Inhibitory Evaluation of Sulfonamide Chalcones on β-Secretase and Acylcholinesterase
Abstract
:1. Introduction
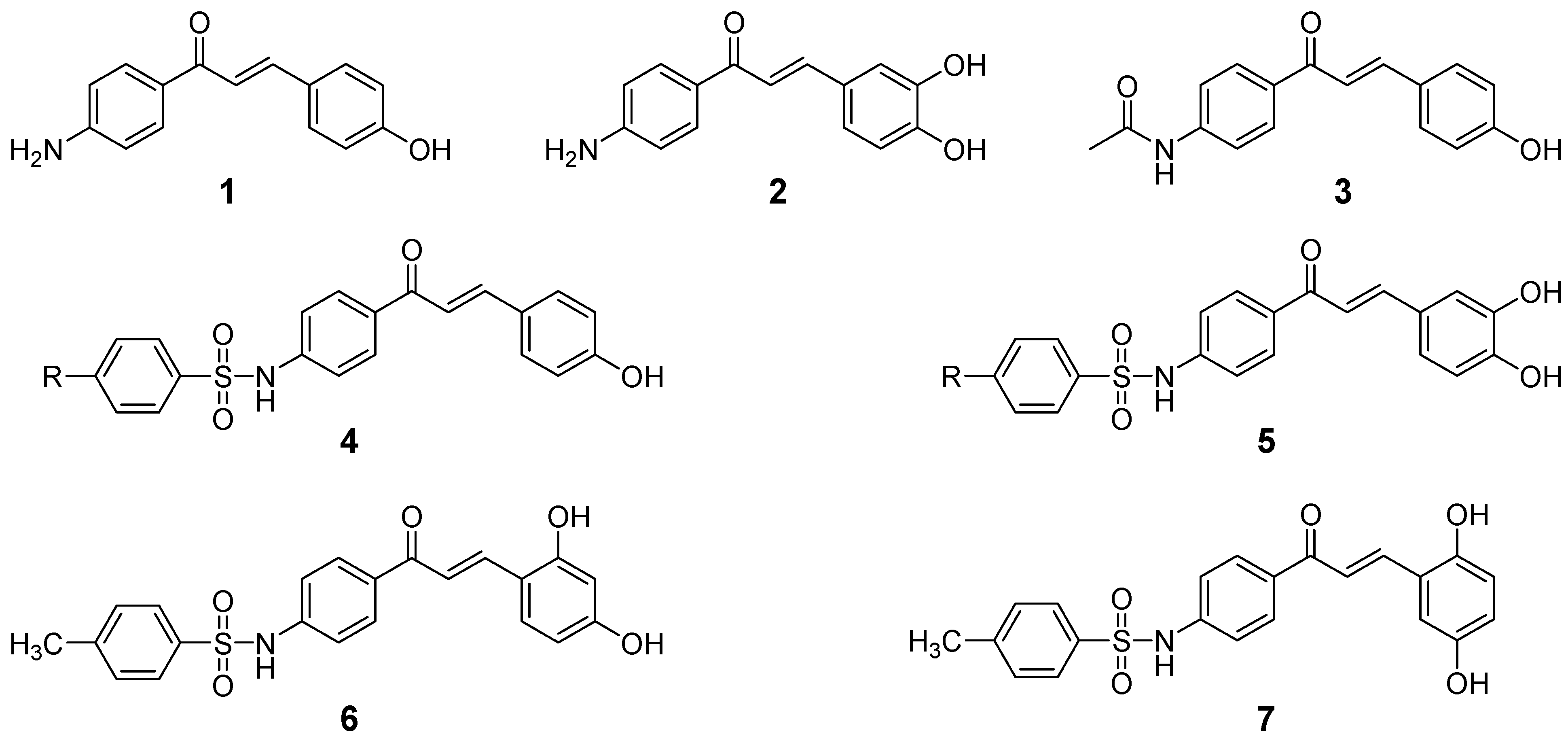
2. Results and Discussion
2.1. Chemistry
| Compound | R1 R2 R3 | IC50 (mM) a | Ki (μM) b |
|---|---|---|---|
| 1 | 48.2 ± 1.2 | 43.8 | |
| 2 | 17.7 ± 0.8 | 10.5 | |
| 3 | >200 | NT | |
| 4a | H OH CH3 | 1.44 ± 0.2 | 0.56 |
| 4b | H H CH3 | 168.7 ± 2.4 | NT |
| 4c | H OH H | 6.28 ± 0.7 | 5.83 |
| 4d | H OH OH | 2.88 ± 0.5 | 1.49 |
| 4e | H OH OCH3 | 79.3 ± 2.3 | 75.2 |
| 4f | H OH NH2 | 5.58 ± 1.4 | 4.19 |
| 4g | H OH NO2 | 107.4 ± 2.7 | 94.8 |
| 4h | H OH F | 119.6 ± 3.2 | NT |
| 5a | OH OH CH3 | 0.21 ± 0.02 | 0.07 |
| 5b | OH OH H | 4.59 ± 1.5 | 3.78 |
| 5c | OH OH OH | 0.62 ± 0.03 | 0.56 |
| 5d | OH OH NH2 | 0.69 ± 0.04 | 0.69 |
| 5e | OH OH NO2 | 101.3 ± 2.4 | 92.4 |
| 5f | OH OH F | 8.95 ± 1.0 | 10.8 |
| 6 | 3.60 ± 0.3 | 1.89 | |
| 7 | 16.87 ± 0.8 | 11.7 |
2.2. Biological Activities
β-Secretase Inhibition Activities
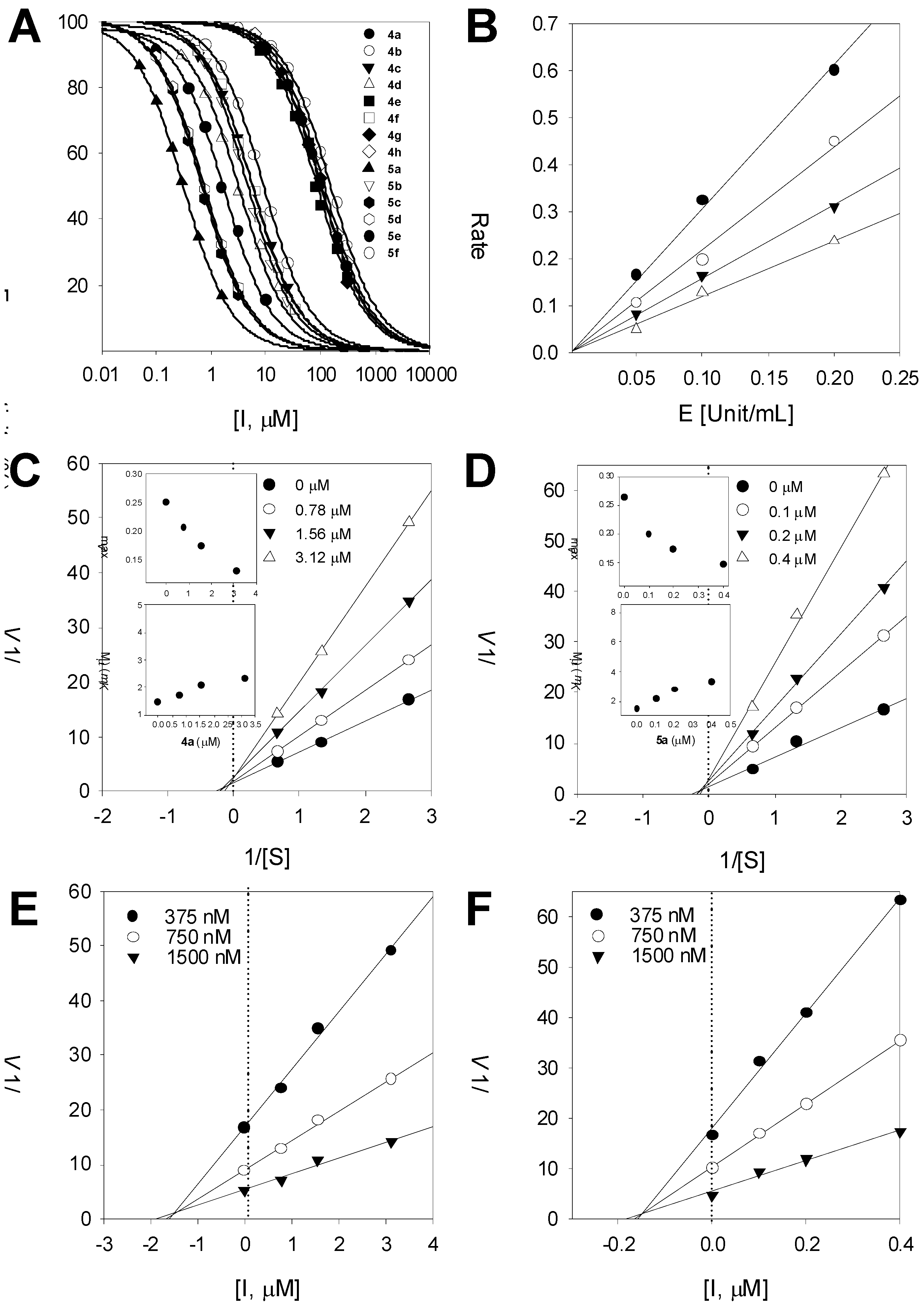
2.3. Inhibitory Kinetics
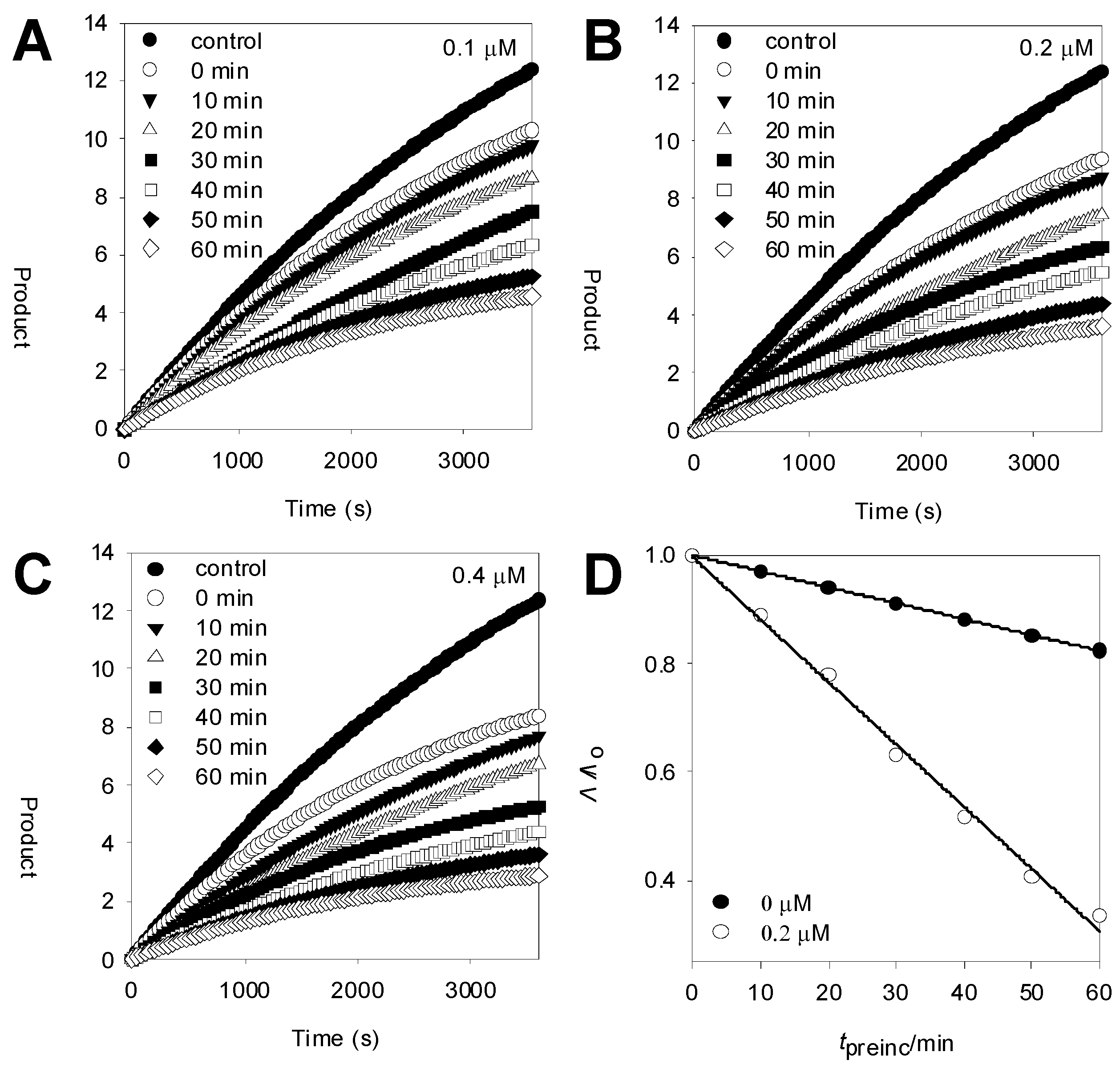
| Compound | IC50a (μM) | Ki (μM)b | IC50a (μM) | Ki (μM)b |
|---|---|---|---|---|
| erythrocytes AChE | equine serum BChE | |||
| 4a | 56.1 | 40.4 | 47.4 | 25.3 |
| 4c | 83.3 | 80.3 | 75.0 | 72.2 |
| 4f | 95.8 | 61.5 | 34.3 | 19.0 |
| 5a | 75.9 | 55.8 | 19.5 | 9.8 |
| 5b | 75.4 | 57.2 | 79.0 | 63.5 |
| 5c | 57.4 | 47.6 | 24.7 | 26.6 |
| Eserin | 0.15 | NT c | 3.7 | NT |
3. Experimental
3.1. General
3.2. Synthesis of Chalcone Derivatives
3.3. Pharmacology
3.3.1. BACE1 Enzyme Assay
3.3.2. Slow and Time-Dependent Inhibitory Activity
3.3.3. Cholinesterase Inhibitory Activity
3.3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
References
- 2012 Alzheimer’s Disease Facts and Figures. Available online: http://www.alz.org/downloads/facts_figures_2012.pdf (accessed on 16 December 2012).
- Sjöbeck, M.; Haglund, M.; Englund, E. Decreasing myelin density reflected increasing white matter pathology in Alzheimer’s disease—A neuropathological study. Int. J. Geriatr. Psychiatry 2005, 20, 919–926. [Google Scholar] [CrossRef]
- Hardy, J.; Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 1991, 12, 383–388. [Google Scholar] [CrossRef]
- Lee, V.M.Y.; Goedert, M.; Trojanowski, J.Q. Neurodegenerative tauopathies. Annu. Rev. Neurosci. 2001, 24, 1121–1259. [Google Scholar] [CrossRef]
- Vassar, R.; Bennett, B.D.; Babu-Khan, S.; Kahn, S.; Mendiaz, E.A.; Denis, P.; Teplow, D.B.; Ross, S.; Amarante, P.; Loeloff, R.; et al. β-Secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 1999, 286, 735–741. [Google Scholar]
- Sinha, S.; Anderson, J.P.; Barbour, R.; Basi, G.S.; Caccavello, R.; Davis, D.; Doan, M.; Dovey, H.F.; Frigon, N.; Hong, J.; et al. Purification and cloning of amyloid precursor protein β-secretase from human brain. Nature 1999, 402, 533–537. [Google Scholar]
- Yan, R.; Bienkowski, M.J.; Shuck, M.E.; Miao, H.; Tory, M.C.; Pauley, A.M.; Brashler, J.R.; Stratman, N.C.; Mathews, W.R.; Buhl, A.E.; et al. Membrane-anchored aspartyl protease with Alzheimer’s disease β-secretase activity. Nature 1999, 402, 537–540. [Google Scholar]
- Hussain, I.; Powell, D.; Howlett, D.R.; Tew, D.G.; Meek, T.D.; Chapman, C.; Gloger, I.S.; Murphy, K.E.; Southan, C.D.; Ryan, D.M.; et al. Identification of a novel aspartic protease (Asp 2) as β-secretase. Mol. Cell. Neurosci. 1999, 14, 419–427. [Google Scholar] [CrossRef]
- Yang, L.B.; Lindholm, K.; Yan, R.; Citron, M.; Xia, W.; Yang, X.L.; Beach, T.; Sue, L.; Wong, P.; Price, D.; et al. Elevated β-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat. Med. 2003, 9, 3–4. [Google Scholar] [CrossRef]
- Fukumoto, H.; Cheung, B.S.; Hyman, B.T.; Irizarry, M.C. β-Secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch. Neurol. 2002, 59, 1381–1389. [Google Scholar] [CrossRef]
- Holsinger, R.M.D.; McLean, C.A.; Beyreuther, K.; Masters, C.L.; Evin, G. Increased expression of the amyloid precursor β-secretase in Alzheimer’s disease. Ann. Neurol. 2002, 51, 783–786. [Google Scholar] [CrossRef]
- McConlogue, L.; Buttini, M.; Anderson, J.P.; Brigham, E.F.; Chen, K.S.; Freedman, S.B.; Games, D.; Johnson-Wood, J.; Lee, M.; Zeller, M.; et al. Partial reduction of BACE1 has dramatic effects on Alzheimer plaque and synaptic pathology in APP transgenic Mice. J. Biol. Chem. 2007, 282, 26326–26334. [Google Scholar]
- Garcia-Ayllon, M.S.; Small, D.H.; Avila, J.; Saez-Valero, J. Revisiting the role of acetylcholinesterase in Alzheimer’s disease: cross-talk with P-tau and β-amyloid. Front. Mol. Neurosci. 2011, 22, 1–9. [Google Scholar]
- Arneric, S.P.; Holladay, M.; Williams, M. Neuronal nicotinic receptors: A perspective on two decades of drug discovery research. Biochem. Pharmacol. 2007, 74, 1092–1101. [Google Scholar]
- Asai, M.; Hattori, C.; Iwata, N.; Saido, T.C.; Sasagawa, N.; Szabo, B.; Hashimoto, Y.; Maruyama, K.; Tanuma, S.; Kiso, Y.; et al. The novel β-secretase inhibitor KMI-429 reduces amyloid β peptide production in amyloid precursor protein transgenic and wild-type mice. J. Neurochem. 2006, 96, 533–540. [Google Scholar] [CrossRef]
- Vassar, R. Beta-secretase (BACE) as a drug target for Alzheimer’s disease. Drug Deliv. Rev. 2002, 54, 1589–1602. [Google Scholar] [CrossRef]
- Lee, S.A.; Ryu, H.W.; Kim, Y.M.; Choi, S.; Lee, M.J.; Kwak, T.K.; Kim, H.J.; Cho, M.; Park, K.H.; Lee, J.W. Blockade of Four-Transmembrane L6 Family Member 5 (TM4SF5)-Mediated Tumorigenicity in Hepatocytes by a Synthetic Chalcone Derivative. Hepatology 2009, 49, 1316–1325. [Google Scholar] [CrossRef]
- Seo, W.D.; Kim, J.H.; Kang, J.E.; Ryu, H.W.; Curtis-Long, M.J.; Lee, H.S.; Yang, M.S.; Park, K.H. Sulfonamide chalcone as a new class of α-glucosidase inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 5514–5516. [Google Scholar]
- Yarishkin, O.V.; Ryu, H.W.; Park, J.Y.; Yang, M.S.; Hong, S.G.; Park, K.H. Sulfonatechalcone as new class voltage-dependent K+ channel blocker. Bioorg. Med. Chem. Lett. 2008, 18, 137–140. [Google Scholar]
- Ryu, H.W.; Curtis-Long, M.J.; Jung, S.; Jeong, I.Y.; Kim, D.S.; Kang, K.Y.; Park, K.H. Anticholinesterase potential of flavonols from paper mulberry (Broussonetia papyrifera) and their kinetic studies. Food Chem. 2012, 132, 1244–1250. [Google Scholar]
- Jeon, S.Y.; Bae, K.; Seong, Y.H.; Song, K.S. Green tea catechins as a BACE1 (β-Secretase) inhibitor. Bioorg. Med. Chem. Lett. 2003, 13, 3905–3908. [Google Scholar] [CrossRef]
- Hwang, E.M.; Ryu, Y.B.; Kim, H.Y.; Kim, D.G.; Hong, S.G.; Lee, J.H.; Curtis-Long, M.J.; Jeong, S.H.; Park, J.Y.; Park, K.H. BACE1 inhibitory effects of lavandulyl flavanones from Sophora flavescens. Bioorg. Med. Chem. 2008, 16, 6669–6674. [Google Scholar]
- Li, Y.Q.; Zhou, F.C.; Bian, J.S.; Shan, F. Comparative Evaluation of Quercetin, Isoquercetin and Rutin as Inhibitors of α-Glucosidase. J. Agric. Food Chem. 2009, 57, 11463–11468. [Google Scholar]
- Zhan, J.W.; Wang, Z.J.; Yan, Y.J.; Xiang, W.S. Comparative Studies on the Interaction of Genistein, 8-Chlorogenistein, and 3',8-Dichlorogenistein with Bovine Serum Albumin. J. Agric. Food Chem. 2011, 59, 7506–7513. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase actiity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar]
- Gabrovska, K.; Marinov, I.; Godjevargova, T.; Portaccio, M.; Lepore, M.; Grano, V.; Diano, N.; Mita, D.G. The influence of the support nature on the kinetics parameters, inhibition constants and reactivation of immobilized acetylcholinesterase. Int. J. Biol. Macromol. 2008, 43, 339–345. [Google Scholar] [CrossRef]
- Kamal, M.A.; Klein, P.; Luo, W.; Li, Y.; Holloway, H.W.; Tweedie, D.; Greig, N.H. Kinetics of human serum butyrylcholinesterase inhibition by a novel experimental Alzheimer therapeutic, dihydrobenzodioxepine cymserine. Neurochem. Res. 2008, 33, 745–753. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds (1–7) are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kang, J.E.; Cho, J.K.; Curtis-Long, M.J.; Ryu, H.W.; Kim, J.H.; Kim, H.J.; Yuk, H.J.; Kim, D.W.; Park, K.H. Inhibitory Evaluation of Sulfonamide Chalcones on β-Secretase and Acylcholinesterase. Molecules 2013, 18, 140-153. https://doi.org/10.3390/molecules18010140
Kang JE, Cho JK, Curtis-Long MJ, Ryu HW, Kim JH, Kim HJ, Yuk HJ, Kim DW, Park KH. Inhibitory Evaluation of Sulfonamide Chalcones on β-Secretase and Acylcholinesterase. Molecules. 2013; 18(1):140-153. https://doi.org/10.3390/molecules18010140
Chicago/Turabian StyleKang, Jae Eun, Jung Keun Cho, Marcus J. Curtis-Long, Hyung Won Ryu, Jin Hyo Kim, Hye Jin Kim, Heung Joo Yuk, Dae Wook Kim, and Ki Hun Park. 2013. "Inhibitory Evaluation of Sulfonamide Chalcones on β-Secretase and Acylcholinesterase" Molecules 18, no. 1: 140-153. https://doi.org/10.3390/molecules18010140
APA StyleKang, J. E., Cho, J. K., Curtis-Long, M. J., Ryu, H. W., Kim, J. H., Kim, H. J., Yuk, H. J., Kim, D. W., & Park, K. H. (2013). Inhibitory Evaluation of Sulfonamide Chalcones on β-Secretase and Acylcholinesterase. Molecules, 18(1), 140-153. https://doi.org/10.3390/molecules18010140