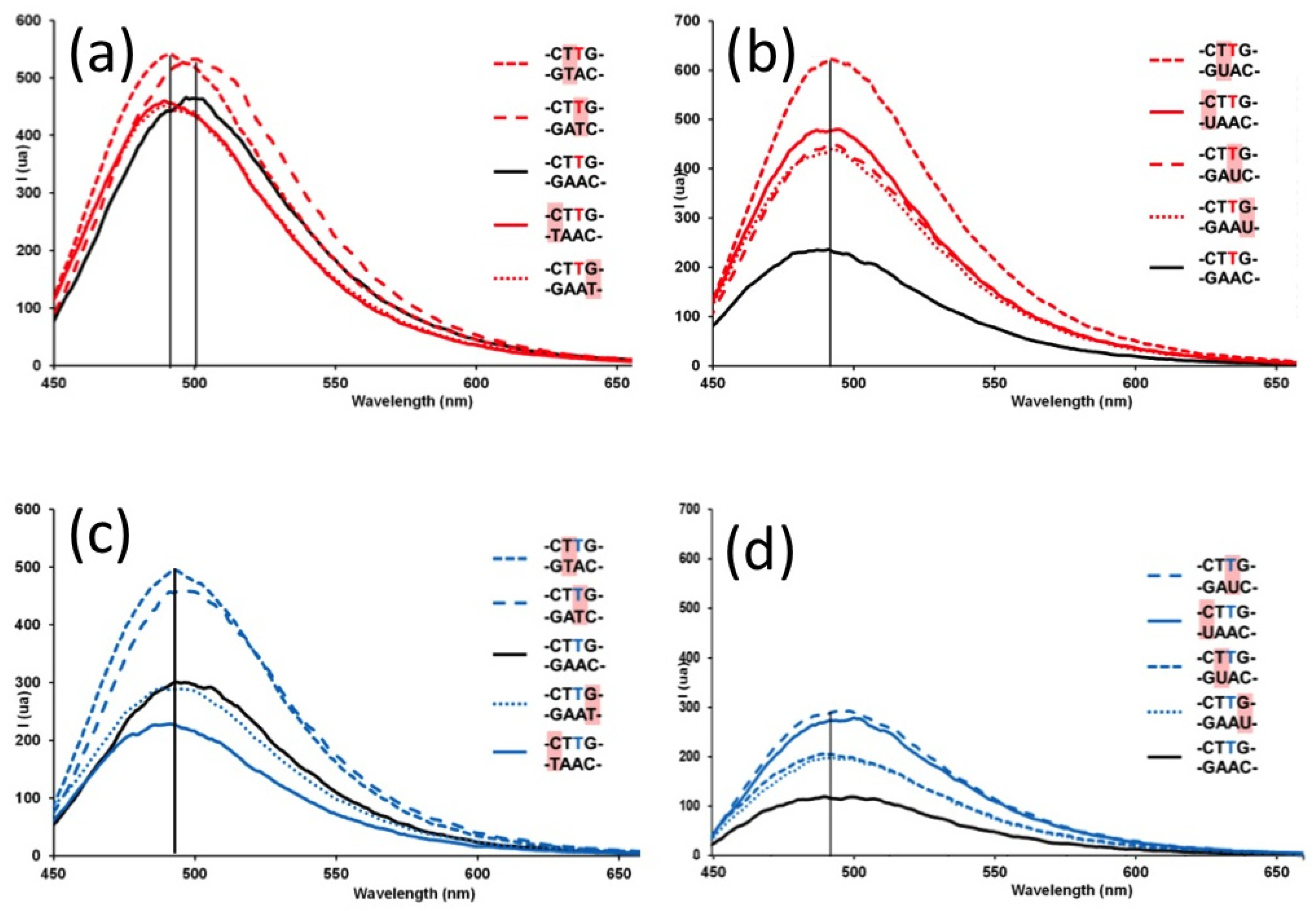
3. Experimental
3.1. General
3.2. Protocol for Streptocyanine Dye Formation with Amino ODN
4. Conclusions
Supplementary Materials
Conflicts of Interest
References
- Ranasinghe, R.T.; Brown, T. Fluorescence based strategies for genetic analysis. Chem. Commun. 2005, 5487–5502. [Google Scholar] [CrossRef]
- Sterath, M.; Marx, A. Genotyping-from genomic DNA to genotype in a single tube. Angew. Chem. Int. Ed. 2005, 44, 7842–7849. [Google Scholar] [CrossRef]
- Johansson, M.K.; Cook, R.M. Intramolecular dimers: A new design strategy for fluorescence-quenched probes. Chem. Eur. J. 2003, 9, 3466–3471. [Google Scholar] [CrossRef]
- Singh, Y.; Murat, P.; Defrancq, E. Recent developments in oligonucleotide conjugation. Chem. Soc. Rev. 2010, 39, 2054–2070. [Google Scholar] [CrossRef]
- Le Droumaguet, C.; Wang, C.; Wang, Q. Fluorogenic click reaction. Chem. Soc. Rev. 2010, 39, 1233–1239. [Google Scholar] [CrossRef]
- Qi, J.; Tung, C.H. Development of benzothiazole “click-on” fluorogenic dyes. Bioorganic Med. Chem. Lett. 2011, 21, 320–323. [Google Scholar] [CrossRef]
- Qi, J.; Han, M.S.; Chang, Y.C.; Tung, C.H. Developing visible fluorogenic “click-on” dyes for cellular imaging. Bioconjugate Chem. 2011, 22, 1758–1762. [Google Scholar]
- Sun, H.; Peng, X. Template-directed fluorogenic oligonucleotide ligation using “click” chemistry: Detection of single nucleotide polymorphism in the human p53 tumor suppressor gene. Bioconjugate Chem. 2013, 24, 1226–1234. [Google Scholar] [CrossRef]
- Gierlich, J.; Burley, G.A.; Gramlich, P.M.E.; Hammond, D.M.; Carell, T. Click Chemistry as a reliable method for the high-density postsynthetic functionalization of alkyne-modified DNA. Org. Lett. 2006, 8, 3639–3642. [Google Scholar] [CrossRef]
- Franzini, R.M.; Kool, E.T. 7-Azidomethoxy-coumarins as profluorophores for templated nucleic acid detection. ChemBioChem 2008, 9, 2981–2988. [Google Scholar] [CrossRef]
- Meguellati, K.; Koripelly, G.; Ladame, S. DNA-templated synthesis of trimethine cyanine dyes: A versatile fluorogenic reaction for sensing G-quadruplex formation. Angew. Chem. Int. Ed. 2010, 49, 2738–2742. [Google Scholar] [CrossRef]
- Huang, Y.; Coull, J.M. Diamine catalyzed hemicyanine dye formation from nonfluorescent precursors through DNA programmed chemistry. J. Am. Chem. Soc. 2008, 130, 3238–3239. [Google Scholar] [CrossRef]
- Obaya, N.; Payrastre, C.; Madaule, Y. Synthesis of new pentacarbon chain streptocyanines (pentamethinium salts). Tetrahedron 2001, 57, 9137–9147, and references therein. [Google Scholar] [CrossRef]
- Izquierdo, A.; Payrastre, C.; Gornitzka, H.; Madaule, Y. Synthesis and reactivity of a new heptacarbon chain carboxonium salt − access to a new class of streptocyanine dyes. Eur. J. Org. Chem. 2003, 2371–2374. [Google Scholar]
- Guieu, V.; Izquierdo, A.; Garcia-Alonso, S.; André, C.; Madaule, Y.; Payrastre, C. Fluorescent streptocyanine dyes: Synthesis and photophysical properties – synthesis of a new hemicarboxonium salt. Eur. J. Org. Chem. 2007, 804–810. [Google Scholar]
- Izquierdo, A.; Guieu, V.; Gornitzka, H.; Madaule, Y.; Payrastre, C. Synthesis and reactivity of a new nonacarbon chain carboxonium salt access to a new class of streptocyanine dyes. Eur. J. Org. Chem. 2004, 2317–2320. [Google Scholar]
- Hellriegel, C.; Kirstein, J.; Braüchle, C.; Latour, V.; Pigot, T.; Olivier, R.; Lacombe, S.; Brown, R.; Guieu, V.; Payrastre, C.; et al. Diffusion of single streptocyanine molecules in the nanoporous network of sol-gel glasses. J. Phys. Chem. B 2004, 108, 14699–14709. [Google Scholar] [CrossRef]
- Maether, M.-P.; Bernat, V.; Maturano, M.; André-Barrès, C.; Ladeira, S.; Valentin, A.; Vial, H.; Payrastre, C. Synthesis and antiplasmodial activity of streptocyanine/peroxide and streptocyanine/4-aminoquinoline hybrid dyes. Org. Biomol. Chem. 2011, 9, 7400–7410. [Google Scholar] [CrossRef]
- Banuls, V.; Escudier, J.-M. Allylsilanes in the preparation of 5’-C-hydroxy or bromo alkylthymidines. Tetrahedron 1999, 55, 5831–5838. [Google Scholar] [CrossRef]
- Caruthers, M.H. Chemical synthesis of DNA and DNA analogs. Acc. Chem. Res. 1991, 24, 278–284. [Google Scholar] [CrossRef]
- Banuls, V.; Escudier, J.M.; Zedde, C.; Claparols, C.; Donnadieu, B.; Plaisancié, H. Stereoselective synthesis of (5'S)-5'-C-(5-Bromo-2-penten-1-yl)-2'-deoxyribofuranosyl thymine, a new convertible nucleoside. Eur. J. Org. Chem. 2001, 4693–4700. [Google Scholar]
- Escudier, J.-M.; Dupouy, C.; Fountain, M.A.; del Mundo, I.M.A.; Jacklin, E.M.; Morrow, J. Synthesis and luminescence properties of a trinucleotide-europium(III) complex conjugate. Org. Biomol. Chem. 2009, 7, 3251–3257. [Google Scholar] [CrossRef]
- Hwang, G.T.; Seo, Y.J.; Kim, B.H. A highly discriminating quencher-free molecular beacon for probing DNA. J. Am. Chem. Soc. 2004, 126, 6528–6529. [Google Scholar] [CrossRef]
- Berndl, S.; Herzig, N.; Kele, P.; Lachmann, D.; Li, X.; Wolfbeis, O.S.; Wagenknecht, H.-A. Comparison of a nucleosidic vs. non-nucleosidic postsynthetic “click” modification of DNA with base-labile fluorescent probes. Bioconjugate Chem. 2009, 20, 558–564. [Google Scholar] [CrossRef]
- Østergaard, M.E.; Hrdlicka, P.J. Pyrene-functionalized oligonucleotides and locked nucleic acids (LNAs): Tools for fundamental research, diagnostics, and nanotechnology. Chem. Soc. Rev. 2011, 40, 5771–5788. [Google Scholar] [CrossRef]
- Astakhova, I.K.; Wengel, J. Interfacing click chemistry with automated oligonucleotide synthesis for the preparation of fluorescent DNA probes containing internal xanthene and cyanine dyes. Chem. Eur. J. 2013, 19, 1112–1122. [Google Scholar] [CrossRef]
- Bethge, L.; Jarikote, V.D.; Seitz, O. New cyanine dyes as base surrogates in PNA: Forced intercalation probes (FIT-probes) for homogeneous SNP detection. Bioorg. Med. Chem. 2008, 16, 114–125. [Google Scholar] [CrossRef]
- Sharma, A.K.; Heemstra, J.M. Small-molecule-dependent split aptamer ligation. J. Am. Chem. Soc. 2011, 133, 12426–12429. [Google Scholar] [CrossRef]
- Percivalle, C.; Bartolo, J.-F.; Ladame, S. Oligonucleotide-templated chemical reactions: Pushing the boundaries of a nature-inspired process. Org. Biomol. Chem. 2013, 11, 16–26. [Google Scholar] [CrossRef]
- Gorska, K.; Winssinger, N. Reactions templated by nucleic acids: More ways to translate oligonucleotide-based instructions into emerging function. Angew. Chem. Int. Ed. 2013, 52, 6820–6843. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Maether, M.-P.; Lapin, K.; Muntean, A.; Payrastre, C.; Escudier, J.-M. Oligonucleotide Labelling Using a Fluorogenic “Click” Reaction with a Hemicarboxonium Salt. Molecules 2013, 18, 12966-12976. https://doi.org/10.3390/molecules181012966
Maether M-P, Lapin K, Muntean A, Payrastre C, Escudier J-M. Oligonucleotide Labelling Using a Fluorogenic “Click” Reaction with a Hemicarboxonium Salt. Molecules. 2013; 18(10):12966-12976. https://doi.org/10.3390/molecules181012966
Chicago/Turabian StyleMaether, Marie-Pierre, Kristie Lapin, Andreea Muntean, Corinne Payrastre, and Jean-Marc Escudier. 2013. "Oligonucleotide Labelling Using a Fluorogenic “Click” Reaction with a Hemicarboxonium Salt" Molecules 18, no. 10: 12966-12976. https://doi.org/10.3390/molecules181012966
APA StyleMaether, M.-P., Lapin, K., Muntean, A., Payrastre, C., & Escudier, J.-M. (2013). Oligonucleotide Labelling Using a Fluorogenic “Click” Reaction with a Hemicarboxonium Salt. Molecules, 18(10), 12966-12976. https://doi.org/10.3390/molecules181012966