In Vitro Anti-Candida Activity of Certain New 3-(1H-Imidazol-1-yl)propan-1-one Oxime Esters
Abstract
:1. Introduction
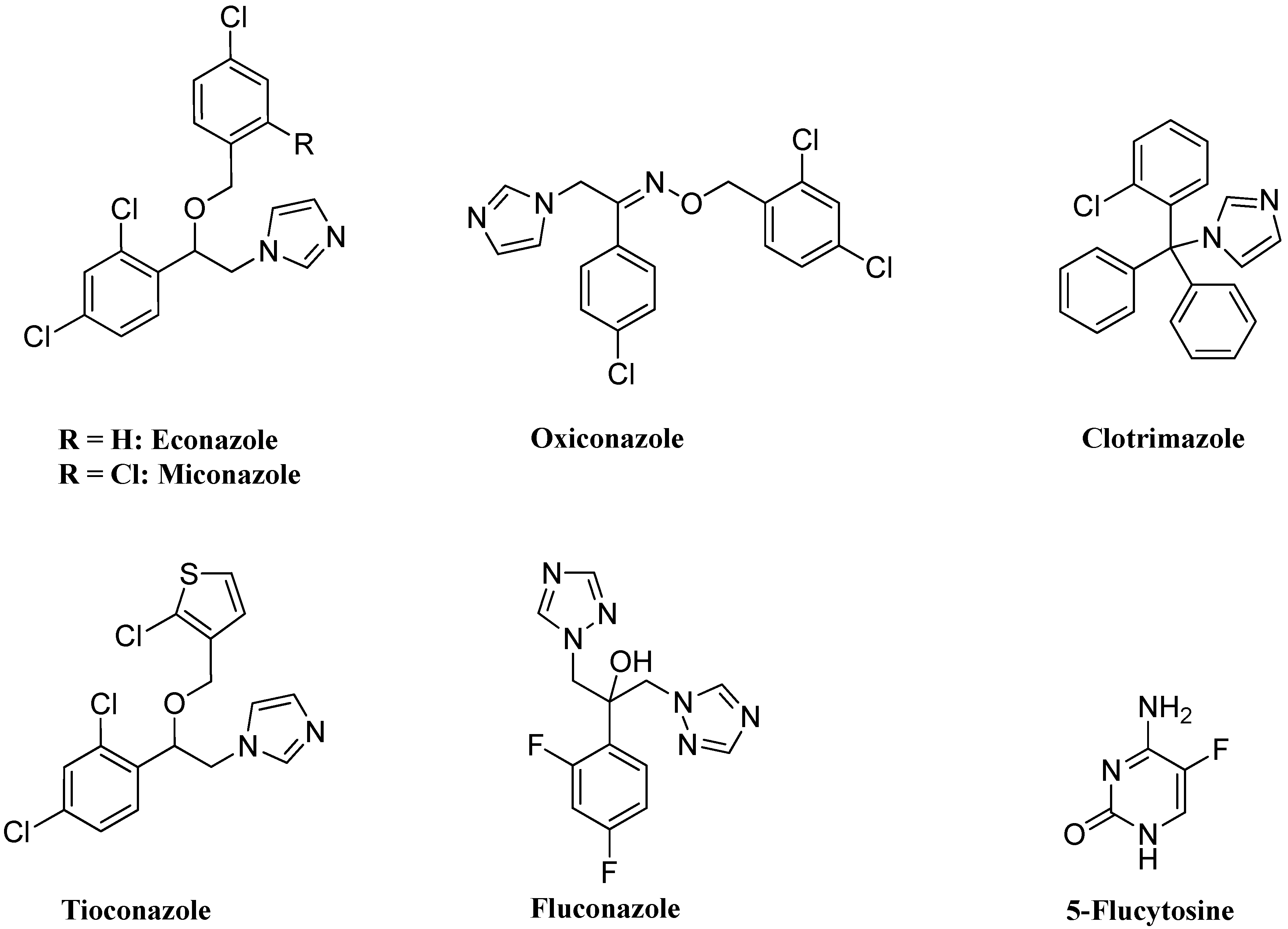
2. Results and Discussion
2.1. Chemistry

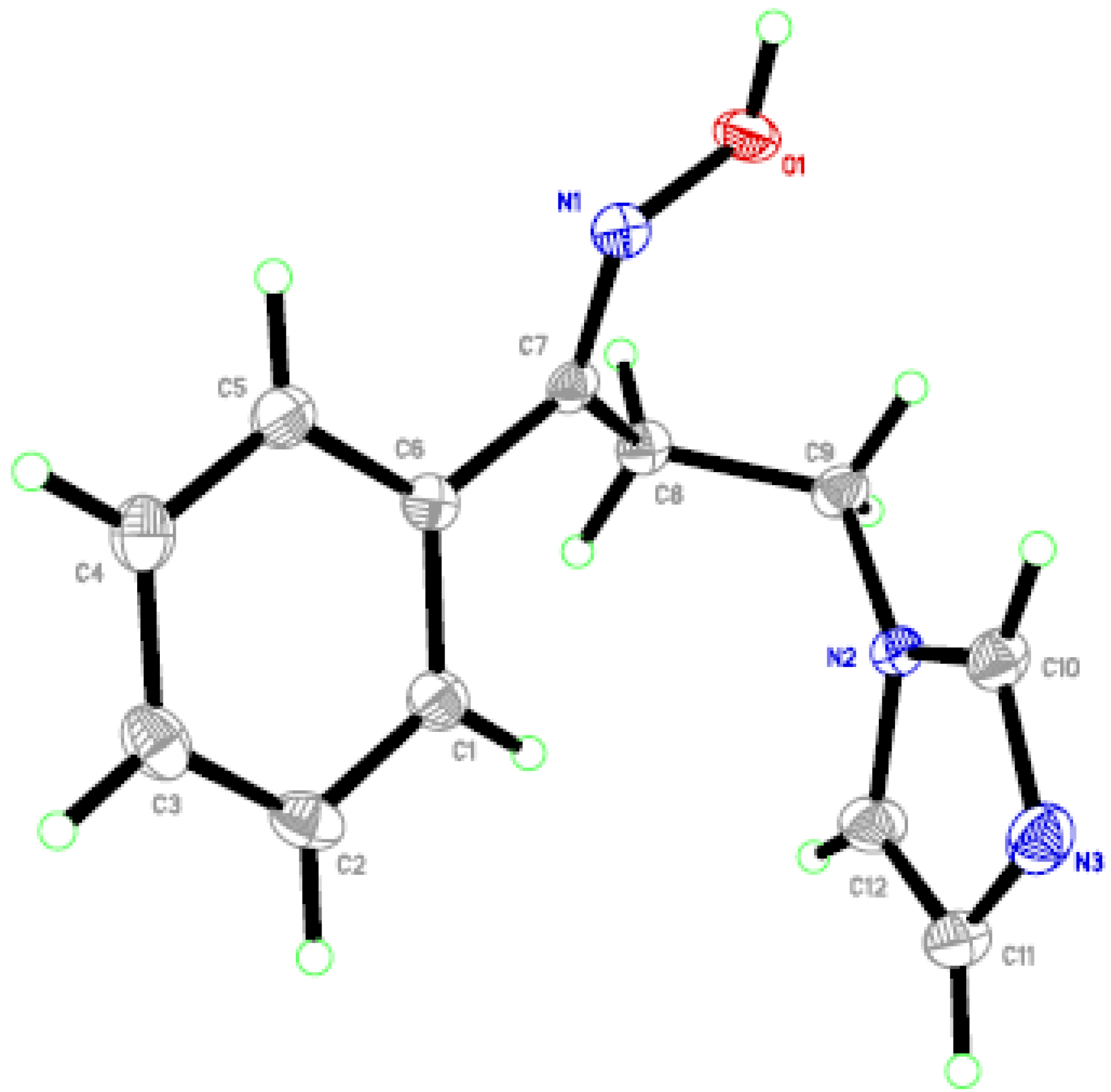
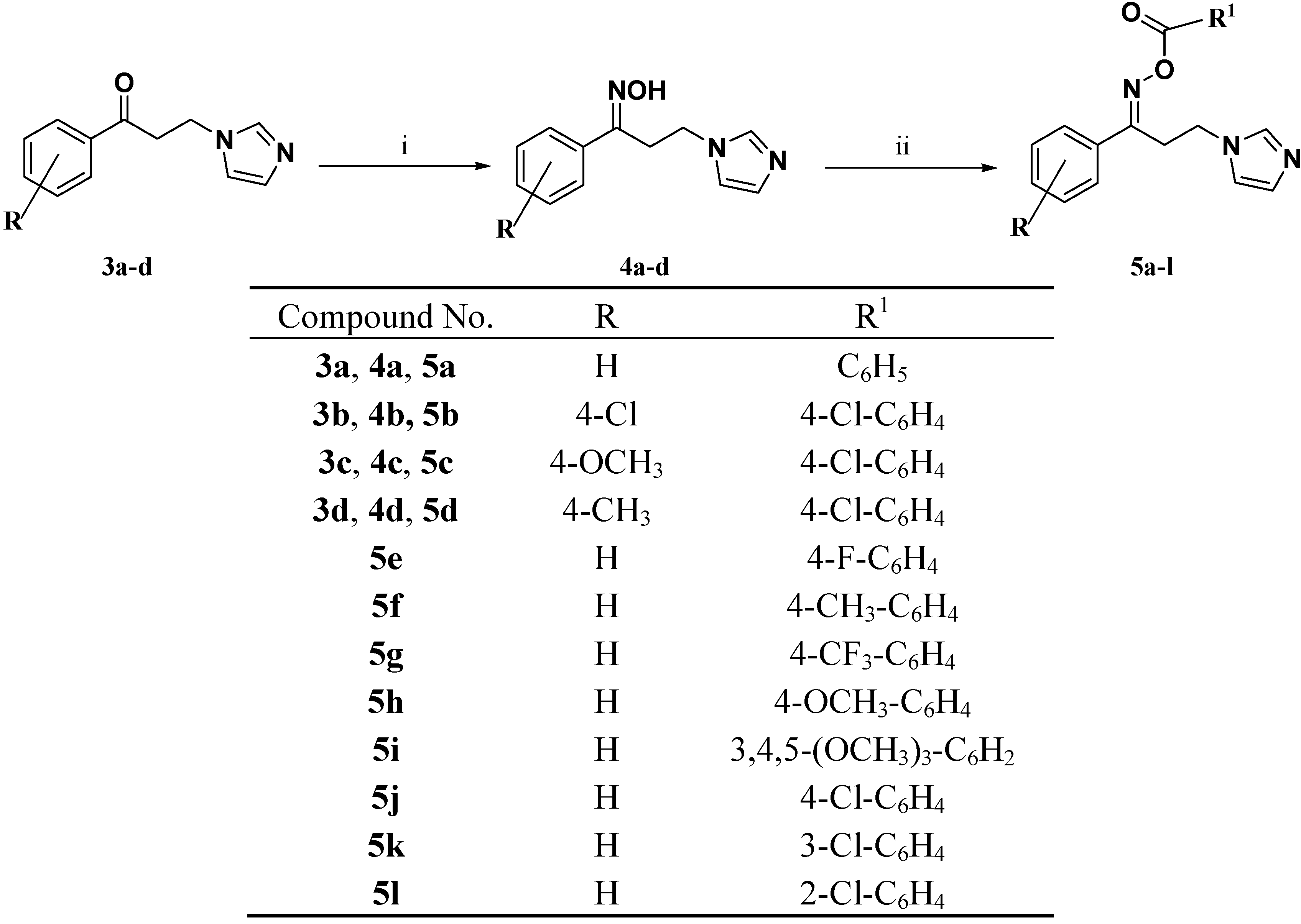
2.2. In Vitro Anti-Candida Activity and SARs
| Compound No | Candida albicans | Candida tropicalis | |||
|---|---|---|---|---|---|
| DIZ ± SD * | MIC (µmol/mL) ** | DIZ ± SD * | MIC (µmol/mL) ** | ||
| 4a | 21 ± 1.0 | 0.5807 | 20 ± 0.5 | 0.5807 | |
| 4b | 13 ± 0.6 | 0.5019 | 8 ± 1.0 | 0.5019 | |
| 4c | 9 ± 1.15 | 0.5099 | 8 ± 1.0 | 0.2549 | |
| 4d | 8 ± 1.0 | 0.5456 | 8 ± 1.0 | 0.5456 | |
| 5a | 11 ± 1.2 | 0.3919 | 8 ± 1.0 | 0.7837 | |
| 5b | 18 ± 1.1 | 0.0805 | 12 ± 1.0 | 0.6439 | |
| 5c | 16 ± 0.4 | 0.3257 | 16 ± 1.2 | 0.3257 | |
| 5d | 18 ± 0.9 | 0.1699 | 14 ± 0.5 | 0.3398 | |
| 5e | 16 ± 0.9 | 0.3708 | 14 ± 0.6 | 0.3708 | |
| 5f | 7 ± 1.0 | 0.3752 | 8 ± 1.0 | 0.1876 | |
| 5g | 13 ± 0.6 | 0.6454 | 14 ± 1.0 | 0.3227 | |
| 5h | 24 ± 1.1 | 0.0112 | 17 ± 1.0 | 0.3582 | |
| 5i | 17 ± 1.1 | 0.3053 | 14 ± 0.5 | 0.3053 | |
| 5j | 25 ± 1.0 | 0.0054 | 25 ± 1.2 | 0.1767 | |
| 5k | 20 ± 0.9 | 0.0221 | 14 ± 0.5 | 0.7069 | |
| 5l | 12 ± 1.0 | 0.7069 | 12 ± 0.7 | 0.3535 | |
| Fluconazole | 15 ± 0.5 | >1.6325 | 16 ± 0.5 | >1.6325 | |
| Miconazole | 38 ± 1.1 | 0.0188 | 24 ± 0.5 | 0.0024 | |
3. Experimental
3.1. Chemistry
3.1.1. General
3.1.2. General Procedure for Preparation of the Ketones 3a–d
3.1.3. General Procedure for Preparation of the Oximes 4a–d
3.1.4. General Procedure for the Synthesis of the Target Oxime Esters 5a–l
3.2. Anti-Candida Activity
3.2.1. Anti-Candida Agents
3.2.2. Media
3.2.3. Organisms
3.2.4. Preparation of Inocula
3.2.5. Disk Diffusion Assay
3.2.6. Antifungal Susceptibility Studies
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Imwidthaya, P.; Poungvarin, N. Cryptococcosis in AIDS. Postgrad. Med. J. 2000, 76, 85–88. [Google Scholar] [CrossRef]
- Sutton, D.A.; Fothergill, A.W.; Rinaldi, M.G. Guide to Clinically Significant Fungi, 1st ed.; Williams and Wilkins: Baltimore, MD, USA, 1998. [Google Scholar]
- Saral, R. Candida and Aspergillus infections in immunocompromised patients: An overview. Rev. Infec. Dis. 1991, 13, 487–492. [Google Scholar] [CrossRef]
- Odds, F.; Brown, A.J.P.; Gow, N.A.R. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Joseph-Horne, T.; Hollomon, D.W. Molecular mechanisms of azole resistance in fungi. FEMS Microbiol. Lett. 1997, 149, 141–149. [Google Scholar] [CrossRef]
- Vanden Bossche, H.; Dromer, F.; Improvisi, I.; Lozano-Chiu, M.; Rex, J.H.; Sanglards, D. Antifungal drug resistance in pathogenic fungi. Med. Mycol. 1998, 36, 119–128. [Google Scholar]
- Marichal, P.; Vanden Bossche, H. Mechanisms of resistance to azole antifungals. Acta Biochim. Pol. 1995, 42, 509–516. [Google Scholar]
- De Luca, L. Naturally occurring and synthetic imidazoles: Their chemistry and their biological activities. Curr. Med. Chem. 2006, 13, 1–23. [Google Scholar]
- Pennisi, M.; Antonelli, M. Clinical aspects of invasive candidiasis in critically III patients. Drugs 2009, 69, 21–28. [Google Scholar] [CrossRef]
- Chen, S.C.; Playford, E.G.; Sorrell, T.C. Antifungal therapy in invasive fungal infections. Curr. Opin. Pharmacol. 2010, 10, 522–530. [Google Scholar] [CrossRef]
- Wang, W.; Sheng, C.; Che, X.; Ji, H.; Miao, Z.; Yao, J.; Zhang, W. Design, synthesis, and antifungalactivity of novel conformationally restricted triazole derivatives. Arch. Pharm. 2009, 342, 732–739. [Google Scholar] [CrossRef]
- Hamdan, J.S.; Hahn, R.C. Antifungal drugs for systemic mycosis: An overview of mechanism of action and resistance. Anti-Infective Agents Med. Chem. 2006, 5, 403–412. [Google Scholar] [CrossRef]
- Rossello, A.; Bertini, S.; Lapucci, A.; Macchia, M.; Martinelli, A.; Rapposelli, S.; Herreros, E.; Macchia, B. Synthesis, Antifungal activity, and molecular modeling studies of new inverted oxime ethers of oxiconazole. J. Med. Chem. 2002, 45, 4903–4912. [Google Scholar] [CrossRef]
- Aboul-Enein, M.N.; El-Azzouny, A.A.; Attia, M.I.; Saleh, O.A.; Kansoh, A.L. Synthesis and Anti-Candida Potential of Certain Novel 1-[(3-Substituted-3-phenyl)propyl]-1H-imidazoles. Arch. Pharm. 2011, 344, 794–801. [Google Scholar] [CrossRef]
- Roman, G.; Mares, M.; Nastasa, V. A novel antifungal agent with broad spectrum: 1-(4-Biphenylyl)-3-(1H-imidazol-1-yl)-1-propanone. Arch. Pharm. 2013, 346, 110–118. [Google Scholar] [CrossRef]
- Walker, K.A.; Hirschfeld, D.R.; Marx, M. Antimycotic imidazoles. 2. Synthesis and antifungal properties of esters of 1-[2-hydroxy(mercapto)-2-phenylethyl]-1H-imidazoles. J. Med. Chem. 1978, 21, 1335–1338. [Google Scholar] [CrossRef]
- Fun, H.-K.; Quah, C.K.; Attia, M.I.; Almutairi, M.S.; Ghoneim, S.W. (E)-N-[3-(Imidazol-1-yl)-1-phenylpropylidene]hydroxylamine. Acta Crystallogr. E-Struct. Rep. 2012, E68, o627. [Google Scholar]
- Pfaller, M.A.; Andes, D.; Diekema, D.J.; Espinel-Ingroff, A.; Sheehan, D. Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: Time for harmonization of CLSI and EUCAST broth microdilution methods. Drug Resist. Update. 2010, 13, 6180–6195. [Google Scholar]
- De Vita, D.; Scipione, L.; Tortorella, S.; Mellini, P.; Di Rienzo, B.; Simonetti, G.; D’Auria, F.D.; Panella, S.; Cirilli, R.; Di Santo, R.; et al. Synthesis and antifungal activity of a new series of 2-(1H-imidazol-1-yl)-1-phenylethanol derivatives. Eur. J. Med. Chem. 2012, 49, 334–342. [Google Scholar] [CrossRef]
- Wan, J.; Peng, Z.-Z.; Li, X.-M.; Ouyang, P.-K.; Zhang, S.-S. 3-(1H-Imidazol-1-yl)-1-(4-methoxyphenyl)propan-1-one. Acta Crystallogr. E-Struct. Rep. 2005, E 61, o2585–o2586. [Google Scholar]
- Selvamurugan, V.; Aidhen, I.S. Simple Synthetic Equivalents for the β-(N,N-Disubstituted)ethylamino Acyl Cation Synthon and their Applications. Synthesis 2001, 2239–2246. [Google Scholar] [CrossRef]
- Attia, M.I.; Ghabbour, H.A.; Almutairi, M.S.; Ghoneim, S.W.; Abdel-Aziz, H.A.; Fun, H.-K. Synthesis and X-ray crystal structure of (1E)-1-(4-chlorophenyl)-N-hydroxy-3-(1H-imidazol-1-yl)propan-1-imine. J. Chem. 2013. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI), Reference method for broth dilution antifungal susceptibility testing of yeasts. In Approved Standard M27-A2, 2nd ed.; CLSI: Villanova, PA, USA, 2002.
- Clinical and Laboratory Standards Institute (CLSI), Performance Standards for Antimicrobial Disk Susceptibility Tests. In Approved Standard, M2-A8, 8thed.; CLSI: Wayne, PA, USA, 2005.
- Sample Availability: Contact the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Attia, M.I.; Zakaria, A.S.; Almutairi, M.S.; Ghoneim, S.W. In Vitro Anti-Candida Activity of Certain New 3-(1H-Imidazol-1-yl)propan-1-one Oxime Esters. Molecules 2013, 18, 12208-12221. https://doi.org/10.3390/molecules181012208
Attia MI, Zakaria AS, Almutairi MS, Ghoneim SW. In Vitro Anti-Candida Activity of Certain New 3-(1H-Imidazol-1-yl)propan-1-one Oxime Esters. Molecules. 2013; 18(10):12208-12221. https://doi.org/10.3390/molecules181012208
Chicago/Turabian StyleAttia, Mohamed I., Azza S. Zakaria, Maha S. Almutairi, and Soraya W. Ghoneim. 2013. "In Vitro Anti-Candida Activity of Certain New 3-(1H-Imidazol-1-yl)propan-1-one Oxime Esters" Molecules 18, no. 10: 12208-12221. https://doi.org/10.3390/molecules181012208
APA StyleAttia, M. I., Zakaria, A. S., Almutairi, M. S., & Ghoneim, S. W. (2013). In Vitro Anti-Candida Activity of Certain New 3-(1H-Imidazol-1-yl)propan-1-one Oxime Esters. Molecules, 18(10), 12208-12221. https://doi.org/10.3390/molecules181012208





