Comparative Evaluation of Different Co-Antioxidants on the Photochemical- and Functional-Stability of Epigallocatechin-3-gallate in Topical Creams Exposed to Simulated Sunlight
Abstract
:1. Introduction
2. Results and Discussion
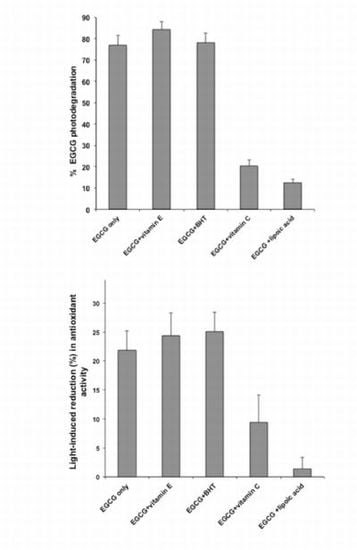
2.1. Photodegradation Studies
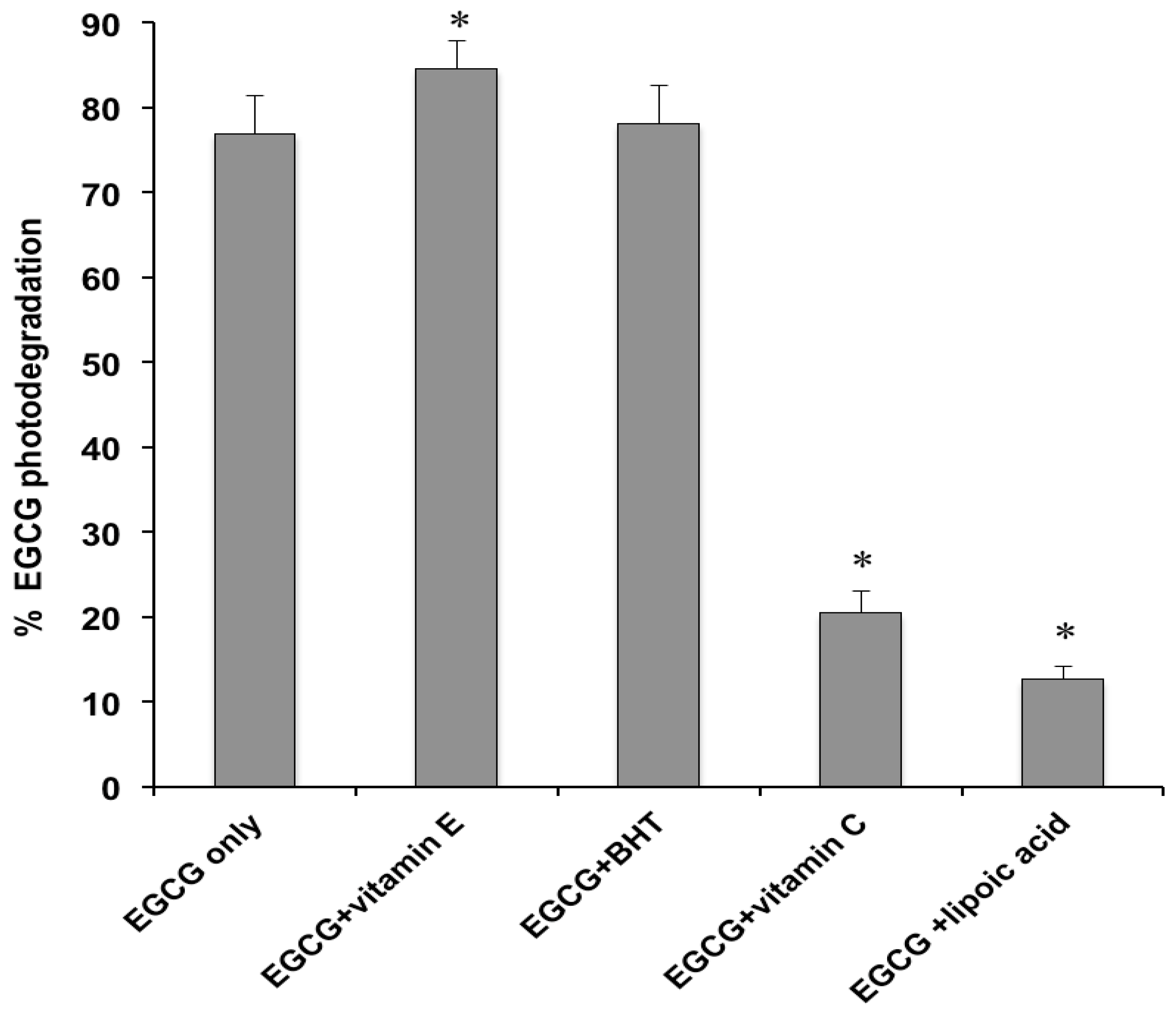
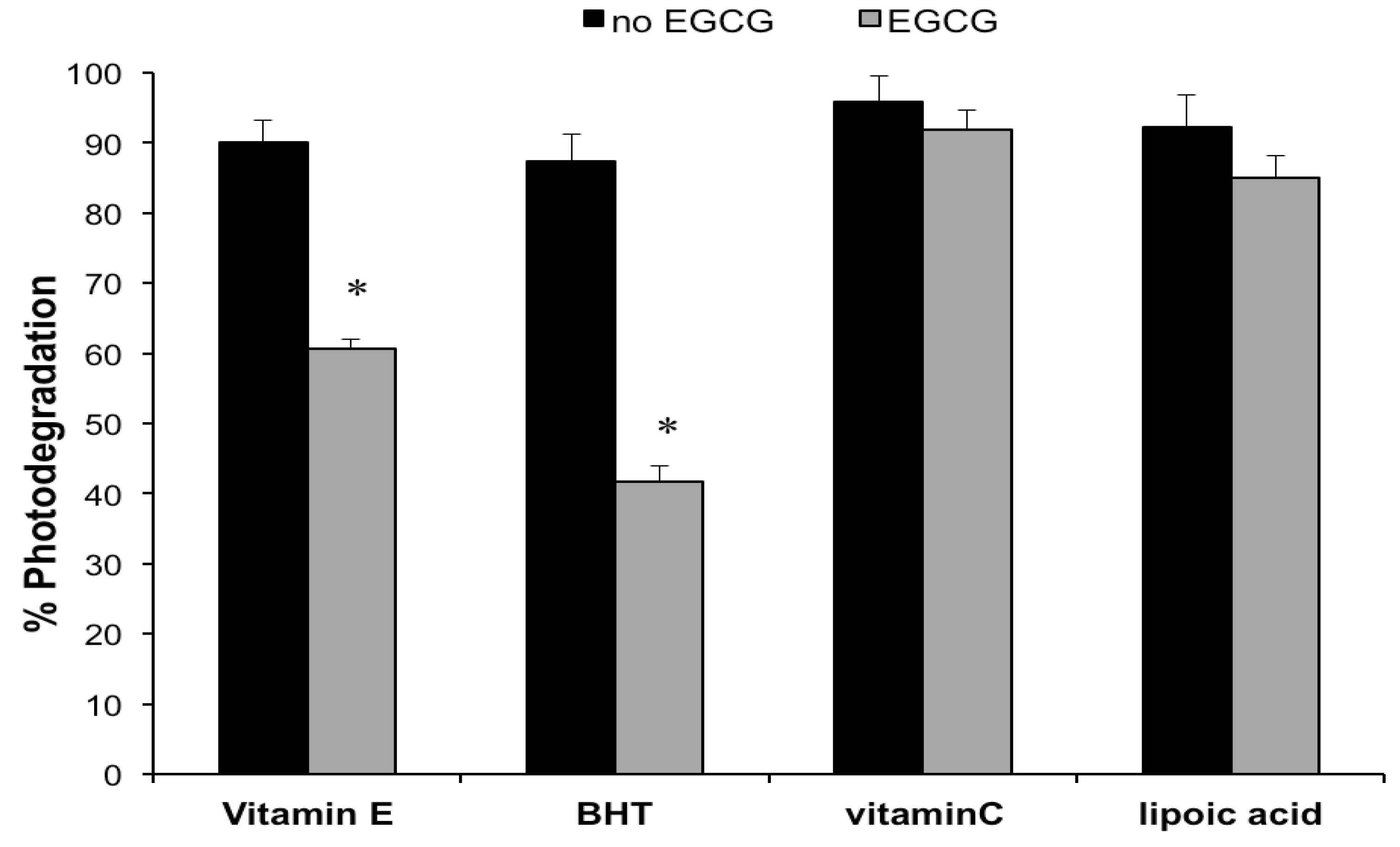
2.2. Antioxidant Activity
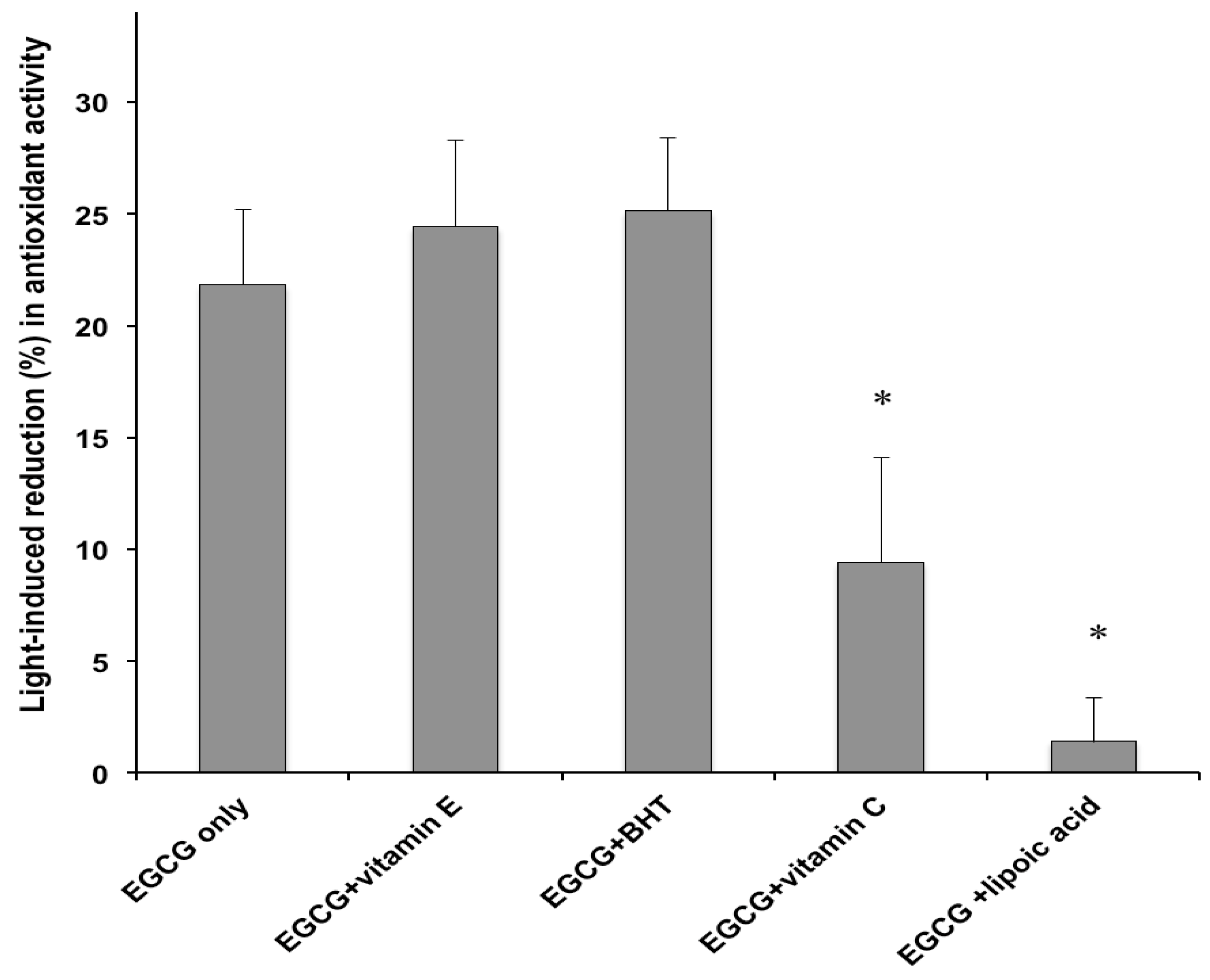
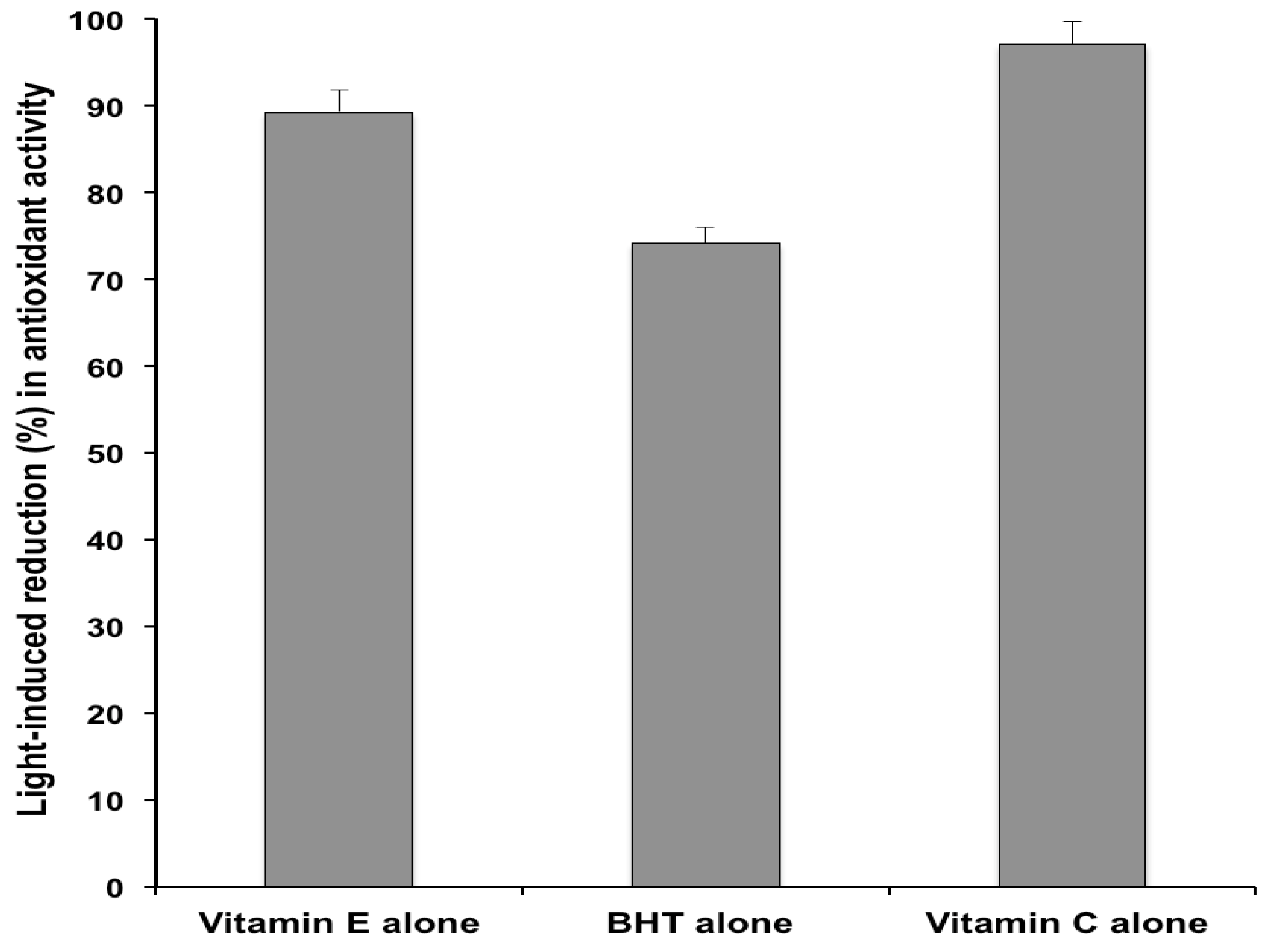
3. Experimental
3.1. Materials
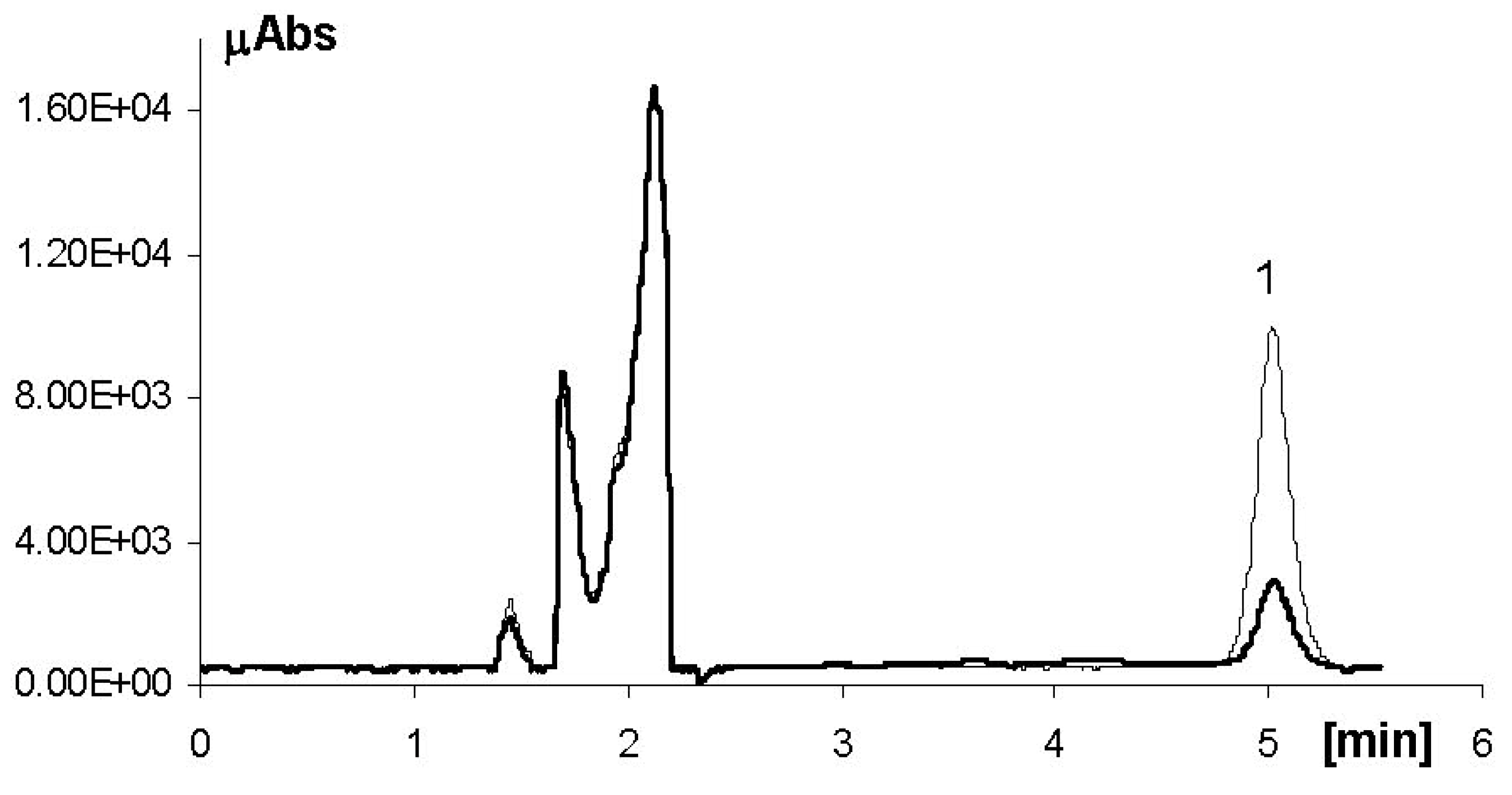
3.2. High-Performance Liquid Chromatography
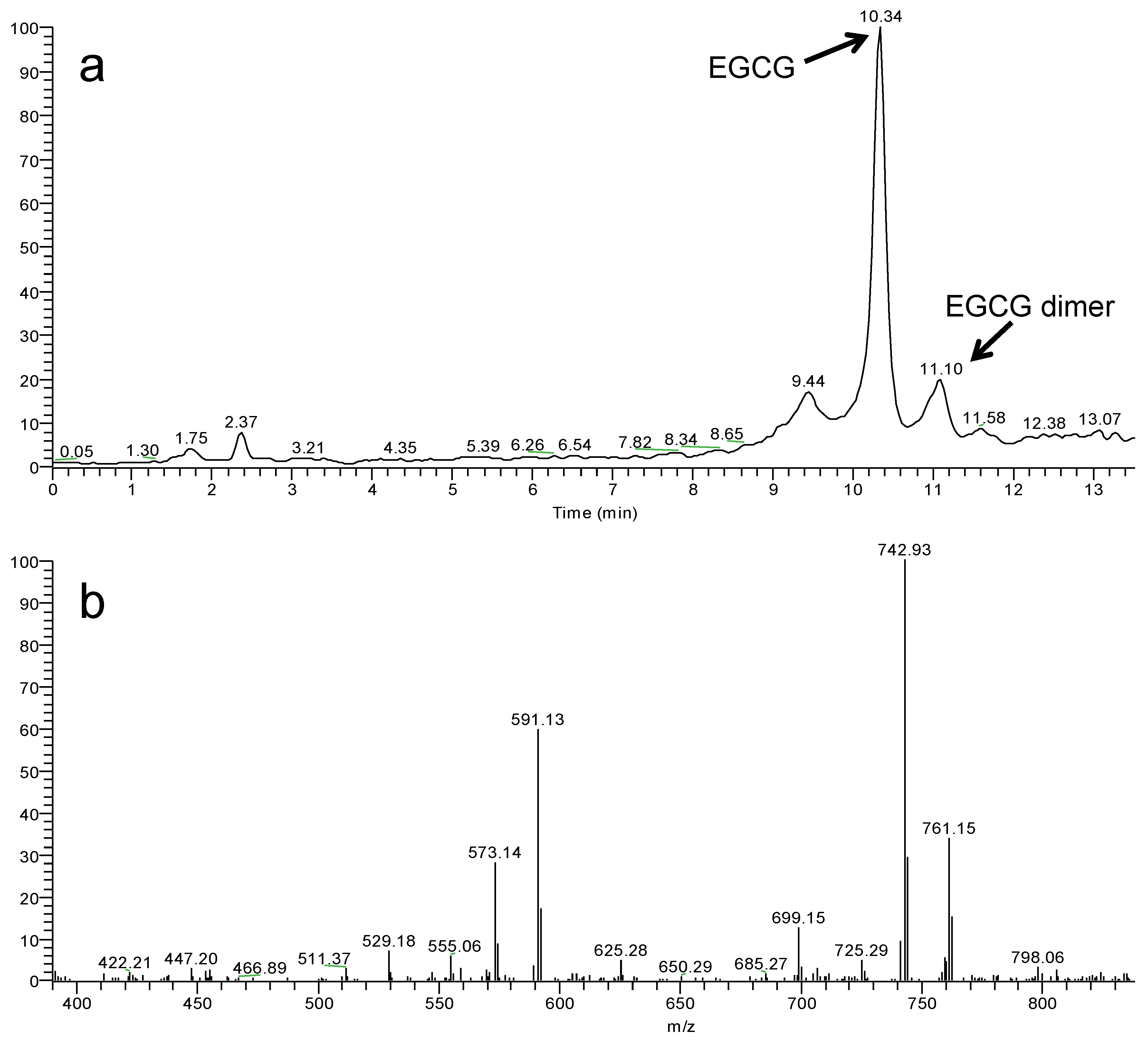
3.3. HPLC-Tandem Mass Spectrometry
3.4. Emulsion Formulations
3.5. Photodegradation Studies
3.6. Antioxidant Activity

3.7. Statistical Analysis
4. Conclusions
- Sample Availability: Samples of (−)-epigallocatechin-3-gallate, vitamin E, butylated hydroxytoluene, vitamin C and α-lipoic acid are available from the authors.
References
- Nagle, D.G.; Ferreira, D.; Zhou, Y.D. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef]
- Mitrica, R.; Dumitru, I.; Ruta, L.L.; Ofiteru, A.M.; Farcasanu, I.C. The dual action of epigallocatechin gallate (EGCG) the main constituent of green tea, against the deleterious effects of visible light and singlet oxygen-generating conditions as seen in yeast cells. Molecules 2012, 17, 10355–10369. [Google Scholar] [CrossRef]
- Kuriyama, S.; Shimazu, T.; Ohmori, K.; Kikuchi, N.; Nakaya, N.; Nishino, Y.; Tsubono, Y.; Tsuji, I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: The Ohsaki study. JAMA 2006, 296, 1255–1265. [Google Scholar] [CrossRef]
- Arts, I.C.; Hollman, P.C. Polyphenols and diseases risk in epidemiological studies. Am. J. Clin. Nutr. 2005, 81, 317–325. [Google Scholar]
- Yusuf, N.; Irby, C.; Katiyar, S.K.; Elmets, C.A. Photoprotective effects of green tea polyphenols. Photoderm. Photoimmunol. Photomed. 2007, 23, 48–56. [Google Scholar] [CrossRef]
- Kuzuhara, T.; Suganuma, M.; Fujiki, H. Green tea catechin as a chemical chaperone in cancer prevention. Cancer Lett. 2008, 261, 12–20. [Google Scholar] [CrossRef]
- Zaveri, N.T. Green tea and its polypenolic catechins: Medicinal uses in cancer and noncancer applications. Life Sci. 2006, 78, 2073–2080. [Google Scholar] [CrossRef]
- Mittal, A.; Piyathilake, C.; Hara, Y.; Katiyar, S. Exceptionally high protection of photocarcinogenesis by topical application in hydrophilic cream in SKH-1 hairless mouse model: Relationship to inhibition of UVB-induced global DNA hypomethylation. Neoplasia 2003, 5, 555–565. [Google Scholar]
- Nichols, J.A.; Katiyar, S. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanism. Arc. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef]
- Li, Y.-H.; Wu, Y.; Wei, H.-C.; Xu, Y.-Y.; Jia, L.-L.; Cheen, J.; Yang, X.-S.; Dong, G.-H.; Gao, X.-H.; Chen, H.-D. Protective effects of green tea extracts on photoaging and photoimmunosuppression. Skin Res. Toxicol. 2009, 15, 338–345. [Google Scholar] [CrossRef]
- Mochizuki, M.; Yamazaki, S.; Kano, K.; Ikeda, T. Kinetic analysis and mechanistic aspects of autoxidation of catechins. Biochim. Biophys. Acta 2002, 1569, 35–44. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, W.B.; Jiang, X.H. Reaction kinetics of degradation and epimerization of epigallocatechin gallate (EGCG) in aqueous system over a wide range of temperature. J. Agric. Food Chem. 2008, 56, 2694–2701. [Google Scholar] [CrossRef]
- Dube, A.; Ng, K.; Nicolazzo, J.A.; Larson, I. Effective use of reducing agents and nanoparticle encapsulation in stabilizing catechins in alkaline solution. Food Chem. 2010, 12, 662–667. [Google Scholar]
- Dvorakova, K.; Dorr, R.T.; Valcic, S.; Timmermann, B.; Alberts, D.S. Pharmacokinetics of the green tea derivative, EGCG, by the topical route of administration in mouse and human skin. Cancer Chemother. Pharmacol. 1999, 43, 331–335. [Google Scholar] [CrossRef]
- Proniuk, S.; Liederer, B.M.; Blanchard, J. Preformulation study of epigallocatechin gallate, a promising antioxidant for topical skin cancer. J. Pharm. Sci. 2002, 91, 111–116. [Google Scholar] [CrossRef]
- Rode, T.; Frauen, M.; Muller, B.W.; Schonrock, U.; Mundt, C.; Hintze, U.; Wenck, H. The influence of antioxidant and chelating agents on the stability of catechins, with particular reference to (−)-epigallocatechin-gallate (EGCG) and (−)-epicatechin (EC) in topical emulsion based formulations. SOFWJ 2002, 128, 24–26. [Google Scholar]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Bianchi, A.; Marchetti, N.; Scalia, S. Photodegradation of (−)-epigallocatechin-3-gallate in topical creams and its photostabilization. J. Pharm. Biomed. Anal. 2011, 56, 692–697. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Zhu, Q.Y.; Wong, Y.F.; Zhang, Z.; Chung, H.Y. Stabilizing effect of ascorbic acid on green tea catechins. J. Agric. Food Chem. 1998, 46, 2512–2516. [Google Scholar] [CrossRef]
- Podda, M.; Zollner, T.M.; Grundmann-Kollmann, M.; Thiele, J.J.; Packer, L.; Kaufmann, R. Activity of alpha-lipoic acid in protection against oxidative stress in skin. Curr. Probl. Dermatol. 2001, 19, 43–51. [Google Scholar]
- Moini, H.; Packer, L.; Saris, N.-E. Antioxidant and prooxidant activities of α-lipoic acid and dihydrolipoic acid. Toxicol. Appl. Pharmacol. 2002, 182, 84–90. [Google Scholar] [CrossRef]
- Block, L.H. Medicated topicals. In Remington: The Science and Practice of Pharmacy; Gennaro, A., Der Marderosian, A., Hanson, G., Medwick, T., Popovich, N., Schnaare, R., Schwartz, J., White, H., Eds.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2000; p. 836. [Google Scholar]
- Sang, S.; Lee, M.-J.; Hou, Z.; Ho, C.-T.; Yang, C.S. Stability of tea polyphenol (−)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J. Agric. Food Chem. 2005, 53, 9478–9484. [Google Scholar]
- Fuchs, J. Potentials and limitations of the natural antioxidants alpha-tocopherol, l-ascorbic acid and β-carotene in cutaneous photoprotection. Free Radic. Biol. Med. 1998, 25, 848–867. [Google Scholar] [CrossRef]
- Allwood, M.C.; Martin, H.J. The photodegradation of vitamins A and E in parental nutrition mixtures during infusion. J. Clin. Nutr. 2000, 19, 339–342. [Google Scholar] [CrossRef]
- Carr, A.; Frei, B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999, 9, 1007–1024. [Google Scholar]
- Wada, N.; Wakami, H.; Konishi, T.; Matsugo, S. The degradation and regeneration of α-lipoic acid under the irradiation of UV light in the existence of homocysteine. J. Clin. Biochem. Nutr. 2009, 44, 218–222. [Google Scholar] [CrossRef]
- Li, B.; Du, W.; Jin, J.; Du, Q. Preservation of (−)-epigallocatechin-3-gallate antioxidant properties loaded in heat treated β-lactoglobulin nanoparticles. J. Agric. Food Chem. 2012, 60, 3477–3484. [Google Scholar] [CrossRef]
- Georgetti, S.R.; Casagrande, R.; Vicentini, F.T.; Verri, W.A.; Fonseca, M.J. Evaluation of antioxidant activity of soybean extract by different in vitro methods and investigation of this activity after incorporation in topical formulations. Eur. J. Pharm. Biopharm. 2006, 64, 99–106. [Google Scholar] [CrossRef]
- Vicentini, F.T.; Vaz, M.M.; Fonseca, Y.M.; Bentley, M.V.; Fonseca, M.J. Characterization and stability study of a water-in-oil microemulsion incorporating quercetin. Drug Dev. Ind. Pharm. 2011, 37, 47–55. [Google Scholar] [CrossRef]
- Su, Y.-L.; Xu, J.-Z.; Ng, C.H.; Leung, L.K.; Huang, Y.; Chen, Z.-Y. Antioxidant activity of tea theaflavins and methylated catechins in canoa oil. J. Am. Oil Chem. Soc. 2004, 81, 269–274. [Google Scholar] [CrossRef]
- Madawala, S.R.P.; Andersson, R.E.; Jastrebova, J.A.; Alameida, M.; Dutta, P.C. Novel conjugates of 1,3-diacylglycerol and lipoic acid: Synthesis, DPPH assay, and RP-LC-MS-APCI analysis. J. Lipids 2011. article ID 419809. [Google Scholar]
- Chaudhuri, R.K. Role of antioxidants in suncare products. In Sunscreens, 3th; Shaath, N., Ed.; Taylor Francis Group: Boca Raton, FL, USA, 2005; pp. 611–621. [Google Scholar]
- Pinnel, S.R. Cutaneous photodamage, oxidative stress, topical antioxidant protection. J. Am. Acad. Dermatol. 2003, 48, 1–19. [Google Scholar] [CrossRef]
- Aguilera, J.; de Gálvez., M.V.; Sánchez, C.; Herrera-Ceballos, E. Changes in photoinduced cutaneous erythema with topical application of a combination of vitamins C and E before and after UV exposure. J. Dermatol. Sci. 2012, 66, 216–220. [Google Scholar] [CrossRef]
- Scalia, S.; Ruberto, G.; Bonina, F. Determination of vitamin A, vitaminE and their esters in tablet preparations using supercritical fluid extraction and HPLC. J. Pharm. Sci. 1995, 84, 433–436. [Google Scholar] [CrossRef]
- Chen, M.; Xia, Q.; Liu, M.; Yang, Y. Cloud-point extraction and reversed phase high-performance liquid chromatography for the determination of synthetic phenolic antioxidants in edible oils. J. Food Sci. 2011, 76, C98–C103. [Google Scholar]
- Poongothai, S.; Ilavarasan, R.; Karrunakaran, C.M. Simultaneous and accurate determination of vitamins B1, B6, B12 and alphalipoic acid in multivitamin capsules by reversed-phase high performance liquid chromatographic method. Int. J. Pharm. Pharm. Sci. 2010, 2, 133–139. [Google Scholar]
- Heudi, O.; Kilinc, T.; Fontannaz, P. Separation of water-soluble vitamins by reversed-phase high performance liquid chromatography with ultraviolet detection: Application to polyvitaminated premixes. J. Chromatogr. A 2005, 1070, 49–56. [Google Scholar] [CrossRef]
- Scalia, S.; Mezzena, M. Photostabilization effect of quercetin on the UV filter combination, butyl metoxydibenzoylmethane-octy methoxycinnamate. Photochem. Photobiol. 2010, 86, 273–278. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Scalia, S.; Marchetti, N.; Bianchi, A. Comparative Evaluation of Different Co-Antioxidants on the Photochemical- and Functional-Stability of Epigallocatechin-3-gallate in Topical Creams Exposed to Simulated Sunlight. Molecules 2013, 18, 574-587. https://doi.org/10.3390/molecules18010574
Scalia S, Marchetti N, Bianchi A. Comparative Evaluation of Different Co-Antioxidants on the Photochemical- and Functional-Stability of Epigallocatechin-3-gallate in Topical Creams Exposed to Simulated Sunlight. Molecules. 2013; 18(1):574-587. https://doi.org/10.3390/molecules18010574
Chicago/Turabian StyleScalia, Santo, Nicola Marchetti, and Anna Bianchi. 2013. "Comparative Evaluation of Different Co-Antioxidants on the Photochemical- and Functional-Stability of Epigallocatechin-3-gallate in Topical Creams Exposed to Simulated Sunlight" Molecules 18, no. 1: 574-587. https://doi.org/10.3390/molecules18010574
APA StyleScalia, S., Marchetti, N., & Bianchi, A. (2013). Comparative Evaluation of Different Co-Antioxidants on the Photochemical- and Functional-Stability of Epigallocatechin-3-gallate in Topical Creams Exposed to Simulated Sunlight. Molecules, 18(1), 574-587. https://doi.org/10.3390/molecules18010574