Promising Low-Toxicity of Viologen-Phosphorus Dendrimers against Embryonic Mouse Hippocampal Cells
Abstract
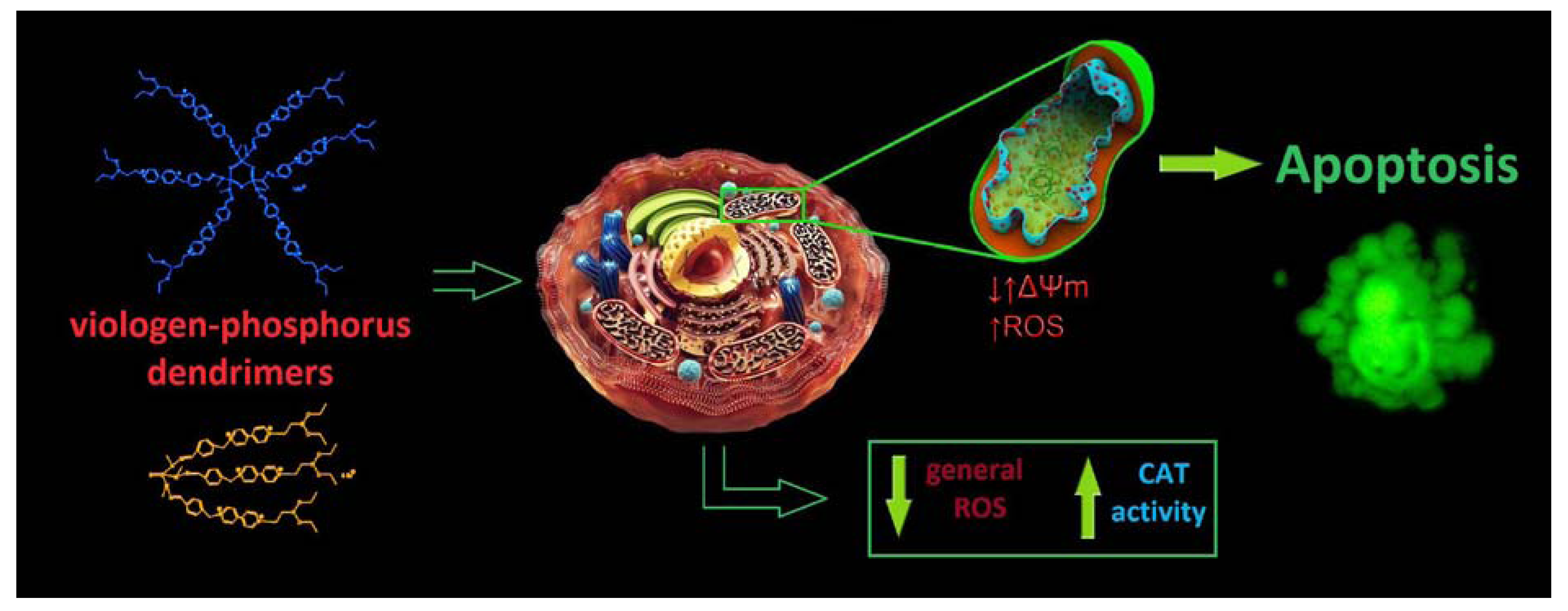
:1. Introduction

2. Results and Discussion
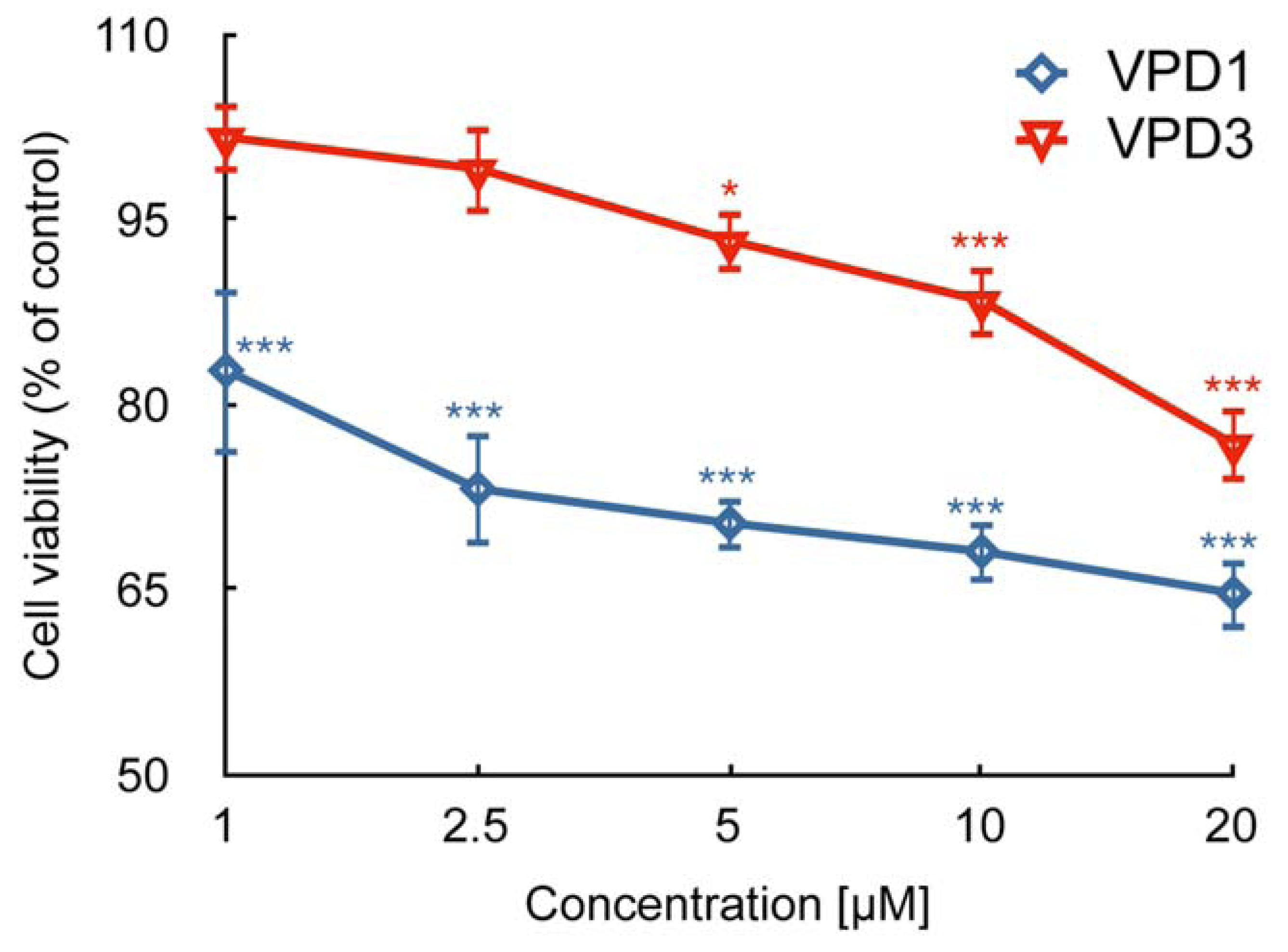
2.1. Cell Viability
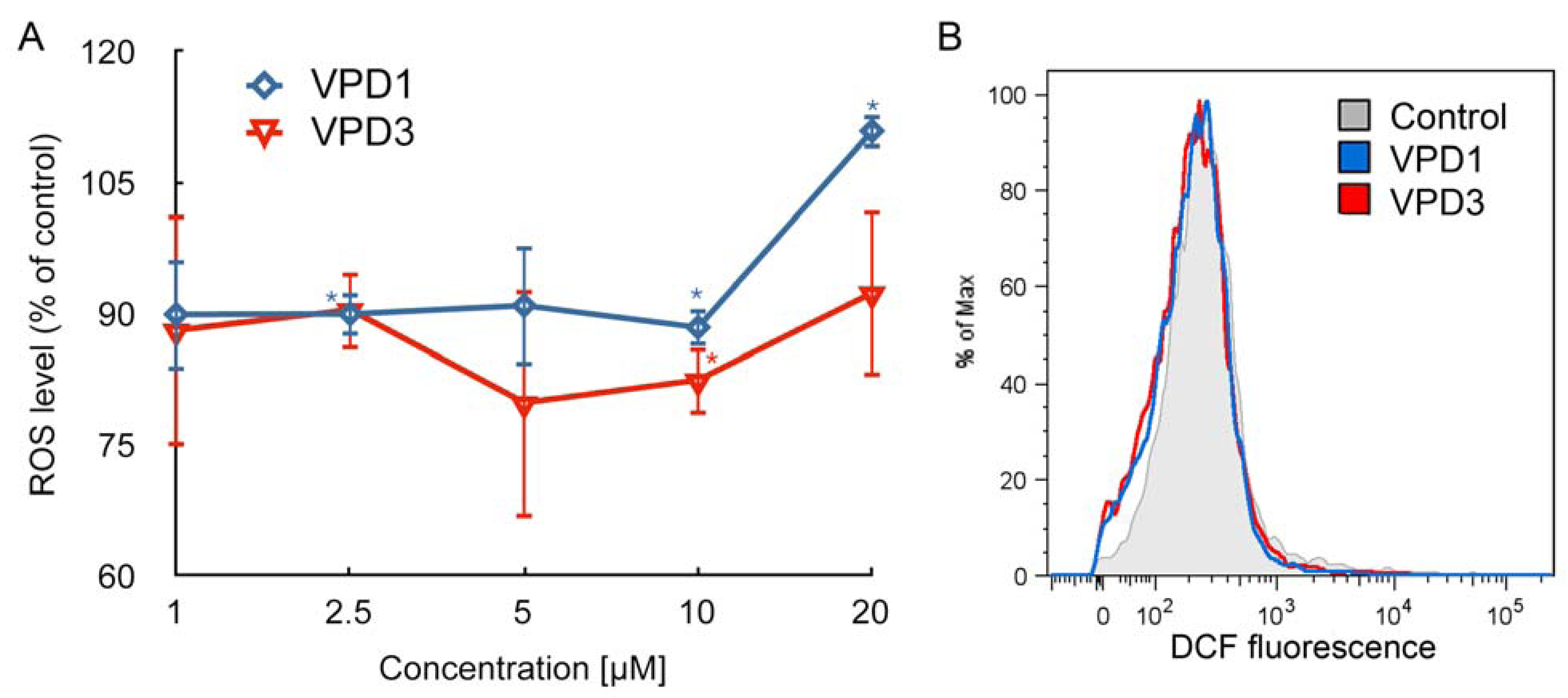
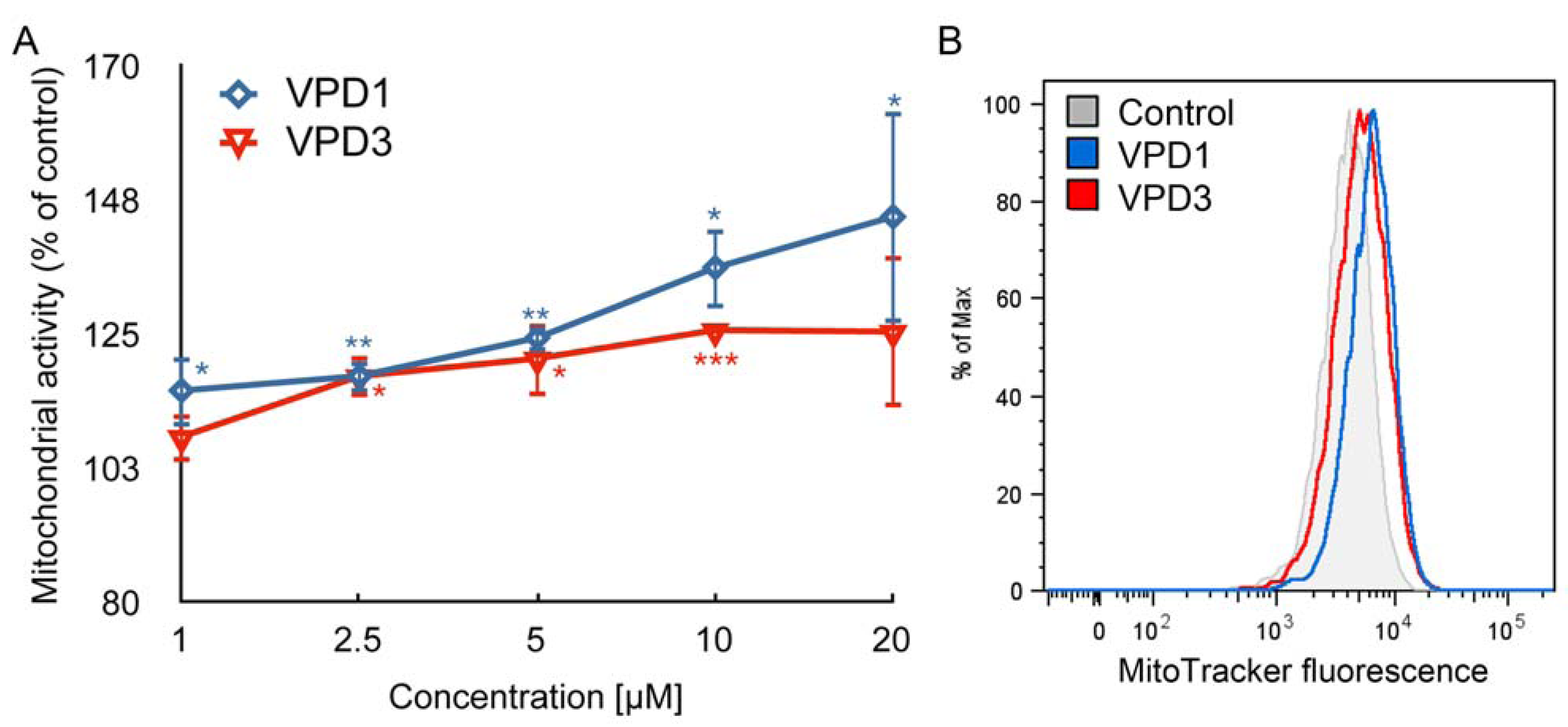
2.2. ROS Formation and Dysfunction of Mitochondria
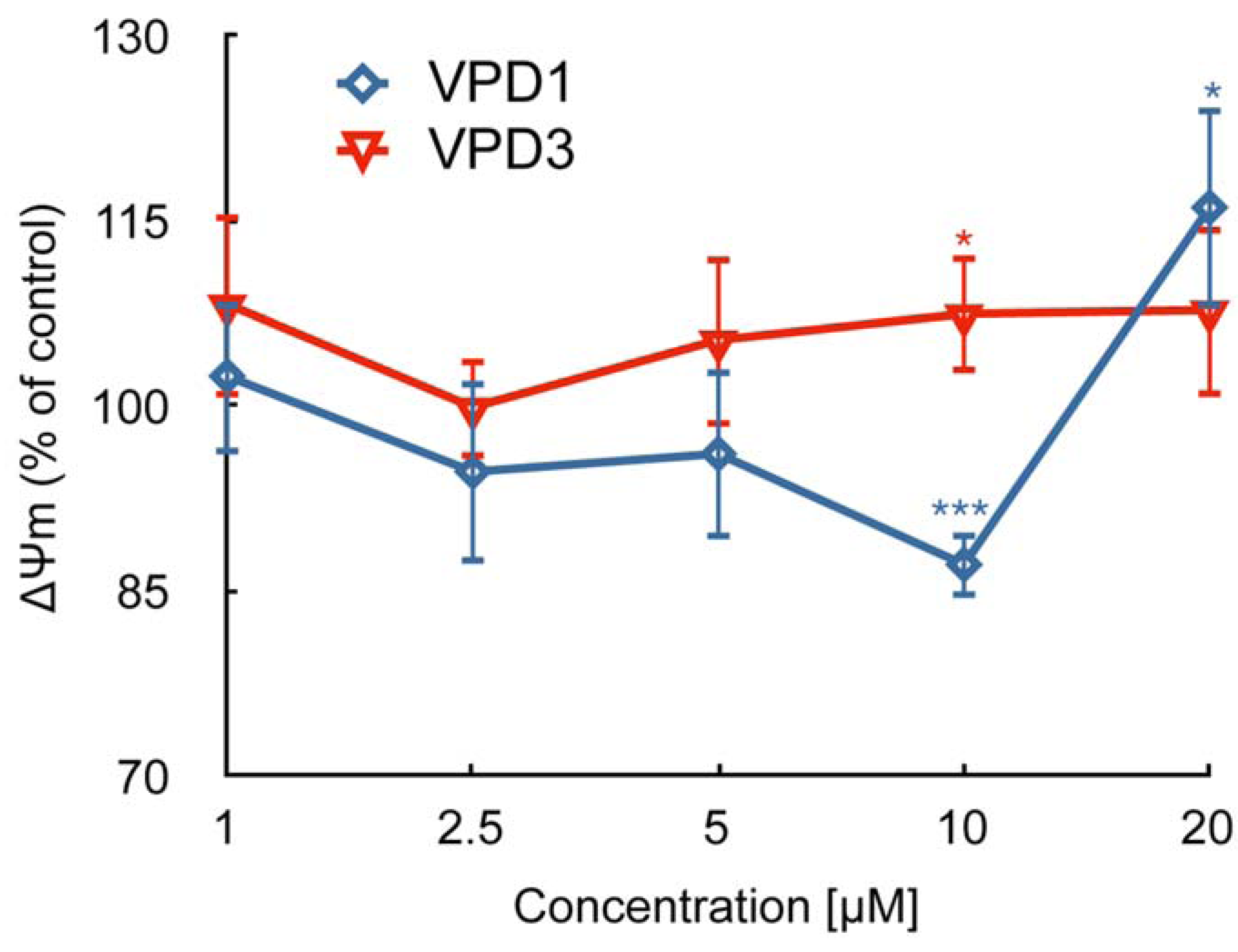
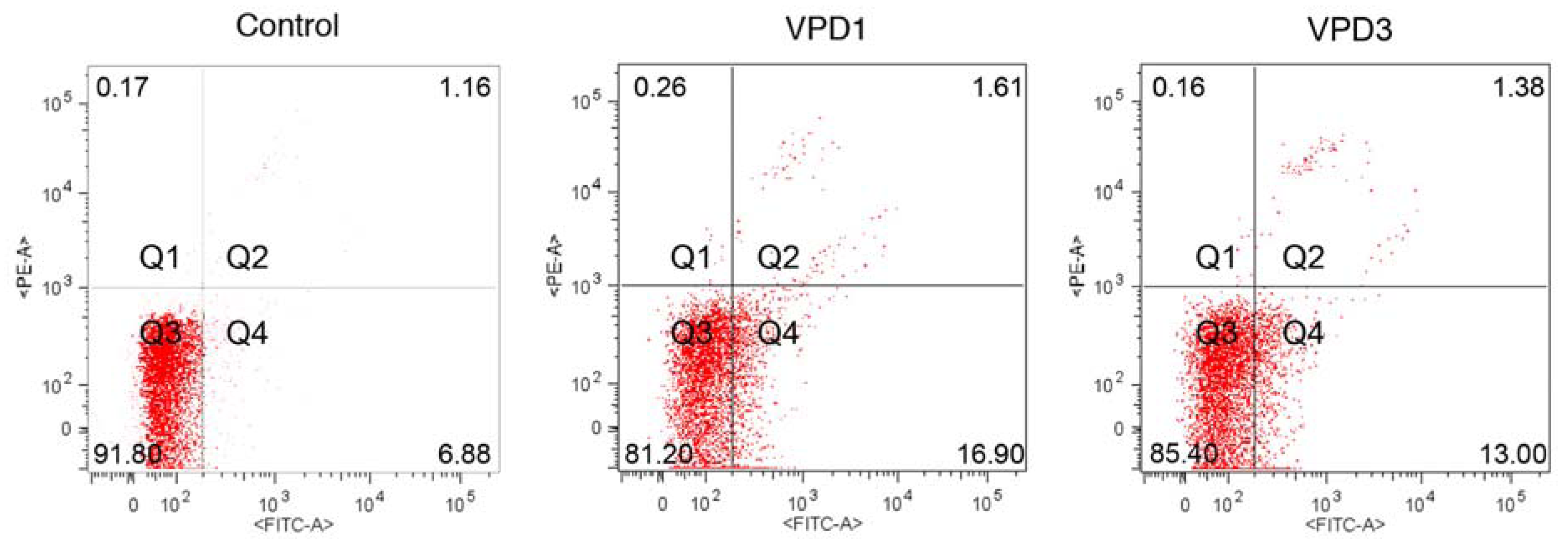
2.3. Cell Death Studies
| Control | VPD1 | |||||
|---|---|---|---|---|---|---|
| C [μM] | 0 | 1 | 2.5 | 5 | 10 | 20 |
| Healthy | 90.06 ± 1.53 | 87.70 ± 2.00 | 88.00 ± 1.06 | 89.37 ± 0.46 | 85.43 ± 0.31 ** | 81.70 ± 1.23 *** |
| Apoptotic | 6.85 ± 0.86 | 9.34 ± 2.10 | 8.27 ± 2.92 | 7.44 ± 0.99 | 11.90 ± 1.01 *** | 13.40 ± 2.40 ** |
| Dead | 2.51 ± 1.29 | 2.91 ± 0.81 | 3.75 ± 2.13 | 2.22 ± 0.57 | 2.77 ± 0.87 | 3.74 ± 1.66 |
| Control | VPD3 | |||||
| C [μM] | 0 | 1 | 2.5 | 5 | 10 | 20 |
| Healthy | 90.06 ± 1.53 | 87.07 ± 2.76 | 86.40 ± 2.87 | 86.57 ± 2.54 * | 87.17 ± 2.02 | 86.50 ± 0.40 ** |
| Apoptotic | 6.85 ± 0.86 | 9.68 ± 0.92 ** | 8.96 ± 0.58 * | 9.49 ± 1.43 * | 8.55 ± 1.36 | 9.42 ± 0.15 ** |
| Dead | 2.51 ± 1.29 | 3.23 ± 1.90 | 5.40 ± 0.85 * | 4.27 ± 0.64 | 4.39 ± 0.48 | 4.05 ± 0.33 |
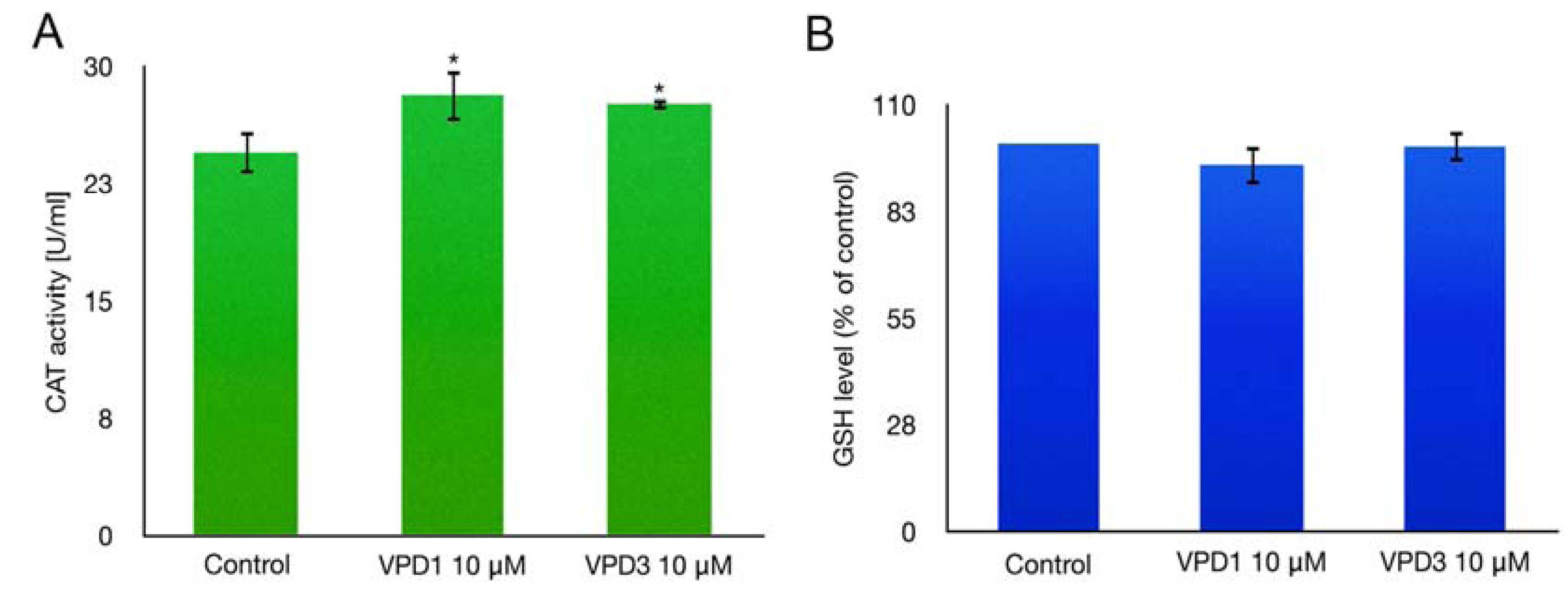
2.4. Catalase (CAT) Activity and GSH Level Measurements
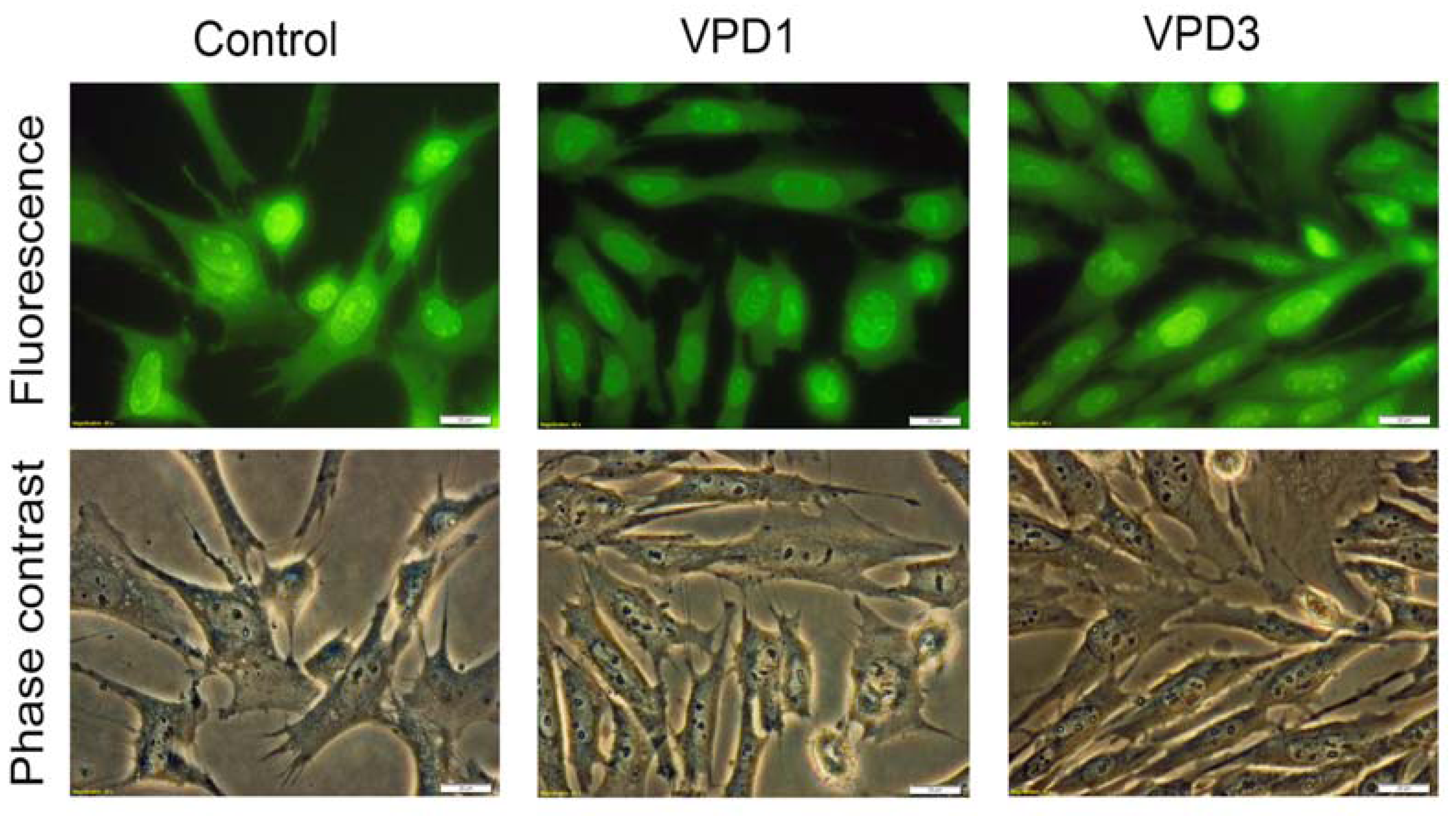
2.5. Analyses of Cell Morphology

2.6. Cell Cycle Analysis
| Control | VPD1 | |||||
|---|---|---|---|---|---|---|
| [%] | 0 | 1 | 2.5 | 5 | 10 | 20 |
| G0/G1 | 51.38 ± 6.25 | 53.19 ± 5,75 | 52.76 ± 3.23 | 50.99 ± 6.24 | 45.62 ± 4.06 | 44.65 ± 5.22 |
| S | 26.33 ± 5.21 | 24.02 ± 4.3 | 26.32 ± 3.58 | 28.28 ± 4.26 | 31.88 ± 6.05 | 36.57 ± 6.34 |
| G2/M | 21.33 ± 1.42 | 22.9 ± 1.23 | 20.56 ± 2.27 | 22.27 ± 2.1 | 22.02 ± 2.20 | 18.62 ± 2.86 |
| Control | VPD3 | |||||
| [%] | 0 | 1 | 2.5 | 5 | 10 | 20 |
| G0/G1 | 51.38 ± 6.25 | 54.07 ± 1.10 | 51.93 ± 2.82 | 50.33 ± 0.99 | 52.50 ± 2.02 | 53.87 ± 4.33 |
| S | 26.33 ± 5.21 | 22.83 ± 0.71 | 24.27 ± 1.10 | 24.67 ± 1.26 | 21.93 ± 0.38 | 21.13 ± 0.84 |
| G2/M | 21.33 ± 1.42 | 22.67 ± 1.18 | 23.00 ± 1.13 | 24.17 ± 2.14 | 24.57 ± 2.89 | 24.7 ± 3.49 |
3. Experimental
3.1. General
3.2. Cell Culture
3.3. Dendrimer Treatment
3.4. Cytotoxicity Assay
3.5. Measurement of Reactive Oxygen Species (ROS)
3.6. Assessment of Oxidative Activity of Mitochondria
3.7. Assessment of Catalase (CAT) Activity
3.8. Assessment of Reduced Glutathione (GSH) Level
3.9. Assessment of Mitochondrial Membrane Potential (ΔΨm)
3.9.1. Determination of Apoptotic and Dead Cells by YO-PRO-1 Iodide/Propidium Iodide (PI) Staining-Flow Cytometry Analyses
3.9.2. Visualisation of Cell Morphology, Apoptosis and Necrosis-Fluorescence/Phase Contrast Microscopy Analysis
3.9.3. Flow Cytometric Analysis of Cell Morphology
3.9.4. Cell Cycle Studies
3.9.5. Statistics
4. Conclusions
Conflicts of Interest
References
- Heinen, S.; Walder, L. Generation-Dependent Intramolecular CT complexation in a dendrimer electron sponge consisting of a viologen skeleton. Angew. Chem. Int. Ed. Engl. 2000, 39, 806–809. [Google Scholar] [CrossRef]
- Ghaddar, T.H.; Wishart, J.F.; Thompson, D.W.; Whitesell, J.K.; Fox, M.A. A dendrimer-based electron antenna: paired electron-transfer reactions in dendrimers with a 4,4'-bipyridine core and naphthalene peripheral groups. J. Am. Chem. Soc. 2002, 124, 8285–8289. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Kaifer, A.E. Preparation, characterization, and electrochemical properties of a new series of hybrid dendrimers containing a viologen core and Frechet and Newkome dendrons. J. Org. Chem. 2008, 73, 5693–5698. [Google Scholar] [CrossRef]
- Asaftei, S.; De Clercq, E. “Viologen” dendrimers as antiviral agents: The effect of charge number and distance. J. Med. Chem. 2010, 53, 3480–3488. [Google Scholar] [CrossRef]
- Asaftei, S.; Huskens, D.; Schols, D. HIV-1 X4 activities of polycationic “viologen” based dendrimers by interaction with the chemokine receptor CXCR4: Study of structure-activity relationship. J. Med. Chem. 2012, 55, 10405–10413. [Google Scholar] [CrossRef]
- Ciepluch, K.; Katir, N.; El Kadib, A.; Felczak, A.; Zawadzka, K.; Weber, M.; Klajnert, B.; Lisowska, K.; Caminade, A.-M.; Bousmina, M.; et al. Biological properties of new viologen-phosphorus dendrimers. Mol. Pharm. 2012, 9, 448–457. [Google Scholar] [CrossRef]
- Bromilow, R.H. Paraquat and sustainable agriculture. Pest. Manag. Sci. 2004, 60, 340–349. [Google Scholar] [CrossRef]
- Tanner, C.M.; Kamel, F.; Ross, G.W.; Hoppin, J.A.; Goldman, S.M.; Korell, M.; Marras, C.; Bhudhikanok, G.S.; Kasten, M.; Chade, A.R.; et al. Rotenone, paraquat, and Parkinson's disease. Environ. Health Perspect. 2011, 119, 866–872. [Google Scholar] [CrossRef]
- Baltazar, T.; Dinis-Oliveira, R.J.; Duarte, J.A.; de Lourdes Bastos, M.; Carvalho, F. Paraquat research: Do recent advances in limiting its toxicity make its use safer? Brit. J. Pharmacol. 2013, 168, 44–45. [Google Scholar] [CrossRef]
- Maksimenko, A.V.; Mandrouguine, V.; Gottikh, M.B.; Bertrand, J.-R.; Majoral, J.-P.; Malvy, C. Optimisation of dendrimer-mediated gene transfer by anionic oligomers. J. Gene. Med. 2003, 5, 61–71. [Google Scholar]
- Krishna, T.R.; Parent, M.; Werts, M.H.V.; Moreaux, L.; Gmouh, S.; Charpak, S.; Caminade, A.-M.; Majoral, J.-P.; Blanchard-Desce, M. Water-soluble dendrimeric two-photon tracers for in vivo imaging. Angew. Chem. Int. Ed. Engl. 2006, 45, 4645–4648. [Google Scholar] [CrossRef]
- Blanzat, M.; Turrin, C.-O.; Aubertin, A.-M.; Couturier-Vidal, C.; Caminade, A.-M.; Majoral, J.-P.; Rico-Lattes, I.; Lattes, A. Dendritic catanionic assemblies: In vitro anti-HIV activity of phosphorus-containing dendrimers bearing galbeta1cer analogues. Chembiochem 2005, 6, 2207–2213. [Google Scholar] [CrossRef]
- Ottaviani, M.F.; Mazzeo, R.; Cangiotti, M.; Fiorani, L.; Majoral, J.-P.; Caminade, A.-M.; Pedziwiatr, E.; Bryszewska, M.; Klajnert, B. Time evolution of the aggregation process of peptides involved in neurodegenerative diseases and preventing aggregation effect of phosphorus dendrimers studied by EPR. Biomacromolecules 2010, 11, 3014–3021. [Google Scholar] [CrossRef]
- Wasiak, T.; Ionov, M.; Nieznanski, K.; Nieznanska, H.; Klementieva, O.; Granell, M.; Cladera, J.; Majoral, J.-P.; Caminade, A.-M.; Klajnert, B. Phosphorus dendrimers affect Alzheimer’s (Aβ1–28) peptide and MAP-Tau protein aggregation. Mol. Pharm. 2012, 9, 458–469. [Google Scholar] [CrossRef]
- Gomulak, P.; Klajnert, B.; Bryszewska, M.; Majoral, J.P.; Caminade, A.M.; Blasiak, J. Cytotoxicity and genotoxicity of cationic phosphorus-containing dendrimers. Curr. Med. Chem. 2012, 19, 6233–6240. [Google Scholar]
- Lazniewska, J.; Milowska, K.; Zablocka, M.; Mignani, S.; Caminade, A.-M.; Majoral, J.-P.; Bryszewska, M.; Gabryelak, T. Mechanism of cationic phosphorus dendrimers toxicity against murine neural cell lines. Mol. Pharm. 2013. [Google Scholar] [CrossRef]
- Lazniewska, J.; Milowska, K.; Katir, N.; El Kadib, A.; Bryszewska, M.; Majoral, J.-P.; Gabryelak, T. Viologen-phosphorus dendrimers exhibit minor toxicity against a murine neuroblastoma cell line. Cell. Mol. Biol. Lett. 2013, 18, 459–478. [Google Scholar] [CrossRef]
- Solassol, J.; Crozet, C.; Perrier, V.; Leclaire, J.; Béranger, F.; Caminade, A.-M.; Meunier, B.; Dormont, D.; Majoral, J.-P.; Lehmann, S. Cationic phosphorus-containing dendrimers reduce prion replication both in cell culture and in mice infected with scrapie. J. Gen. Virol. 2004, 85, 1791–1799. [Google Scholar] [CrossRef]
- Klajnert, B.; Cangiotti, M.; Calici, S.; Majoral, J.P.; Caminade, A.M.; Cladera, J.; Bryszewska, M.; Ottaviani, M.F. EPR study of the interactions between dendrimers and peptides involved in Alzheimer’s and prion diseases. Macromol. Biosci. 2007, 7, 1065–1074. [Google Scholar] [CrossRef]
- Ghaemmaghami, S.; Ullman, J.; Ahn, M.; St Martin, S.; Prusiner, S.B. Chemical induction of misfolded prion protein conformers in cell culture. J. Biol. Chem. 2010, 285, 10415–10423. [Google Scholar] [CrossRef]
- Mignani, S.; Kazzouli, S.E.; Bousmina, M. Dendrimer space concept for innovative nanomedicine: A futuristic vision for medicinal chemistry. Prog. Polym. Sci. 2013, 38, 993–1008. [Google Scholar] [CrossRef]
- Milowska, K.; Grochowina, J.; Katir, N.; El Kadib, A.; Majoral, J.-P.; Bryszewska, M.; Gabryelak, T. Viologen-phosphorus dendrimers inhibit α-synuclein fibrillation. Mol. Pharm. 2013, 10, 1131–1137. [Google Scholar] [CrossRef]
- Ciepluch, K.; Weber, M.; Katir, N.; Caminade, A.-M.; El Kadib, A.; Klajnert, B.; Majoral, J.-P.; Bryszewska, M. Effect of viologen-phosphorus dendrimers on acetylcholinesterase and butyrylcholinesterase activities. Int. J. Biol. Macromol. 2013, 54C, 119–124. [Google Scholar]
- Perez-Martinez, F.C.; Ocana, A.V.; Perez-Carrion, M.D.; Cena, V. Dendrimers as vectors for genetic material delivery to the nervous system. Curr. Med. Chem. 2012, 19, 5101–5108. [Google Scholar] [CrossRef]
- Patel, S.K.; Gajbhiye, V.; Jain, N.K. Synthesis, characterization and brain targeting potential of paclitaxel loaded thiamine-PPI nanoconjugates. J. Drug Targeting 2012, 20, 841–849. [Google Scholar] [CrossRef]
- Albertazzi, L.; Gherardini, L.; Brondi, M.; Sulis Sato, S.; Bifone, A.; Pizzorusso, T.; Ratto, G.M.; Bardi, G. In vivo distribution and toxicity of PAMAM dendrimers in the central nervous system depend on their surface chemistry. Mol. Pharm. 2013, 10, 249–260. [Google Scholar] [CrossRef]
- Dai, H.; Navath, R.S.; Balakrishnan, B.; Guru, B.R.; Mishra, M.K.; Romero, R.; Kannan, R.M.; Kannan, S. Intrinsic targeting of inflammatory cells in the brain by polyamidoamine dendrimers upon subarachnoid administration. Nanomedicine 2010, 5, 1317–1329. [Google Scholar] [CrossRef]
- Iezzi, R.; Guru, B.R.; Glybina, I.V.; Mishra, M.K.; Kennedy, A.; Kannan, R.M. Dendrimer-based targeted intravitreal therapy for sustained attenuation of neuroinflammation in retinal degeneration. Biomaterials 2012, 33, 979–988. [Google Scholar]
- Kannan, S.; Dai, H.; Navath, R.S.; Balakrishnan, B.; Jyoti, A.; Janisse, J.; Romero, R.; Kannan, R.M. Dendrimer-based postnatal therapy for neuroinflammation and cerebral palsy in a rabbit model. Sci. Transl. Med. 2012, 4, 130ra46. [Google Scholar] [CrossRef]
- Gingerich, S.; Kim, G.L.; Chalmers, J.A.; Koletar, M.M.; Wang, X.; Wang, Y.; Belsham, D.D. Estrogen receptor α and G-protein coupled receptor 30 mediate the neuroprotective effects of 17β-estradiol in novel murine hippocampal cell models. Neuroscience 2010, 170, 54–66. [Google Scholar] [CrossRef]
- Majd, S.; Chegini, F.; Chataway, T.; Zhou, X.-F.; Gai, W. Reciprocal induction between α-synuclein and β-amyloid in adult rat neurons. Neurotox. Res. 2013, 23, 69–78. [Google Scholar] [CrossRef]
- Naha, P.C.; Davoren, M.; Lyng, F.M.; Byrne, H.J. Reactive oxygen species (ROS) induced cytokine production and cytotoxicity of PAMAM dendrimers in J774A.1 cells. Toxicol. Appl. Pharmacol. 2010, 246, 91–99. [Google Scholar] [CrossRef]
- Malik, N.; Wiwattanapatapee, R.; Klopsch, R.; Lorenz, K.; Frey, H.; Weener, J.W.; Meijer, E.W.; Paulus, W.; Duncan, R. Dendrimers: relationship between structure and biocompatibility in vitro, and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J. Control. Release 2000, 65, 133–148. [Google Scholar] [CrossRef]
- Stasko, N.A.; Johnson, C.B.; Schoenfisch, M.H.; Johnson, T.A.; Holmuhamedov, E.L. Cytotoxicity of polypropylenimine dendrimer conjugates on cultured endothelial cells. Biomacromolecules 2007, 8, 3853–3859. [Google Scholar] [CrossRef]
- Albertazzi, L.; Serresi, M.; Albanese, A.; Beltram, F. Dendrimer internalization and intracellular trafficking in living cells. Mol. Pharm. 2010, 7, 680–688. [Google Scholar] [CrossRef]
- Hong, S.; Leroueil, P.R.; Janus, E.K.; Peters, J.L.; Kober, M.-M.; Islam, M.T.; Orr, B.G.; Baker, J.R.; Banaszak Holl, M.M. Interaction of polycationic polymers with supported lipid bilayers and cells: Nanoscale hole formation and enhanced membrane permeability. Bioconjugate Chem. 2006, 17, 728–734. [Google Scholar] [CrossRef]
- Leroueil, P.R.; Hong, S.; Mecke, A.; Baker, J.R.; Orr, B.G.; Banaszak Holl, M.M. Nanoparticle interaction with biological membranes: does nanotechnology present a Janus face? Acc. Chem. Res. 2007, 40, 335–342. [Google Scholar] [CrossRef]
- Albertazzi, L.; Fernandez-Villamarin, M.; Riguera, R.; Fernandez-Megia, E. Peripheral functionalization of dendrimers regulates internalization and intracellular trafficking in living cells. Bioconjugate Chem. 2012, 23, 1059–1068. [Google Scholar] [CrossRef]
- Mathers, J.; Fraser, J.A.; McMahon, M.; Saunders, R.D.C.; Hayes, J.D.; McLellan, L.I. Antioxidant and cytoprotective responses to redox stress. Biochem. Soc. Symp. 2004, 71, 157–176. [Google Scholar]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef]
- Wang, W.; Xiong, W.; Wan, J.; Sun, X.; Xu, H.; Yang, X. The decrease of PAMAM dendrimer-induced cytotoxicity by PEGylation via attenuation of oxidative stress. Nanotechnology 2009, 20, 105103. [Google Scholar] [CrossRef]
- Kuo, J.-H.S.; Jan, M.-S.; Lin, Y.-L. Interactions between U-937 human macrophages and poly(propyleneimine) dendrimers. J. Control. Release 2007, 120, 51–59. [Google Scholar] [CrossRef]
- Janaszewska, A.; Mączyńska, K.; Matuszko, G.; Appelhans, D.; Voit, B.; Klajnert, B.; Bryszewska, M. Cytotoxicity of PAMAM, PPI and maltose modified PPI dendrimers in Chinese hamster ovary (CHO) and human ovarian carcinoma (SKOV3) cells. New J. Chem. 2012, 36, 428–437. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Lyng, F.M.; Garcia, A.; Davoren, M.; Byrne, H.J. Mechanistic studies of in vitro cytotoxicity of poly(amidoamine) dendrimers in mammalian cells. Toxicol. Appl. Pharmacol. 2010, 248, 259–268. [Google Scholar] [CrossRef]
- Mukherjee, S.P.; Byrne, H.J. Polyamidoamine dendrimer nanoparticle cytotoxicity, oxidative stress, caspase activation and inflammatory response: experimental observation and numerical simulation. Nanomed. Nanotech. Biol. Med. 2012, 9, 202–211. [Google Scholar] [CrossRef]
- Simon, H.-U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar]
- Giovannini, C.; Matarrese, P.; Scazzocchio, B.; Sanchez, M.; Masella, R.; Malorni, W. Mitochondria hyperpolarization is an early event in oxidized low-density lipoprotein-induced apoptosis in Caco-2 intestinal cells. FEBS Lett. 2002, 523, 200–206. [Google Scholar] [CrossRef]
- Lee, J.-H.; Cha, K.E.; Kim, M.S.; Hong, H.W.; Chung, D.J.; Ryu, G.; Myung, H. Nanosized polyamidoamine (PAMAM) dendrimer-induced apoptosis mediated by mitochondrial dysfunction. Toxicol. Lett. 2009, 190, 202–207. [Google Scholar] [CrossRef]
- Thomas, T.P.; Majoros, I; Kotlyar, A.; Mullen, D; Banaszak Holl, M.M.; Baker, J.R. Cationic poly(amidoamine) dendrimer induces lysosomal apoptotic pathway at therapeutically relevant concentrations. Biomacromolecules 2009, 10, 3207–3214. [Google Scholar] [CrossRef]
- Kitchens, K.M.; Kolhatkar, R.B.; Swaan, P.W.; Ghandehari, H. Endocytosis inhibitors prevent poly(amidoamine) dendrimer internalization and permeability across Caco-2 cells. Mol. Pharm. 2008, 5, 364–369. [Google Scholar] [CrossRef]
- Parimi, S.; Barnes, T.J.; Callen, D.F.; Prestidge, C.A. Mechanistic insight into cell growth, internalization, and cytotoxicity of PAMAM dendrimers. Biomacromolecules 2010, 11, 382–389. [Google Scholar] [CrossRef]
- Klajnert, B.; Sadowska, M.; Bryszewska, M. The effect of polyamidoamine dendrimers on human erythrocyte membrane acetylcholinesterase activity. Bioelectrochemistry 2004, 65, 23–26. [Google Scholar] [CrossRef]
- Ciolkowski, M.; Rozanek, M.; Szewczyk, M.; Klajnert, B.; Bryszewska, M. The influence of PAMAM-OH dendrimers on the activity of human erythrocytes ATPases. Biochim. Biophys. Acta 2011, 1808, 2714–2723. [Google Scholar] [CrossRef]
- Ciolkowski, M.; Rozanek, M.; Bryszewska, M.; Klajnert, B. The influence of PAMAM dendrimers surface groups on their interaction with porcine pepsin. Biochim. Biophys. Acta 2013, 1834, 1982–1987. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Bernas, T.; Dobrucki, J. Mitochondrial and nonmitochondrial reduction of MTT: interaction of MTT with TMRE, JC-1, and NAO mitochondrial fluorescent probes. Cytometry 2002, 47, 236–242. [Google Scholar] [CrossRef]
- Janaszewska, A.; Ciolkowski, M.; Wróbel, D.; Petersen, J.F.; Ficker, M.; Christensen, J.B.; Bryszewska, M.; Klajnert, B. Modified PAMAM dendrimer with 4-carbomethoxypyrrolidone surface groups reveals negligible toxicity against three rodent cell-lines. Nanomed. Nanotech. Biol. Med. 2013, 9, 461–464. [Google Scholar] [CrossRef]
- Bartosz, G. Use of spectroscopic probes for detection of reactive oxygen species. Clin. Chim. Acta 2006, 368, 53–76. [Google Scholar] [CrossRef]
- Agnello, M.; Morici, G.; Rinaldi, A.M. A method for measuring mitochondrial mass and activity. Cytotechnology 2008, 56, 145–149. [Google Scholar] [CrossRef]
- Salvioli, S.; Ardizzoni, A.; Franceschi, C.; Cossarizza, A. JC-1, but not DiOC6(3) or rhodamine 123, is a reliable fluorescent probe to assess delta psi changes in intact cells: implications for studies on mitochondrial functionality during apoptosis. FEBS Lett. 1997, 411, 77–82. [Google Scholar] [CrossRef]
- Michałowicz, J.; Sicińska, P. Chlorophenols and chlorocatechols induce apoptosis in human lymphocytes (in vitro). Toxicol. Lett. 2009, 191, 246–252. [Google Scholar] [CrossRef]
- Ribble, D.; Goldstein, N.B.; Norris, D.A.; Shellman, Y.G. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol. 2005, 5, 12. [Google Scholar]
- Chang, K.-L.; Kung, M.-L.; Chow, N.-H.; Su, S.-J. Genistein arrests hepatoma cells at G2/M phase: involvement of ATM activation and upregulation of p21waf1/cip1 and Wee1. Biochem. Pharmacol. 2004, 67, 717–726. [Google Scholar] [CrossRef]
- Milowska, K.; Grochowina, J.; Katir, N.; El Kadib, A.; Majoral, J.-P.; Bryszewska, M.; Gabryelak, T. Interaction between viologen-phosphorus dendrimers and α-synuclein. J. Luminesc. 2013, 134, 132–137. [Google Scholar] [CrossRef]
- Milowska, K.; Gabryelak, T.; Bryszewska, M.; Caminade, A.-M.; Majoral, J.-P. Phosphorus-containing dendrimers against α-synuclein fibril formation. Int. J. Biol. Macromol. 2012, 50, 1138–1143. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds (VPD1 and VPD3) are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lazniewska, J.; Janaszewska, A.; Miłowska, K.; Caminade, A.-M.; Mignani, S.; Katir, N.; Kadib, A.E.; Bryszewska, M.; Majoral, J.-P.; Gabryelak, T.; et al. Promising Low-Toxicity of Viologen-Phosphorus Dendrimers against Embryonic Mouse Hippocampal Cells. Molecules 2013, 18, 12222-12240. https://doi.org/10.3390/molecules181012222
Lazniewska J, Janaszewska A, Miłowska K, Caminade A-M, Mignani S, Katir N, Kadib AE, Bryszewska M, Majoral J-P, Gabryelak T, et al. Promising Low-Toxicity of Viologen-Phosphorus Dendrimers against Embryonic Mouse Hippocampal Cells. Molecules. 2013; 18(10):12222-12240. https://doi.org/10.3390/molecules181012222
Chicago/Turabian StyleLazniewska, Joanna, Anna Janaszewska, Katarzyna Miłowska, Anne-Marie Caminade, Serge Mignani, Nadia Katir, Abdelkrim El Kadib, Maria Bryszewska, Jean-Pierre Majoral, Teresa Gabryelak, and et al. 2013. "Promising Low-Toxicity of Viologen-Phosphorus Dendrimers against Embryonic Mouse Hippocampal Cells" Molecules 18, no. 10: 12222-12240. https://doi.org/10.3390/molecules181012222
APA StyleLazniewska, J., Janaszewska, A., Miłowska, K., Caminade, A.-M., Mignani, S., Katir, N., Kadib, A. E., Bryszewska, M., Majoral, J.-P., Gabryelak, T., & Klajnert-Maculewicz, B. (2013). Promising Low-Toxicity of Viologen-Phosphorus Dendrimers against Embryonic Mouse Hippocampal Cells. Molecules, 18(10), 12222-12240. https://doi.org/10.3390/molecules181012222