Brønsted Acidic Ionic Liquid Accelerated Halogenation of Organic Compounds with N-Halosuccinimides (NXS)
Abstract
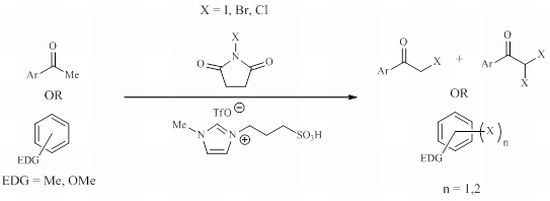
:1. Introduction
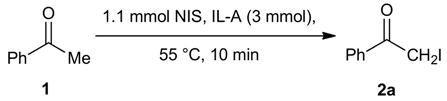
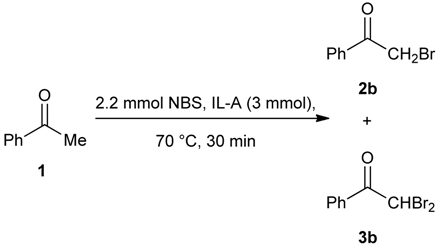
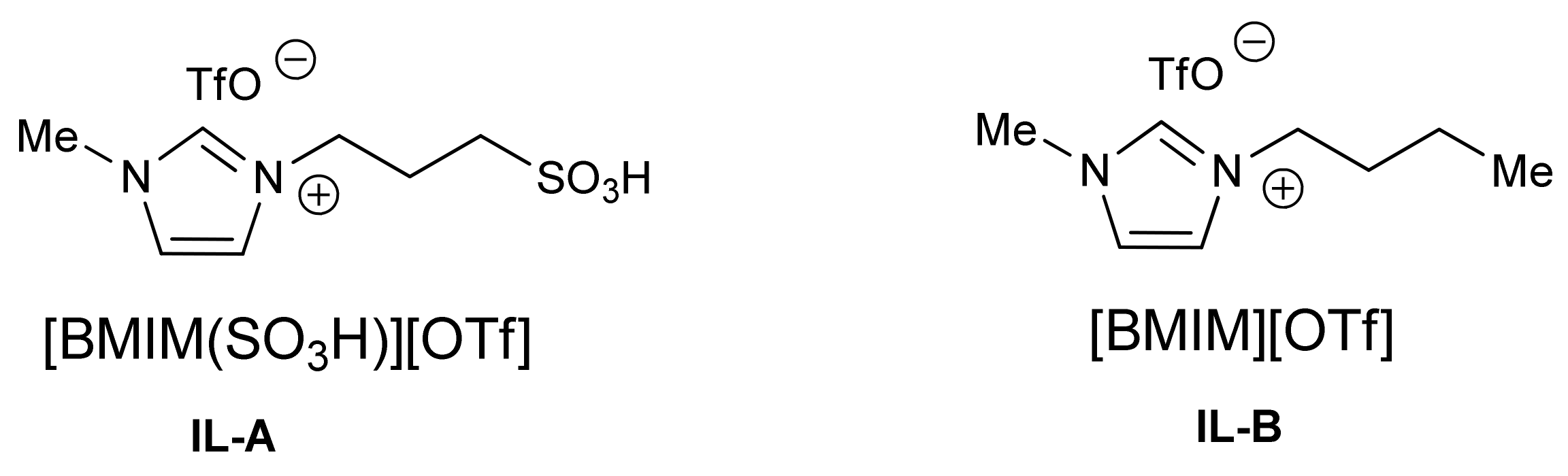
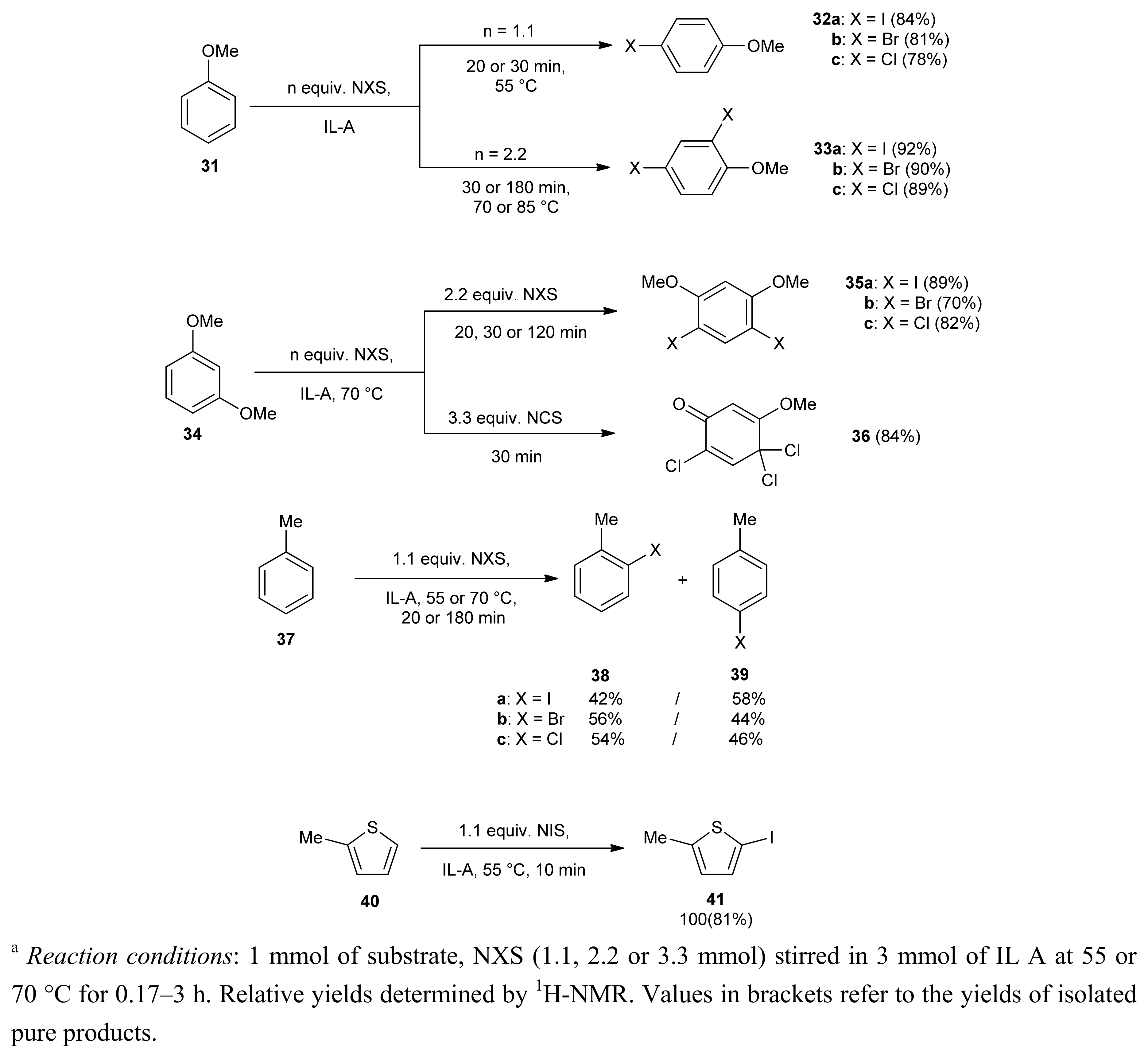
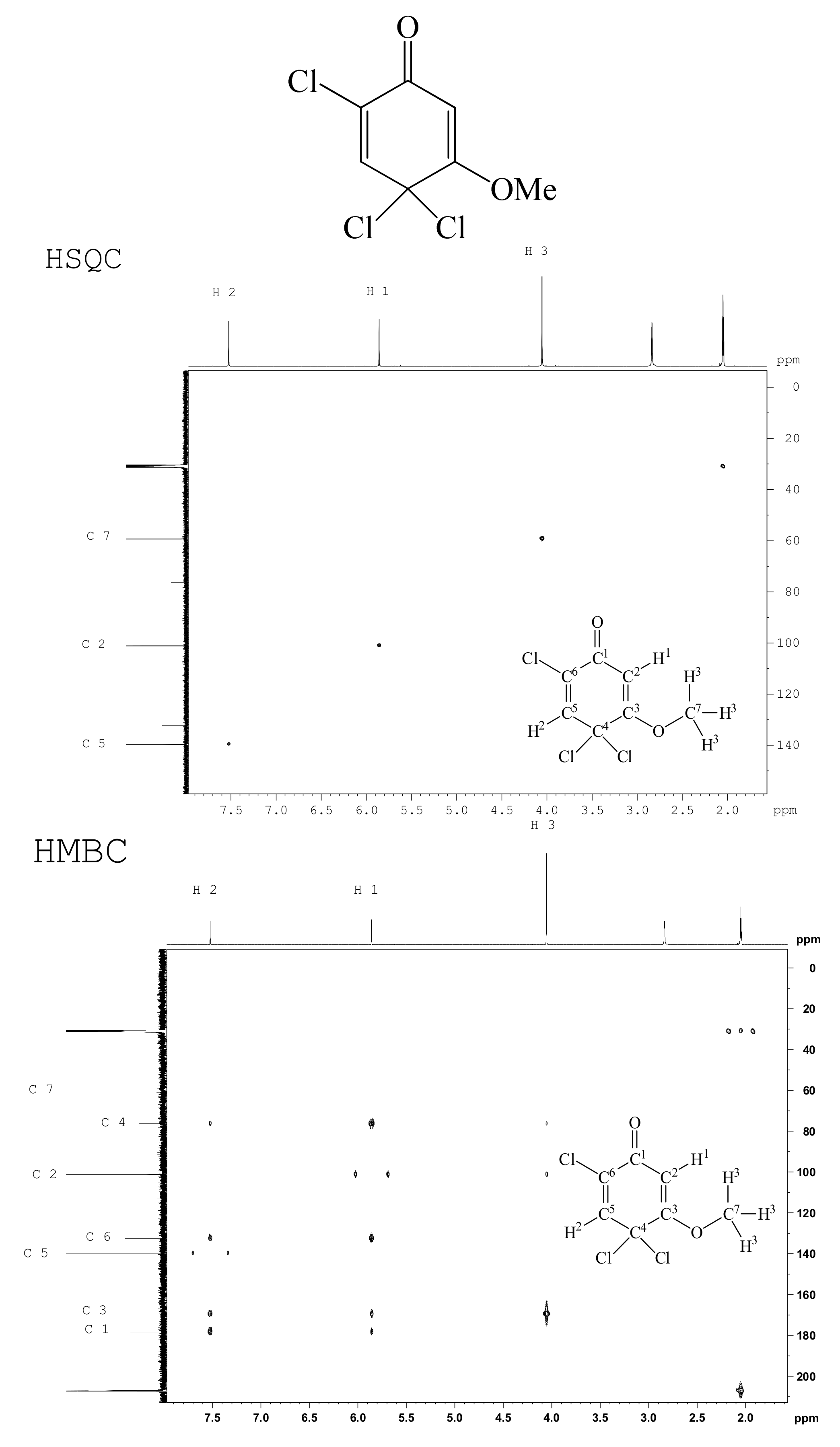
2. Results and Discussion
3. Experimental
3.1. General
3.2. Representative Procedure for Halogenation of Aromatic Compounds
3.3. Scale up Experiment
3.4. Recycling/Reuse of IL-A in Iodination of Acetophenone (1) with NIS
4. Conclusions
Acknowledgments
References
- French, A.N.; Bissmire, D.; Wirth, T. Iodine electrophiles in stereoselective reactions: ecent developments and synthetic applications. Chem. Soc. Rev. 2004, 33, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Stavber, S.; Jereb, M.; Zupan, M. Electrophilic Iodination of Organic Compounds Using Elemental Iodine or Iodides. Synthesis 2008, 1487–1513. [Google Scholar] [CrossRef]
- Podgoršek, A.; Zupan, M.; Iskra, J. Oxidative Halogenation with “Green” Oxidants: Oxygen and Hydrogen Peroxide. Angew. Chem. Int. Ed. 2009, 48, 8424–8450. [Google Scholar] [CrossRef] [PubMed]
- Zhdankin, V.V.; Stang, P.J. Chemistry of Polyvalent Iodine. Chem. Rev. 2008, 108, 5299–5358. [Google Scholar] [CrossRef] [PubMed]
- Golebiewski, W.M.; Gucma, M. Applications of N-Chlorosuccinimide in Organic Synthesis. Synthesis 2007, 3599–3619. [Google Scholar] [CrossRef]
- Tanemura, K.; Suzuki, T.; Nishida, Y.; Satsumabayashi, K.; Horaguchi, T. Halogenation of Aromatic Compounds by N-chloro-, N-bromo-, and N-iodosuccinimide. Chem. Lett. 2003, 32, 932–933. [Google Scholar] [CrossRef]
- Pravst, I.; Zupan, M.; Stavber, S. Halogenation of ketones with N-halosuccinimides under solvent-free reaction conditions. Tetrahedron 2008, 64, 5191–5199. [Google Scholar] [CrossRef]
- Bucos, M.; Villalonga-Barber, C.; Micha-Screttas, M.; Steele, B.R.; Screttas, C.G.; Heropoulos, G.A. Microwave assisted solid additive effects in simple dry chlorination reactions with n-chlorosuccinimide. Tetrahedron 2010, 66, 2061–2065. [Google Scholar] [CrossRef]
- Mo, F.; Yan, J.M.; Qiu, D.; Li, F.; Zhang, Y.; Wang, J. Gold-Catalyzed Halogenation of Aromatics by N-Halosuccinimides. Angew. Chem. Int. Ed. 2010, 49, 2028–2032. [Google Scholar] [CrossRef] [PubMed]
- Borodkin, G.I.; Shubin, V.G. Electrophilic Reactions of Aromatic and Heteroaromatic Compounds in Ionic Liquids. Russ. J. Org. Chem. 2006, 42, 1745–1770. [Google Scholar] [CrossRef]
- Pavlinac, J.; Zupan, M.; Laali, K.K.; Stavber, S. Halogenation of organic compounds in ionic liquids. Tetrahedron 2009, 65, 5625–5662. [Google Scholar] [CrossRef]
- Kumar, V.; Yap, J.; Muroyama, A.; Malhotra, S.V. Highly Efficient Method for C-5 Halogenation of Pyrimidine-Based Nucleosides in Ionic Liquids. Synthesis 2009, 3957–3962. [Google Scholar]
- Laali, K.K.; Borodkin, G.I. First application of ionic liquids in electrophilic fluorination of arenes; SelectfluorTM (F-TEDA-BF4) for “green” fluorination. J. Chem. Soc. Perkin Trans. 2002, 2, 953–957. [Google Scholar] [CrossRef]
- Hubbard, A.; Okazaki, T.; Laali, K.K. Chlorination of Aromatics with Trichloroisocyanuric Acid (TCICA) in Brønsted-Acidic Imidazolium Ionic liquid [BMIM(SO3H)][OTf]: An Economical, Green Protocol for the Synthesis of Chloroarenes. Aust. J. Chem. 2007, 60, 923–927. [Google Scholar] [CrossRef]
- Chiappe, C.; Leandri, E.; Tebano, M. [Hmim][NO3]—An efficient solvent and promoter in the oxidative aromatic chlorination. Green Chem. 2006, 8, 742–745. [Google Scholar] [CrossRef]
- Chiappe, C.; Sanzone, A. Using the ‘Chemical Tunability’ of Ionic Liquids to Increase Sustainability in the Electrophilic Bromination of Unsaturated Compounds. Synthesis 2011, 2392–2396. [Google Scholar] [CrossRef]
- Borikar, S.P.; Daniel, T.; Paul, V. An efficient, rapid, and regioselective bromination of anilines and phenols with 1-butyl-3-methylpyridinium tribromide as a new reagent/solvent under mild conditions. Tetrahedron Lett. 2009, 50, 1007–1009. [Google Scholar] [CrossRef]
- Salazar, J.; Dorta, R. Pentylpyridinium Tribromide: A Vapor Pressure Free Room Temperature Ionic Liquid Analogue of Bromine. Synlett 2004, 1318–1320. [Google Scholar] [CrossRef]
- Borikar, S.P.; Daniel, T. Aromatic Bromination of Aldehydes and Ketones Using 1,3-Di-n-butylimidazolium Tribromide [BBIm]Br3 Ionic Liquids under Solvent-Free Conditions. J. Iran. Chem. Soc. 2011, 8, 531–536. [Google Scholar] [CrossRef]
- Pavlinac, J.; Laali, K.K.; Zupan, M.; Stavber, S. Iodination of Organic Compounds with Elemental Iodine in the Presence of Hydrogen Peroxide in Ionic Liquid Media. Aust. J. Chem. 2008, 61, 946–955. [Google Scholar] [CrossRef]
- Lee, J.C.; Kim, J.; Park, H.J.; Kwang, B.; Lee, S.B. Direct Metal-free α-Iodination of Arylketones Induced by Iodine or Iodomethane with HTIB in Ionic Liquid. Bull. Korean Chem. Soc. 2010, 31, 1385–1386. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Rafiee, F.; Ruoho, A. Efficient and Selective Iodination of Benzylic Alcohols Using NaI/Brønsted Ionic Liquid System at Room Temperature. Synth. Commun. 2011, 41, 603–611. [Google Scholar] [CrossRef]
- Bailey, L.; Handy, S.T. Aromatic iodination using N-iodosaccharin in room temperature ionic liquids. Tetrahedron Lett. 2011, 52, 2413–2414. [Google Scholar] [CrossRef]
- Cole, A.C.; Jensen, J.L.; Ntai, I.; Tran, K.L.T.; Weaver, K.J.; Forbes, D.C.; Davis, J.H., Jr. Novel Brønsted Acidic Ionic Liquids and Their Use as Dual Solvent–Catalysts. J. Am. Chem. Soc. 2002, 124, 5962–5963. [Google Scholar] [CrossRef] [PubMed]
- Kore, R.; Srivastava, R. Influence of –SO3H functionalization (N-SO3H or N-R-SO3H, where R = alkyl/benzyl) on the activity of Brönsted acidic ionic liquids in the hydration reaction. Tetrahedron Lett. 2012, 53, 3245–3249. [Google Scholar] [CrossRef]
- Akbari, J.; Heydari, A. A sulfonic acid functionalized ionic liquid as a homogeneous and recyclable catalyst for the one-pot synthesis of α-aminophosphonates. Tetrahedron Lett. 2009, 50, 4236–4238. [Google Scholar] [CrossRef]
- Garima; Srivastava, V.P.; Yadav, L.D.S. Direct sulfonylation of Baylis-Hillman alcohols and diarylmethanols with TosMIC in ionic liquid-[Hmim]HSO4: an unexpected reaction. Tetrahedron Lett. 2011, 52, 4622–4626. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Ghayeb, Y.; Sheikhan, N.; Ruoho, A.E. Brønsted acidic ionic liquid as an efficient and reusable catalyst for one-pot synthesis of 1-amidoalkyl 2-naphthols under solvent-free conditions. Tetrahedron Lett. 2009, 50, 5649–5651. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, Q.; Su, W. A novel sulfonic acid functionalized ionic liquid catalyzed multicomponent synthesis of 10,11-dihydrochromeno[4,3-b]chromene-6,8(7H,9H)-dione derivatives in water. Tetrahedron Lett. 2011, 52, 2601–2604. [Google Scholar] [CrossRef]
- Chen, X.; Liu, R.; Xu, Y.; Zou, G. Tunable protic ionic liquids as solvent-catalysts for improved synthesis of multiply substituted 1,2,4-triazoles from oxadiazoles and organoamines. Tetrahedron 2012, 68, 4813–4819. [Google Scholar] [CrossRef]
- Laali, K.K.; Gettwert, V.J. Electrophilic Nitration of Aromatics in Ionic Liquid Solvents. J. Org. Chem. 2001, 66, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Sarca, V.D.; Laali, K.K. Triflic acid-promoted transacylation and deacylation reactions in ionic liquid solvents. Green Chem. 2004, 6, 245–248. [Google Scholar] [CrossRef]
- Laali, K.K.; Sarca, V.D.; Okazaki, T.; Brock, A.; Der, P. Triflic acid-catalyzed adamantylation in [BMIM][OTf] ionic liquid; synthetic scope and mechanistic insight. Org. Biomol. Chem. 2005, 3, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Sarca, V.D.; Laali, K.K. Facile benzylation of aromatics in ionic liquid solvents promoted by TfOH, Sc(OTf)3, and Yb(OTf)3·xH2O; New life for a classic transformation. Green Chem. 2006, 8, 615–620. [Google Scholar] [CrossRef]
- Laali, K.K.; Okazaki, T.; Bunge, S.D. N-(Trifluoromethylsulfonyl)aryloxytrifluoromethyl-sulfoximines [ArO-SO(CF3)=NTf] and N-Aryltriflimides Ar-N(Tf)2 by Thermal and Photolytic Dediazoniation of [ArN2][BF4] in [BMIM][Tf2N] Ionic Liquid: Exploiting the Ambident Nucleophilic Character of a “Nonnucleophilic” Anion. J. Org. Chem. 2007, 72, 6758–6762. [Google Scholar] [PubMed]
- Hubbard, A.; Okazaki, T.; Laali, K.K. Halo- and Azidodediazoniation of Arenediazonium Tetrafluoroborates with Trimethylsilyl Halides and Trimethylsilyl Azide and Sandmeyer-Type Bromodediazoniation with Cu(I)Br in [BMIM][PF6] Ionic Liquid. J. Org. Chem. 2008, 73, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Kalkhambkar, R.G.; Laali, K.K. Arenediazonium salts immobilized in imidazolium ionic liquids as electrophilic partners in the Pd(OAc)2-catalyzed Matsuda–Heck arylation. Tetrahedron Lett. 2011, 52, 1733–1737. [Google Scholar] [CrossRef]
- Kalkhambkar, R.G.; Laali, K.K. Pd(OAc)2-catalyzed cross-coupling of polyfluoroarenes with simple aromatics in imidazolium ionic liquids (ILs) without oxidant and additive and with recycling/reuse of the IL. Tetrahedron Lett. 2011, 52, 5525–5529. [Google Scholar] [CrossRef]
- Aridoss, G.; Laali, K.K. Condensation of propargylic alcohols with 1,3-dicarbonyl compounds and 4-hydroxycoumarins in ionic liquids (ILs). Tetrahedron Lett. 2011, 52, 6859–6864. [Google Scholar] [CrossRef]
- Aridoss, G.; Sarca, V.D.; Ponder, J.F., Jr.; Crowe, J.; Laali, K.K. Electrophilic chemistry of propargylic alcohols in imidazolium ionic liquids: Propargylation of arenes and synthesis of propargylic ethers catalyzed by metallic triflates [Bi(OTf)3, Sc(OTf)3, Yb(OTf)3], TfOH, or B(C6F5)3. Org. Biomol. Chem. 2011, 9, 2518–2529. [Google Scholar] [CrossRef] [PubMed]
- Aridoss, G.; Laali, K.K. Ethylammonium Nitrate (EAN)/Tf2O and EAN/TFAA: Ionic Liquid Based Systems for Aromatic Nitration. J. Org. Chem. 2011, 76, 8088–8094. [Google Scholar] [CrossRef] [PubMed]
- Aridoss, G.; Laali, K.K. Highly Efficient Synthesis of 5-Substituted 1H-Tetrazoles Catalyzed by Cu–Zn Alloy Nanopowder, Conversion into 1,5- and 2,5-Disubstituted Tetrazoles, and Synthesis and NMR Studies of New Tetrazolium Ionic Liquids. Eur. J. Org. Chem. 2011, 6343–6355. [Google Scholar] [CrossRef]
- Kumar, G.G.K.S.N.; Aridoss, G.; Laali, K.K. Condensation of propargylic alcohols with indoles and carbazole in [bmim][PF6]/Bi(NO3)3·5H2O: a simple high yielding propargylation method with recycling and reuse of the ionic liquid. Tetrahedron Lett. 2012, 53, 3066–3069. [Google Scholar] [CrossRef]
- Eberson, L.; Hatshorn, M.P.; Radner, F.; Persson, O. Radical cation mechanism of aromatic halogenation by halogens or iodine chloride in 1,1,1,3,3,3-hexafluoropropan-2-ol. J. Chem. Soc. Perkin Trans. 1998, 2, 59–70. [Google Scholar] [CrossRef]
- Fabbrini, M.; Galli, C.; Gentili, P.; Macchitella, D.; Petride, H. Aromatic iodination: a new investigation on the nature of the mechanism. J. Chem. Soc. Perkin Trans. 2001, 2, 1516–1521. [Google Scholar] [CrossRef]
- Fokin, A.A.; Schreiner, P.R.; Gunchenko, P.A.; Peleshanko, S.A.; Shubina, T.E.; Isaev, S.D.; Tarasenko, P.V.; Kulik, N.I.; Schiebel, H.-M.; Yurchenko, A.G. Oxidative Single-Electron Transfer Activation of σ-Bonds in Aliphatic Halogenation Reactions. J. Am. Chem. Soc. 2000, 122, 7317–7326. [Google Scholar] [CrossRef]
- Vasilyev, A.V.; Lindeman, S.V.; Kochi, J.K. Molecular structures of the metastable charge-transfer complexes of benzene (and toluene) with bromine as the pre-reactive intermediates in electrophilic aromatic bromination. New J. Chem. 2002, 26, 582–592. [Google Scholar] [CrossRef]
- Stavber, G.; Iskra, J.; Zupan, M.; Stavber, S. Aerobic Oxidative Iodination of Organic Compounds with Iodide Catalyzed by Sodium Nitrite. Adv. Synth. Catal. 2008, 350, 2921–2929. [Google Scholar] [CrossRef]
- Podgoršek, A.; Stavber, S.; Zupan, M.; Iskra, J. Bromination of ketones with H2O2–HBr “on water”. Green Chem. 2007, 9, 1212–1218. [Google Scholar] [CrossRef]
- Ram, R.N.; Manoj, T.P. Copper(I)-Promoted Synthesis of Chloromethyl Ketones from Trichloromethyl Carbinols. J. Org. Chem. 2008, 73, 5633–5635. [Google Scholar] [CrossRef] [PubMed]
- Kajigaeshi, S.; Kakinami, T.; Tokiyama, H.; Hirakawa, T.; Okamoto, T. Synthesis of Dibromoacetyl Derivatives by Use of Benzyltrimethylammonium Tribromide. Bull. Chem. Soc. Jpn. 1987, 60, 2667–2668. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, B.; Cai, H.; Zhu, W.; Zou, X. Simple and efficient methods for selective preparation of α-mono or α,α-dichloro ketones and β-ketoesters by using DCDMH. Green Chem. 2009, 11, 275–278. [Google Scholar] [CrossRef]
- Okamoto, T.; Kakinami, T.; Nishimura, T.; Hermawan, I.; Kajigaeshi, S. Preparation of Aromatic Iodoacetyl Derivatives by Direct Iodination with a Potassium Iodide-Potassium Iodate-Sulfuric Acid System. Bull. Chem. Soc. Jpn. 1992, 65, 1731–1733. [Google Scholar] [CrossRef]
- Arcoria, A.; Fisichella, S.; Maccarone, E.; Scarlata, G. Reactions of Triethyl Phosphite with 2-Haloacetyl-furan, -thiophene, -pyrrole and -N-methylpyrrole. J. Heterocyclic. Chem. 1975, 12, 215–218. [Google Scholar] [CrossRef]
- Kourounakis, A.P.; Matralis, A.N.; Nikitakis, A. Design of more potent squalene synthase inhibitors with multiple activities. Bioorg. Med. Chem. 2010, 18, 7402–7412. [Google Scholar] [CrossRef] [PubMed]
- Song, G.-L.; Zhu, H.-J.; Chen, L.; Liu, S.; Luo, Z.-H. Novel Disubstituted Phenylene-Linked Bis-imidazole Derivatives: Facile Synthesis and Optical Properties. Helv. Chim. Acta 2010, 93, 2397–2405. [Google Scholar] [CrossRef]
- Terent’ev, A.O.; Khodykin, S.V.; Krylov, I.B.; Ogibin, Y.N.; Nikishin, G.I. A Convenient Synthesis of 2,2-Dibromo-1-arylethanones by Bromination of 1-Arylethanones with the H2O2-HBr System. Synthesis 2006, 1087–1092. [Google Scholar] [CrossRef]
- Kim, K.; Cho, J.; Yoon, S.C. Reactions of Tetrasulfur Tetranitride with Aryl Dibromomethyl Ketones: One-pot Synthesis of 3-Aroylformamido-4-aryl-1,2,5-thiadiazoles and their Reactions. J. Chem. Soc. Perkin Trans. 1995, 1, 253–259. [Google Scholar] [CrossRef]
- Kinbara, K.; Harada, Y.; Saigo, K. A high-performance, tailor-made resolving agent: remarkable enhancement of resolution ability by introducing a naphthyl group into the fundamental skeleton. J. Chem. Soc. Perkin Trans. 2 2000, 2, 1339–1347. [Google Scholar] [CrossRef]
- Nobrega, J.A.; Gonçalves, S.M.C.; Peppe, C. Selective Preparation of α,α-dichloroketones with Copper(II) chloride. Synth. Commun. 2002, 32, 3711–3717. [Google Scholar] [CrossRef]
- Kurosawa, K.; Yamaguchi, K. The Reaction of Acetophenones with Manganese(III) Acetate. Bull. Chem. Soc. Jpn. 1981, 54, 1757–1760. [Google Scholar] [CrossRef]
- Jereb, M.; Stavber, S.; Zupan, M. Direct α-Iodination of Aryl Alkyl Ketones by Elemental Iodine Activated by 1-Chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane Bis(tetrafluoroborate). Synthesis 2003, 853–858. [Google Scholar] [CrossRef]
- Shang, G.; Liu, D.; Allen, S.E.; Yang, Q.; Zhang, X. Asymmetric Hydrogenation of α-Primary and Secondary Amino Ketones: Efficient Asymmetric Syntheses of (–)-Arbutamine and (–)-Denopamine. Chem. Eur. J. 2007, 13, 7780–7784. [Google Scholar] [CrossRef] [PubMed]
- Lindh, J.; Sjöberg, P.J.R.; Larhed, M. Synthesis of Aryl Ketones by Palladium(II)-Catalyzed Decarboxylative Addition of Benzoic Acids to Nitriles. Angew. Chem. Int. Ed. 2010, 49, 7733–7737. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-H.; Lv, P.-C.; Xue, J.-Y.; Song, B.-A.; Zhu, H.-L. Novel 2,4,5-trisubstituted oxazole derivatives: Synthesis and antiproliferative activity. Eur. J. Med. Chem. 2009, 44, 3930–3935. [Google Scholar] [CrossRef] [PubMed]
- Maraš, N.; Polanc, S.; Kočevar, M. Microwave-assisted methylation of phenols with tetramethylammonium chloride in the presence of K2CO3 or Cs2CO3. Tetrahedron 2008, 64, 11618–11624. [Google Scholar] [CrossRef]
- Muraki, T.; Togo, H.; Yokoyama, M. Reactivity and Synthetic Utility of 1-(Arenesulfonyloxy)benziodoxolones. J. Org. Chem. 1999, 64, 2883–2889. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, H.; Hattori, S.; Takemaru, K.; Miki, Y. Hypervalent Iodine(III)–LiX Combination in Fluoroalcohol Solvent for Aromatic Halogenation of Electron-Rich Arenecarboxylic Acids. Synlett 2011, 1563–1566. [Google Scholar] [CrossRef]
- Pahari, P.; Rohr, J. Total Synthesis of Psoralidin, an Anticancer Natural Product. J. Org. Chem. 2009, 74, 2750–2754. [Google Scholar] [CrossRef] [PubMed]
- Testaferri, L.; Tiecco, M.; Tingoli, M.; Chianelli, D.; Montanucci, M. The Reactions of Unactivated Aryl Halides with Sodium Methoxide in HMPA. Tetrahedron 1983, 39, 193–197. [Google Scholar] [CrossRef]
- Zhou, C.-Y.; Li, J.; Peddibhotla, S.; Romo, D. Mild Arming and Derivatization of Natural Products via an In(OTf)3-Catalyzed Arene Iodination. Org. Lett. 2010, 12, 2104–2107. [Google Scholar] [CrossRef] [PubMed]
- Gavara, L.; Boisse, T.; Rigo, B.; Hénichart, J.-P. A new method of bromination of aromatic rings by an iso-amyl nitrite/HBr system. Tetrahedron 2008, 64, 4999–5004. [Google Scholar] [CrossRef]
- O’Connell, J.L.; Simpson, J.S.; Dumanski, P.G.; Simpson, G.W.; Easton, C.J. Aromatic chlorination of ω-phenylalkylamines and ω-phenylalkylamides in carbon tetrachloride and α,α,α-trifluorotoluene. Org. Biomol. Chem. 2006, 4, 2716–2723. [Google Scholar] [CrossRef] [PubMed]
- Reich, H.J.; Whipple, W.L. Mechanism of the lithium–iodine exchange in an iodothiophene. Can. J. Chem. 2005, 83, 1577–1587. [Google Scholar] [CrossRef]
Sample Availability: Samples of all the compounds except 16, 17, 20, 23, 28, 38a–c, 39a–c and 41 are available from the authors. |
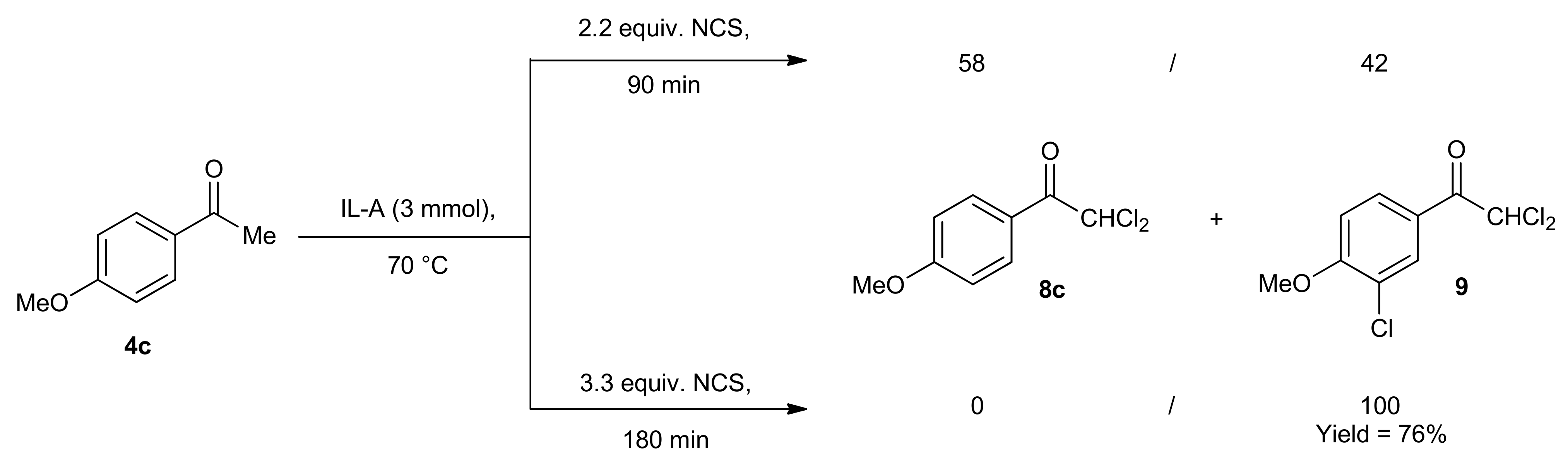
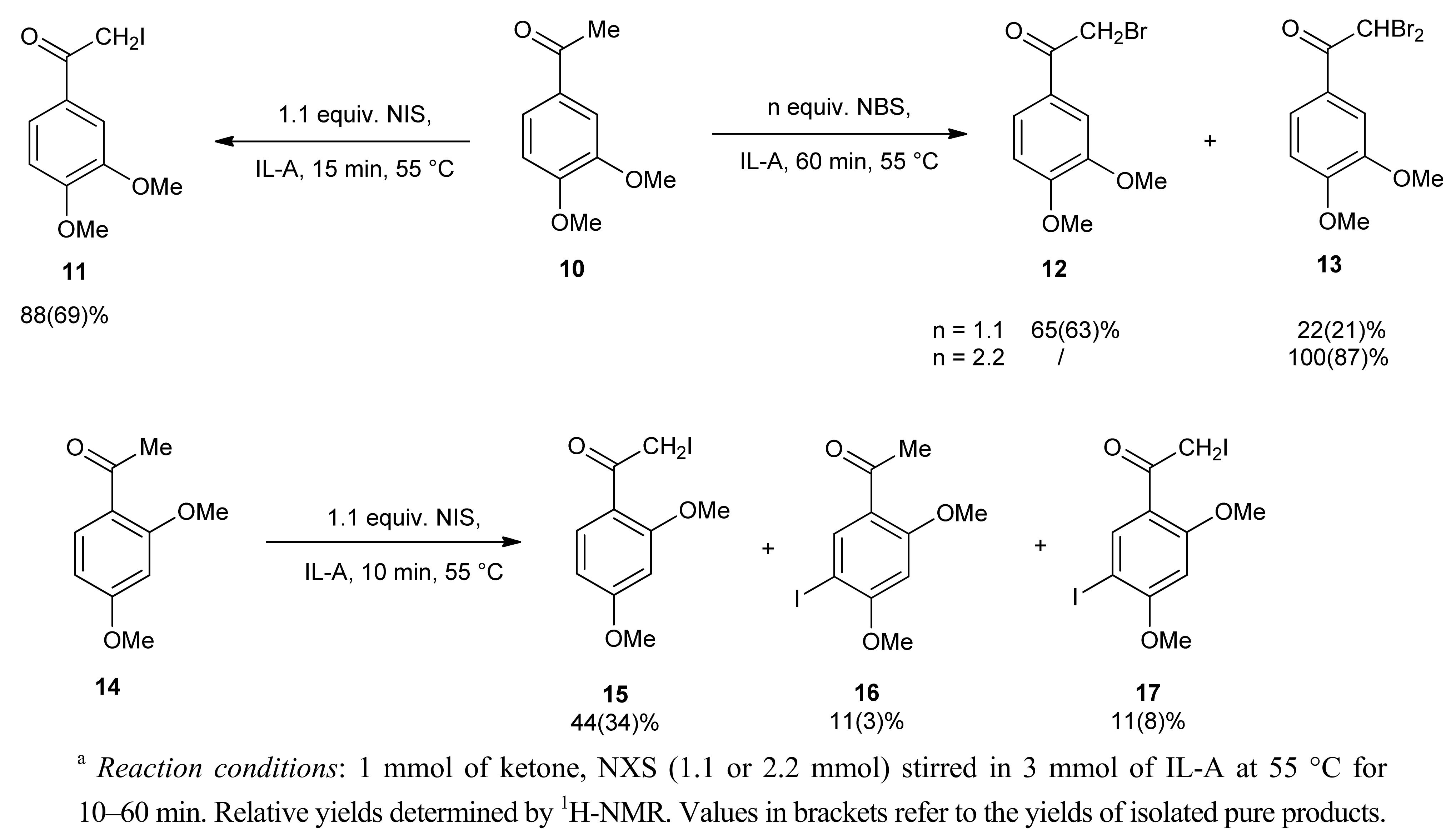
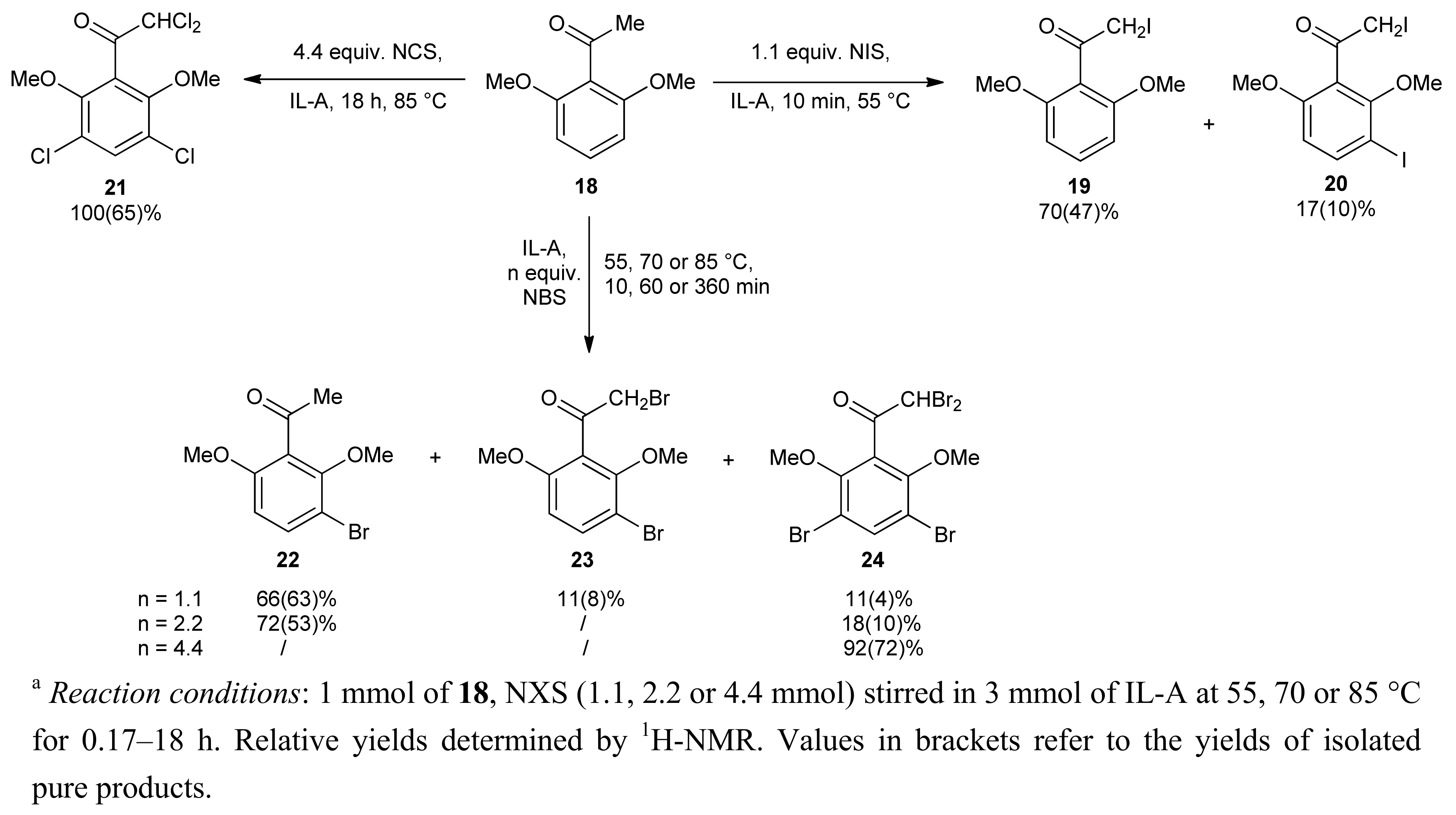
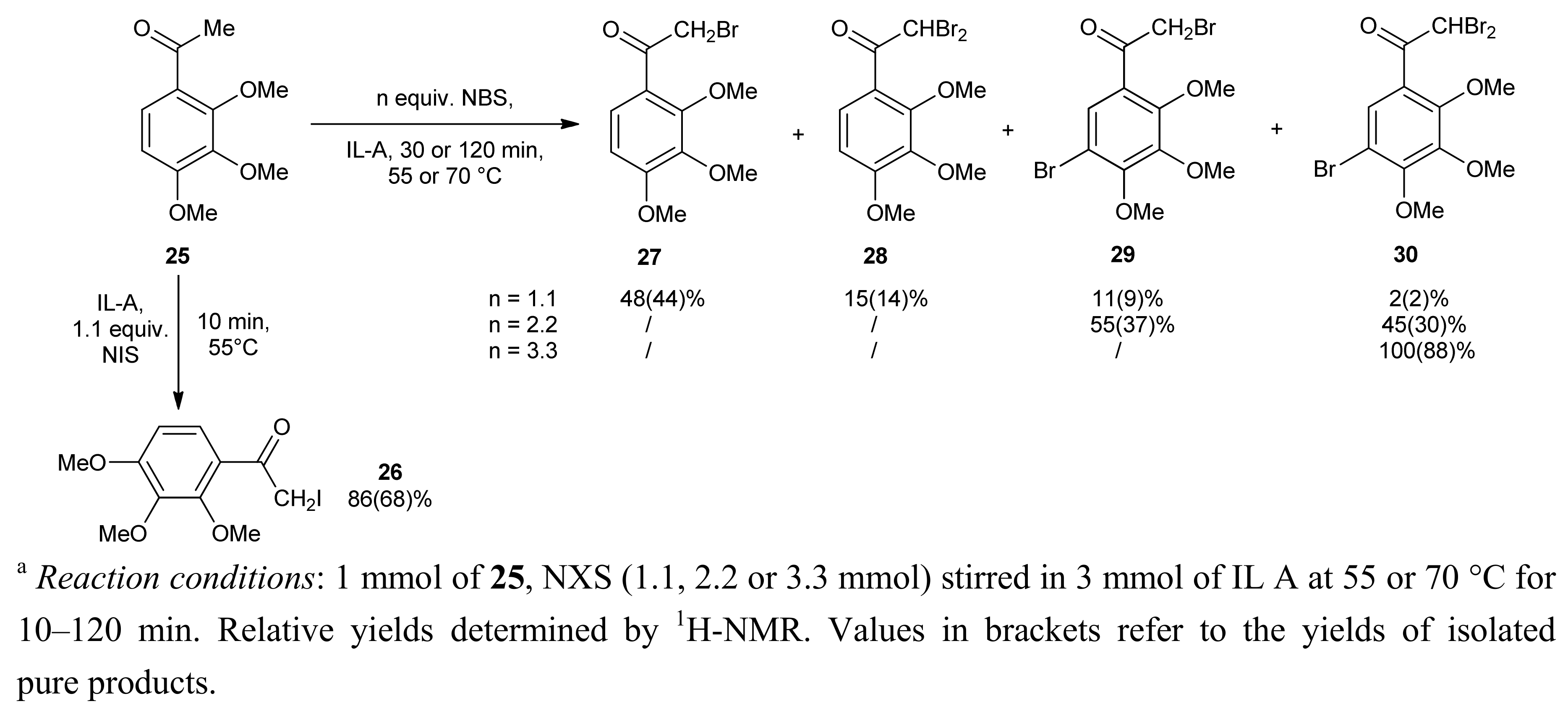
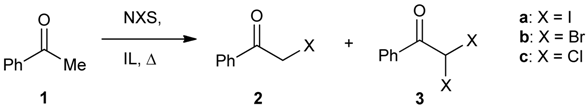
| Entry | X | NXS (equiv.) | IL | T (°C) | Reaction time (min) | Conversion of 1 b (%) | 2 / 3 |
|---|---|---|---|---|---|---|---|
| 1 | I | 1.1 | IL-B | 55 | 10 | 13 | 100 / 0 |
| 2 | I | 1.1 | IL-A | 55 | 10 | 98 | 93 / 7 |
| 3 | Br | 1.1 | IL-B | 55 | 20 | 22 | 100 / 0 |
| 4 | Br | 1.1 | IL-A | 55 | 20 | 95 | 86 / 14 |
| 5 | Br | 2.2 | IL-B | 70 | 30 | 60 | 100 / 0 |
| 6 | Br | 2.2 | IL-A | 70 | 30 | 100 | 0 / 100 |
| 7 | Cl | 1.1 | IL-B | 70 | 30 | 97 | 85 / 15 |
| 8 | Cl | 1.1 | IL-A | 70 | 30 | 47 | 90 / 10 |
| 9 | Cl | 1.1 | IL-A | 70 | 90 | 63 | 15 / 85 |
| 10 | Cl | 2.2 | IL-B | 70 | 30 | 92 | 91 / 9 |
| 11 | Cl | 2.2 | IL-A | 70 | 30 | 100 | 0 / 100 |
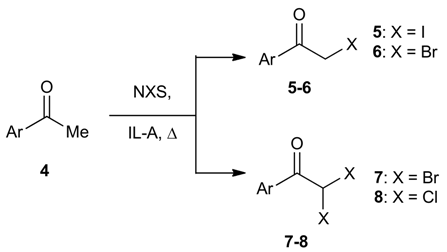
| Entry | Ar | Reaction conditionsa NXS (mmol) / T (°C) / t (min) | Products | Yield (%) b | |
|---|---|---|---|---|---|
| 5 or 6 | 7 or 8 | ||||
| 1 |  | NIS (1.1) / 55 / 10 | 5a | 94 (84) | |
| 2 | NBS (1.1) / 55 / 20 | 6a / 7a | 72 (66) | 19 (18) | |
| 3 | NBS (2.2) / 70 / 60 | 7a | 96 (94) | ||
| 4 | NCS (2.2) / 70 / 150 | 8a | 97 (86) | ||
| 5 | 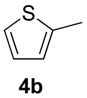 | NIS (1.1) / 55 / 10 | 5b | 91 (80) | |
| 6 | NBS (1.1) / 55 / 10 | 6b / 7b | 75 (72) | 16 (15) | |
| 7 | NBS (2.2) / 70 / 30 | 7b | 95 (85) | ||
| 8 | NCS (2.2) / 70 / 60 | 8b | 96 (67) | ||
| 9 |  | NIS (1.1) / 55 / 10 | 5c | 92 (80) | |
| 10 | NBS (1.1) / 55 / 20 | 6c / 7c | 71 (62) | 22 (20) | |
| 11 | NBS (2.2) / 70 / 30 | 7c | 100 (91) | ||
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vražič, D.; Jereb, M.; Laali, K.K.; Stavber, S. Brønsted Acidic Ionic Liquid Accelerated Halogenation of Organic Compounds with N-Halosuccinimides (NXS). Molecules 2013, 18, 74-96. https://doi.org/10.3390/molecules18010074
Vražič D, Jereb M, Laali KK, Stavber S. Brønsted Acidic Ionic Liquid Accelerated Halogenation of Organic Compounds with N-Halosuccinimides (NXS). Molecules. 2013; 18(1):74-96. https://doi.org/10.3390/molecules18010074
Chicago/Turabian StyleVražič, Dejan, Marjan Jereb, Kenneth K. Laali, and Stojan Stavber. 2013. "Brønsted Acidic Ionic Liquid Accelerated Halogenation of Organic Compounds with N-Halosuccinimides (NXS)" Molecules 18, no. 1: 74-96. https://doi.org/10.3390/molecules18010074
APA StyleVražič, D., Jereb, M., Laali, K. K., & Stavber, S. (2013). Brønsted Acidic Ionic Liquid Accelerated Halogenation of Organic Compounds with N-Halosuccinimides (NXS). Molecules, 18(1), 74-96. https://doi.org/10.3390/molecules18010074