Optimization of Ultrasonic Assisted Extraction of Antioxidants from Black Soybean (Glycine max var) Sprouts Using Response Surface Methodology
Abstract
:1. Introduction
2. Results and Discussions
2.1. Results of Single-Factor Experiments
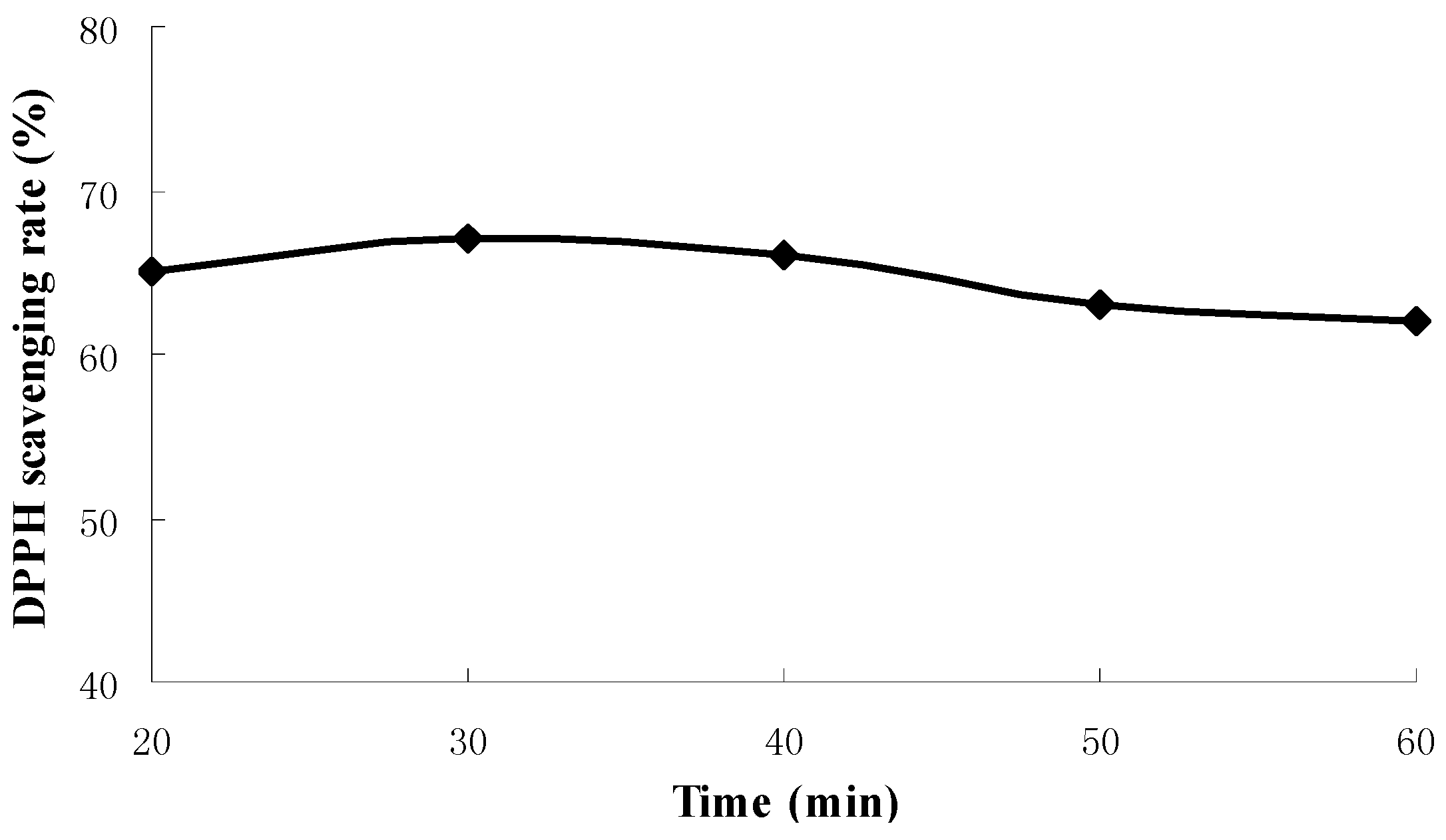
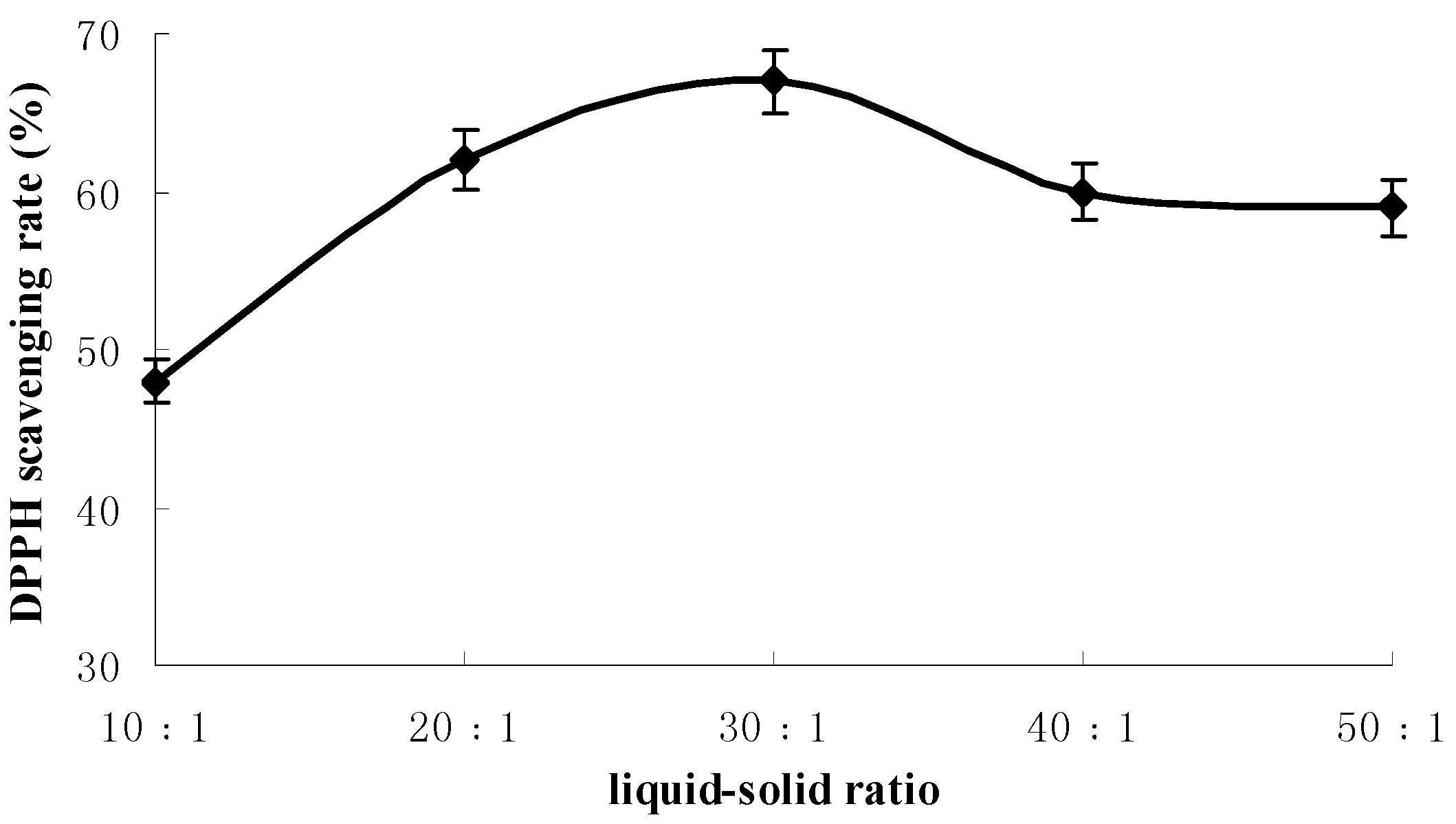
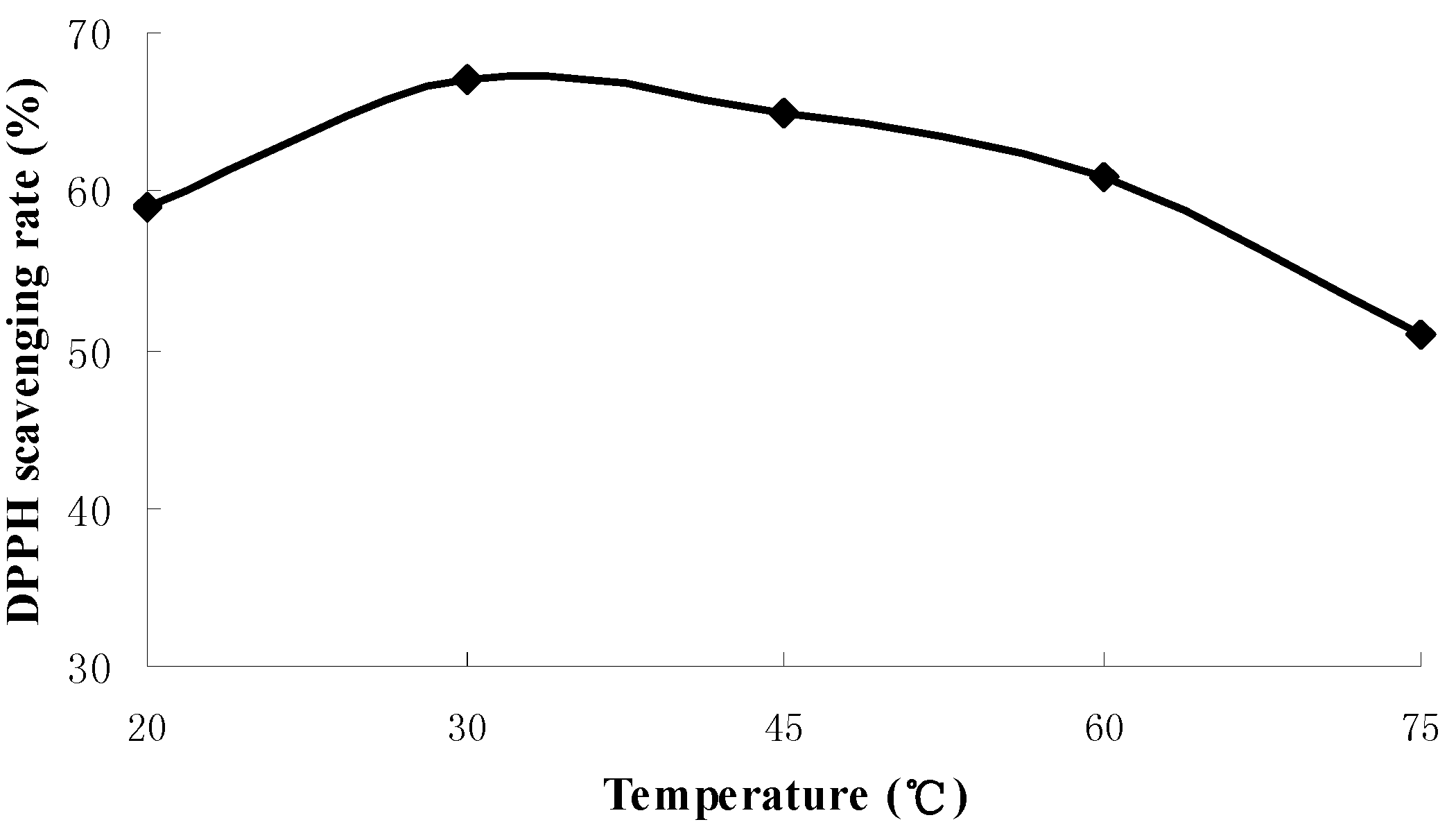
2.2. Results of Response Surface Methodology Experiments
| Run | Factor1 | Factor2 | Factor3 | Clearance rate |
|---|---|---|---|---|
| A: Temperature (°C) | B: Time (min) | C: liquid-solid ratio | Y: (%) | |
| 1 | 30 | 40 | 30:1 | 66.52 |
| 2 | 45 | 30 | 30:1 | 67.56 |
| 3 | 60 | 20 | 30:1 | 62.98 |
| 4 | 60 | 30 | 20:1 | 62.43 |
| 5 | 30 | 20 | 30:1 | 65.28 |
| 6 | 45 | 20 | 40:1 | 61.37 |
| 7 | 45 | 20 | 20:1 | 60.38 |
| 8 | 60 | 30 | 40:1 | 60.88 |
| 9 | 45 | 30 | 30:1 | 65.57 |
| 10 | 30 | 30 | 40:1 | 60.10 |
| 11 | 45 | 30 | 30:1 | 65.55 |
| 12 | 30 | 30 | 20:1 | 62.75 |
| 13 | 45 | 40 | 20:1 | 60.17 |
| 14 | 60 | 40 | 30:1 | 61.25 |
| 15 | 45 | 40 | 40:1 | 60.77 |
| 16 | 45 | 30 | 30:1 | 65.25 |
| 17 | 45 | 30 | 30:1 | 65.55 |
| Source | Sum of Squares | DF | Mean Square | F Value | Prob > F | Significance |
|---|---|---|---|---|---|---|
| Model | 85.45 | 9 | 9.49 | 4.21 | 0.0357 | significant |
| A | 6.32 | 1 | 6.32 | 2.80 | 0.1382 | |
| B | 0.21 | 1 | 0.21 | 0.094 | 0.7685 | |
| C | 0.85 | 1 | 0.85 | 0.38 | 0.5584 | |
| A2 | 1.10 | 1 | 1.10 | 0.49 | 0.5081 | |
| B2 | 8.00 | 1 | 8.00 | 3.54 | 0.1018 | |
| C2 | 62.26 | 1 | 62.26 | 27.59 | 0.0012 | |
| AB | 2.21 | 1 | 2.21 | 0.98 | 0.3558 | |
| AC | 0.30 | 1 | 0.30 | 0.13 | 0.7251 | |
| BC | 0.038 | 1 | 0.038 | 0.017 | 0.9004 | |
| Residual | 15.80 | 7 | 2.26 | |||
| Lack of Fit | 12.26 | 3 | 4.09 | 4.63 | 0.0864 | Not significant |
| Pure Error | 3.53 | 4 | 0.88 | |||
| Cor Total | 101.25 | 16 |
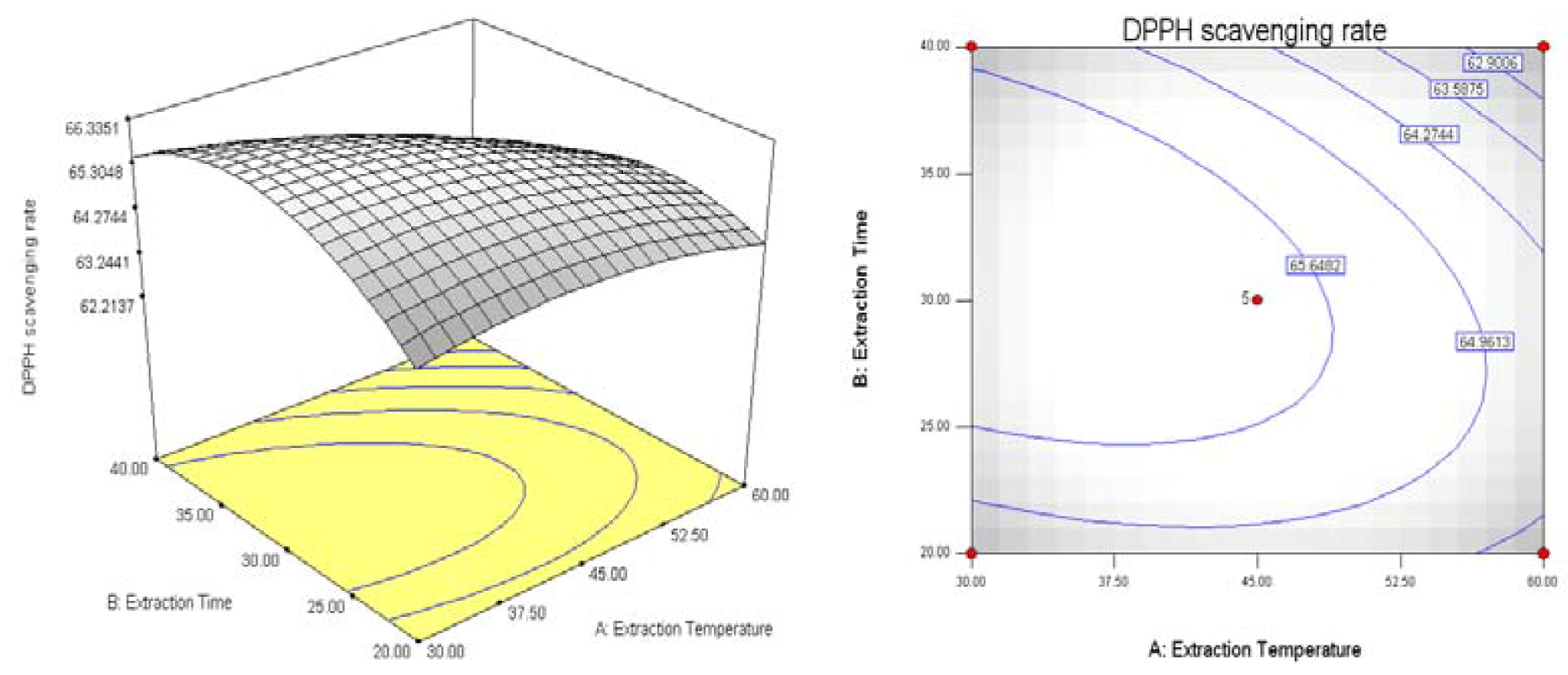
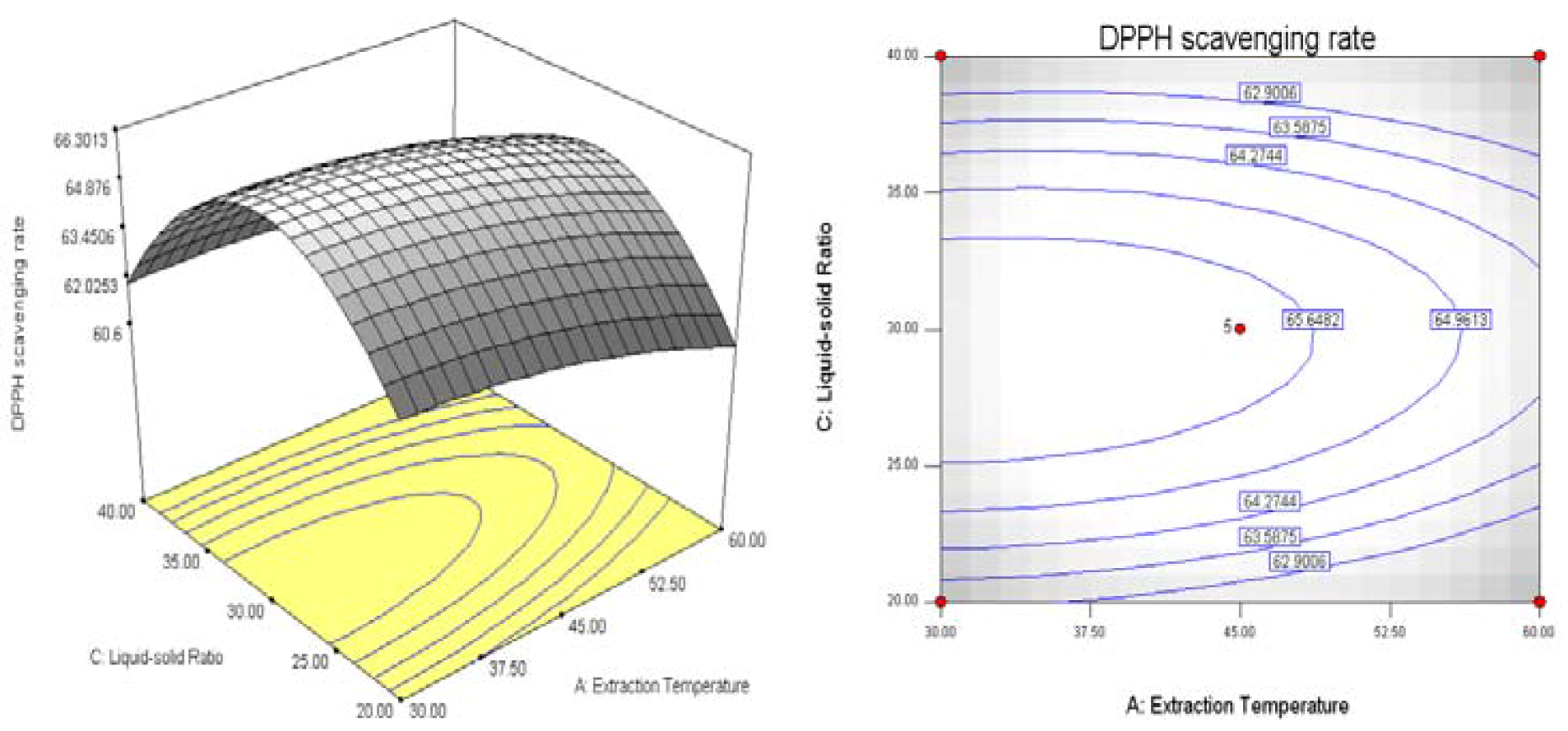
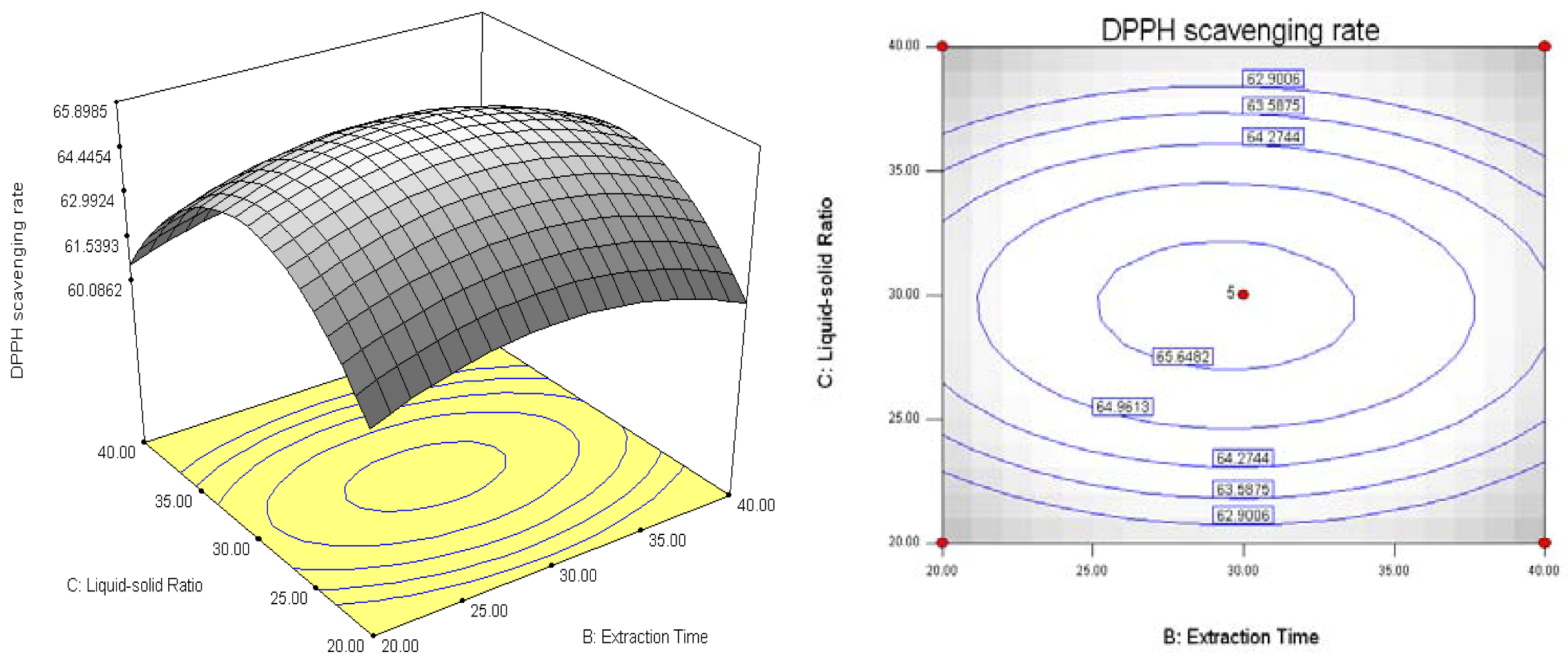
3. Experimental
3.1. Materials and Reagents
3.2. Sample Preparation
3.3. Antioxidants Extraction
3.4. Determination of the Antioxidant Capacity
3.5. Single-Factor Experiments
3.6. Response Surface Methodology Experiments
| Levels | Independent variables | ||
|---|---|---|---|
| A Temperature/°C | B Time/min | C Liquid-solid ratio | |
| −1 | 30 | 20 | 20:1 |
| 0 | 45 | 30 | 30:1 |
| 1 | 60 | 40 | 40:1 |
3.7. Statistical Methods
4. Conclusions
Acknowledgments
- Sample Availability: Samples of the compounds are available from the authors.
References
- Xu, Y.; Xu, P.; Wang, X. Studies on extraction technology and stability of black soybean polysaccharides. Food Res. Dev. 2009, 30, 49–52. [Google Scholar]
- Wang, Y.; Ren, H.; Li, Z. Study on antioxidative activity evaluation on medicinal black soybean pigments by scavenging DPPH. Sci. Technol. Food Ind. 2009, 2009, 102–105. [Google Scholar]
- Ren, H.; Wang, C.; Song, Y. Study on the Isolation and Antioxidant Activity of Black-soybean Peptides. Nat. Prod. Res. Dev. 2009, 21, 136–139. [Google Scholar]
- Xu, J.; Zhang, M.; Liu, X. Correlation BetweenAntioxidation, and Content of Total Phenolics and Anthocyanin in Black Soybean Accessions. Sci. Agric. Sin. 2006, 39, 1545–1552. [Google Scholar]
- Huang, G. Effect of germination on nutritional properties of seeds. Food Nutr. China 2005, 12, 25–27. [Google Scholar]
- Xu, M.; Dong, J.; Zhu, M. Effects of germination conditions on ascorbic acid level and yield of soybeans sprouts. J. Sci. Food Agric. 2005, 85, 943–947. [Google Scholar] [CrossRef]
- Sun, X.; Sun, S. Free isoflavone glycosides measurement of black soybeans on germination period. Shandong Agric. Sci. 2008, 8, 110–111. [Google Scholar]
- Lai, J.; Xin, C.; Zhao, Y.; Feng, B.; He, C.; Dong, Y.; Fang, Y.; Wei, S. Study of Active Ingredients in Black Soybean Sprouts and Their Safety in Cosmetic Use. Molecules 2012, 17, 11669–11679. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, Y.; Han, M.; Yang, L. Aqueous ionic liquid based ultrasonic assisted extraction of eight ginsenosides from ginseng root. Ultrason. Sonochem. 2013, 20, 680–684. [Google Scholar] [CrossRef]
- Ma, T.; Liu, L.; Yang, Y.; Zu, S.; Wang, R. Study on ionic liquid-based ultrasonic-assisted extraction of biphenyl cyclooctene lignans from the fruit of Schisandra chinensis Baill. Anal. Chim. Acta 2011, 689, 110–116. [Google Scholar] [CrossRef]
- Tadeo, C.; Sanchez, B.; Albero, A.I.; Garcia, V. Application of ultrasound-assisted extraction to the determination of contaminants in food and soil samples. J. Chromatogr. A 2010, 1217, 2415–2440. [Google Scholar] [CrossRef]
- Zeng, L.; Xia, Z. The improvement and influence of ultrasonic and microwave irradiation on the extraction of traditional Chinese medicine. Chem. Res. Appl. 2002, 14, 245–249. [Google Scholar]
- Mao, S.; Wang, J.; Shi, D. Statistical Handbook; Science Press: Beijing, China, 2003; pp. 78–86. [Google Scholar]
- Hilde, H.W.; Nigel, B. The optimization of solid-liquid extraction of antioxidants from apple pomace by response surface methodology. J. Food Eng. 2010, 96, 134–140. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Ding, L. Optimization of Polysaccharide Extraction from Angelica sinensis Using Response Surface Methodology. Food Sci. 2012, 33, 146–149. [Google Scholar]
- Song, X.; Gao, Y.; Yuan, F. Optimization of antioxidant peptide production from goat placenta powder using RSM. Food Sci. Technol. 2008, 33, 237–241. [Google Scholar]
- Liu, C.; Jia, S.; Xu, J. Optimization of Enzymatic Hydrolysis for Protein from Actinidia arguta Sieb. et Zucc by Response Surface Methodology and Antioxidant Activity of Polypeptides. Food Sci. 2012, 33, 33–38. [Google Scholar]
- Rajendran, V.; Karuppan, M. Ultrasound-assisted alkaline pretreatment of sugarcane bagasse for fermentable sugar production: Optimization through response surface methodology. Bioresour. Technol. 2012, 112, 293–299. [Google Scholar] [CrossRef]
- McCue, P.; Shetty, K. Clonal herbal extracts as elicitors of phenolic synthesis in dark-germinated mungbeans for improving nutritional value with implications for food safety. Food Biochem. 2002, 26, 209–232. [Google Scholar] [CrossRef]
- McCue, P.; Shetty, K. A biochemical analysis of mungbean (Vigna radiata) response to microbial polysaccharides and potential phenolic-enhancing effects for nutraceutical applications. Food Biotechnol. 2002, 16, 57–79. [Google Scholar] [CrossRef]
- Li, D.; Song, J.; Liu, C. Optimization of technology for ultrasonic-assisted extraction of pigments from black soybean hulls. Trans. Chin. Soc. Agric. Eng. 2009, 25, 273–278. [Google Scholar]
- Gao, X.; Wang, R.; Yang, J. Research on micro-wave extraction technology and properties of black bean red pigment. J. Henan Univ. Technol. (Nat. Sci. Ed.) 2006, 27, 25–28. [Google Scholar]
- Zhang, L.; Yan, Q.; Wang, Y. Study on Isolation and Antioxidant Activity of Soybean Bioactive Peptides. Food Sci. 2007, 5, 208–210. [Google Scholar]
- Li, J.; Li, M.; Chen, J. Microwave-assisted Extraction of Polysaccharide from Kiwifruit (Actinidia deliciosa) Roots. Food Sci. 2010, 31, 42–45. [Google Scholar]
- Zhang, C.; Wu, S.; Xu, N. Optimization of ultrasonic-assisted extraction of polysaccharides from the stem of Actinidia arguta. Sci. Technol. Food Ind. 2012, 33, 297–299. [Google Scholar]
- Zhao, J.; Lai, J.; He, C. Extraction technology of active ingredients in plant Rhodiola and its function in cosmetics. China Surfactant Deterg. Cosmet. 2009, 12, 402–404. [Google Scholar]
- He, R.; Gao, W.; Ceng, X.; Zhu, S. Optimization of Synthesis of Soluble Soybean Polysaccharide-Iron (II) Complex by Response Surface Methodology. Food Sci. 2012, 33, 98–102. [Google Scholar]
- Wu, Y.; Cui, S.; Tang, J. Optimization of extraction process of crude polysaccharides from boat-fruited Sterculia seeds by response surface methodology. Food Chem. 2007, 105, 1599–1605. [Google Scholar] [CrossRef]
- Mehrnoush, A.; Mustafa, S.; Sarker, M.Z.I.; Yazid, A.M.M. Optimization of the conditions for extraction of serine protease from Kesinai Plant (Streblus asper) leaves using response surface methodology. Molecules 2011, 16, 9245–9260. [Google Scholar] [CrossRef]
- Choon, Y.; Nyuk, L.; Yus, A.; Rosnita, A.T.; Chung, L. Optimization of total phenolic content extracted from Garcinia mangostana Linn. hull using response surface methodology versus artificial neural network. Ind. Crops Prod. 2012, 40, 247–253. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lai, J.; Xin, C.; Zhao, Y.; Feng, B.; He, C.; Dong, Y.; Fang, Y.; Wei, S. Optimization of Ultrasonic Assisted Extraction of Antioxidants from Black Soybean (Glycine max var) Sprouts Using Response Surface Methodology. Molecules 2013, 18, 1101-1110. https://doi.org/10.3390/molecules18011101
Lai J, Xin C, Zhao Y, Feng B, He C, Dong Y, Fang Y, Wei S. Optimization of Ultrasonic Assisted Extraction of Antioxidants from Black Soybean (Glycine max var) Sprouts Using Response Surface Methodology. Molecules. 2013; 18(1):1101-1110. https://doi.org/10.3390/molecules18011101
Chicago/Turabian StyleLai, Jixiang, Can Xin, Ya Zhao, Bing Feng, Congfen He, Yinmao Dong, Yun Fang, and Shaomin Wei. 2013. "Optimization of Ultrasonic Assisted Extraction of Antioxidants from Black Soybean (Glycine max var) Sprouts Using Response Surface Methodology" Molecules 18, no. 1: 1101-1110. https://doi.org/10.3390/molecules18011101
APA StyleLai, J., Xin, C., Zhao, Y., Feng, B., He, C., Dong, Y., Fang, Y., & Wei, S. (2013). Optimization of Ultrasonic Assisted Extraction of Antioxidants from Black Soybean (Glycine max var) Sprouts Using Response Surface Methodology. Molecules, 18(1), 1101-1110. https://doi.org/10.3390/molecules18011101




