Mechanistic Study of the Spiroindolones: A New Class of Antimalarials
Abstract
:1. Introduction
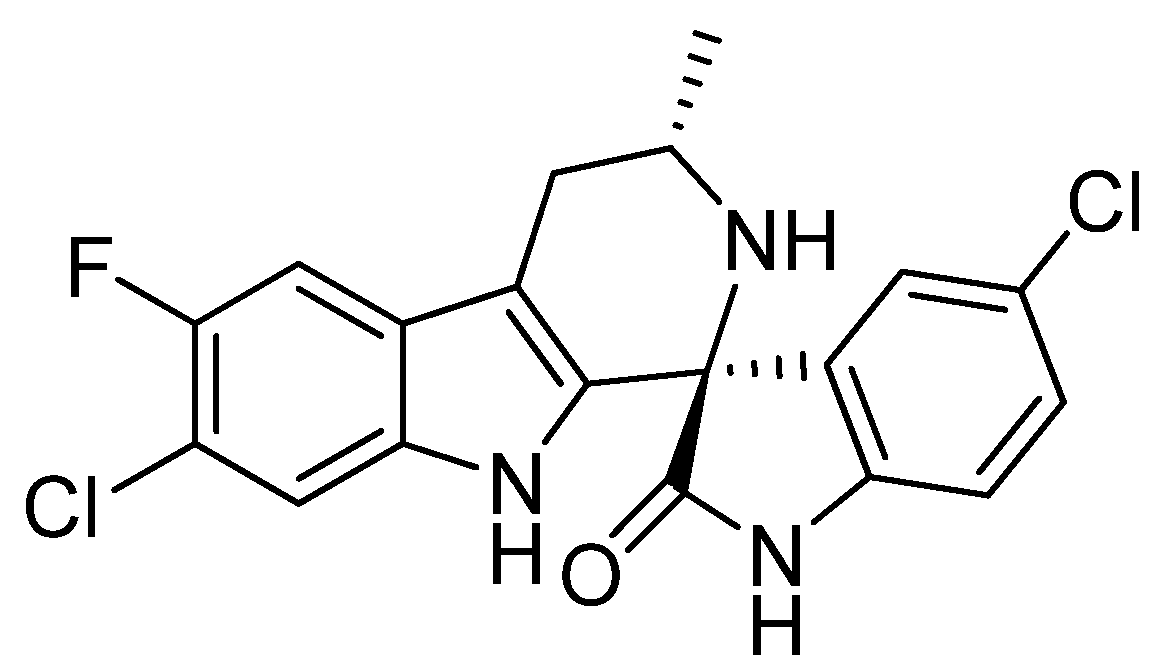
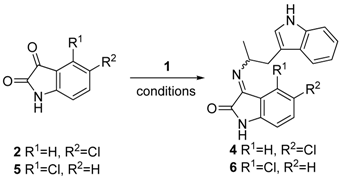
2. Results and Discussion
| Entry | Isatin | Conditions a | Yield | Product |
|---|---|---|---|---|
| 1 | 2 | 2.85 M, EtOH, 80 °C | 58% | 4E:4Z (23:1) b |
| 2 | 2 | 0.95 M, EtOH, 80 °C | 58% | 4E:4Z (1:20) b |
| 3 | 5 | 0.95 M, EtOH, 80 °C | 55% | 6Zc |

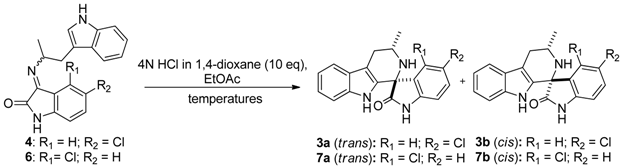
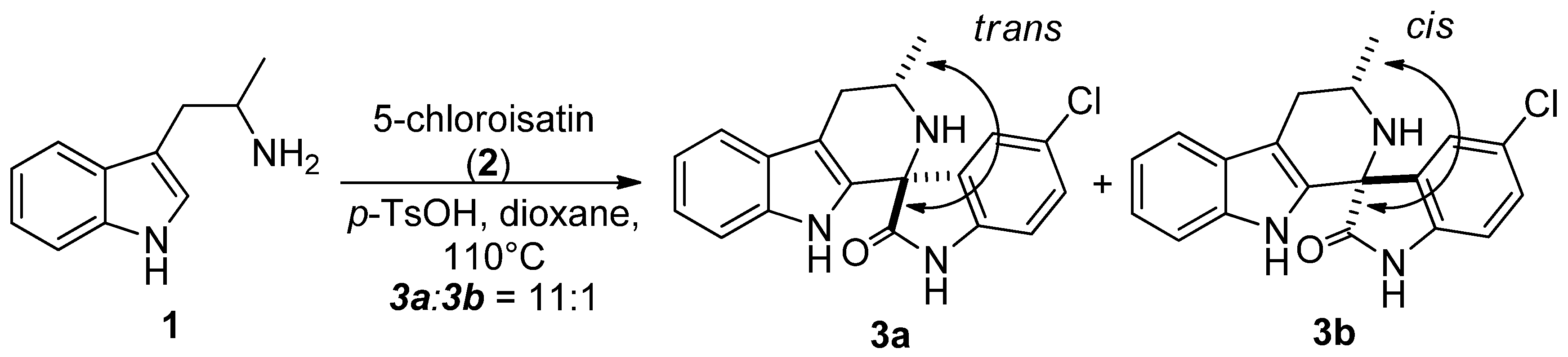
| Entry | Imine | Product | trans:cis ratio a (yield %) b | ||
|---|---|---|---|---|---|
| −78 °C c | r.t. d | 110 °C e | |||
| 1 | 4E:4Z (1:20) | 3a/3b | 18:1(100) | 12:1(100) | 10:1(83) |
| 2 | 4E:4Z (23:1) | 3a/3b | 1:20(95) | 1:2(100) | 7:1(97) |
| 3 | 4E:4Z (1:3) | 3a/3b | 1:1(100) | 3:1(94) | 11:1(100) |
| 4 | 6Z | 7a/7b | 165:1(93) | 37:1(98) | 12:1(92) |

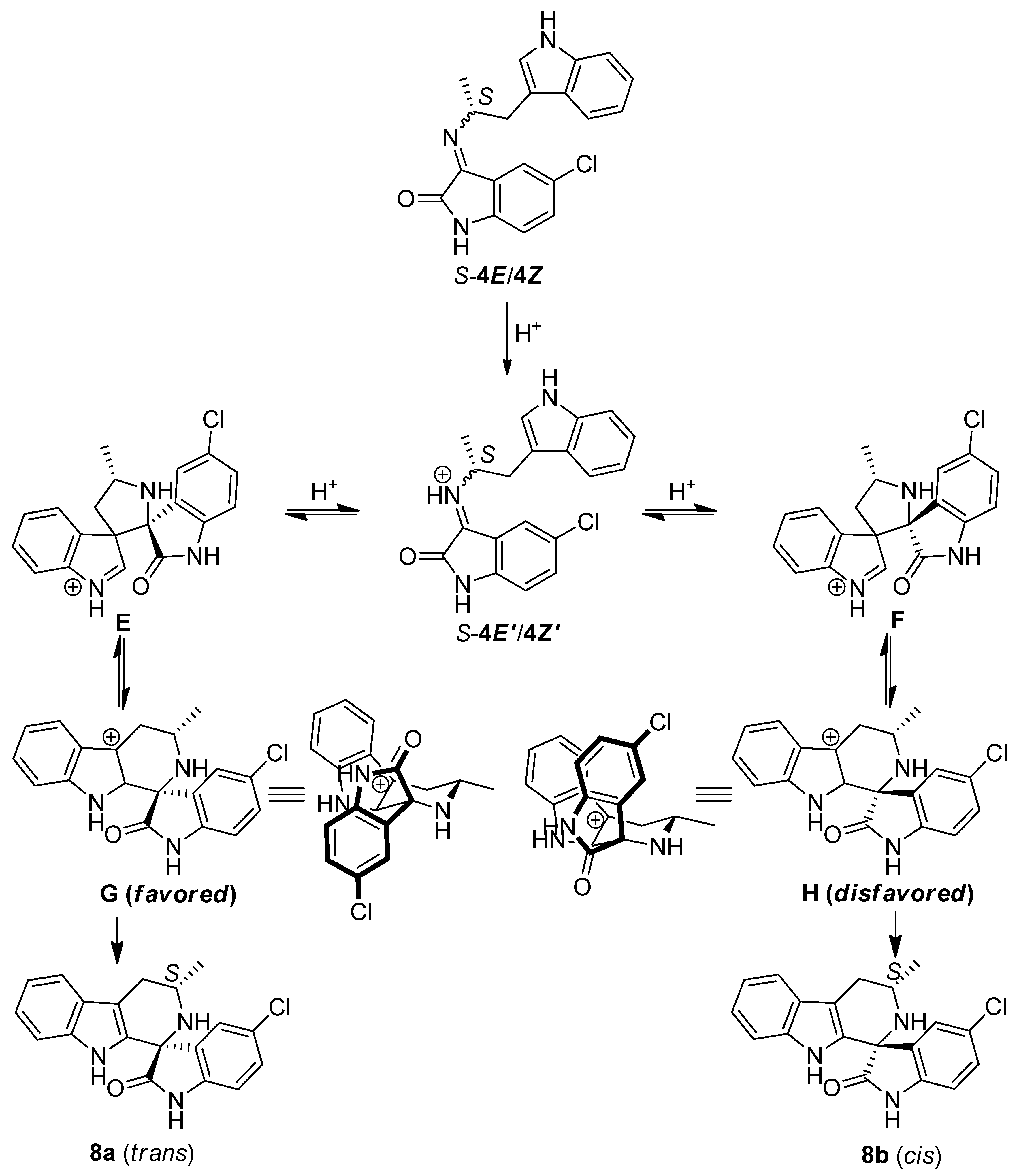
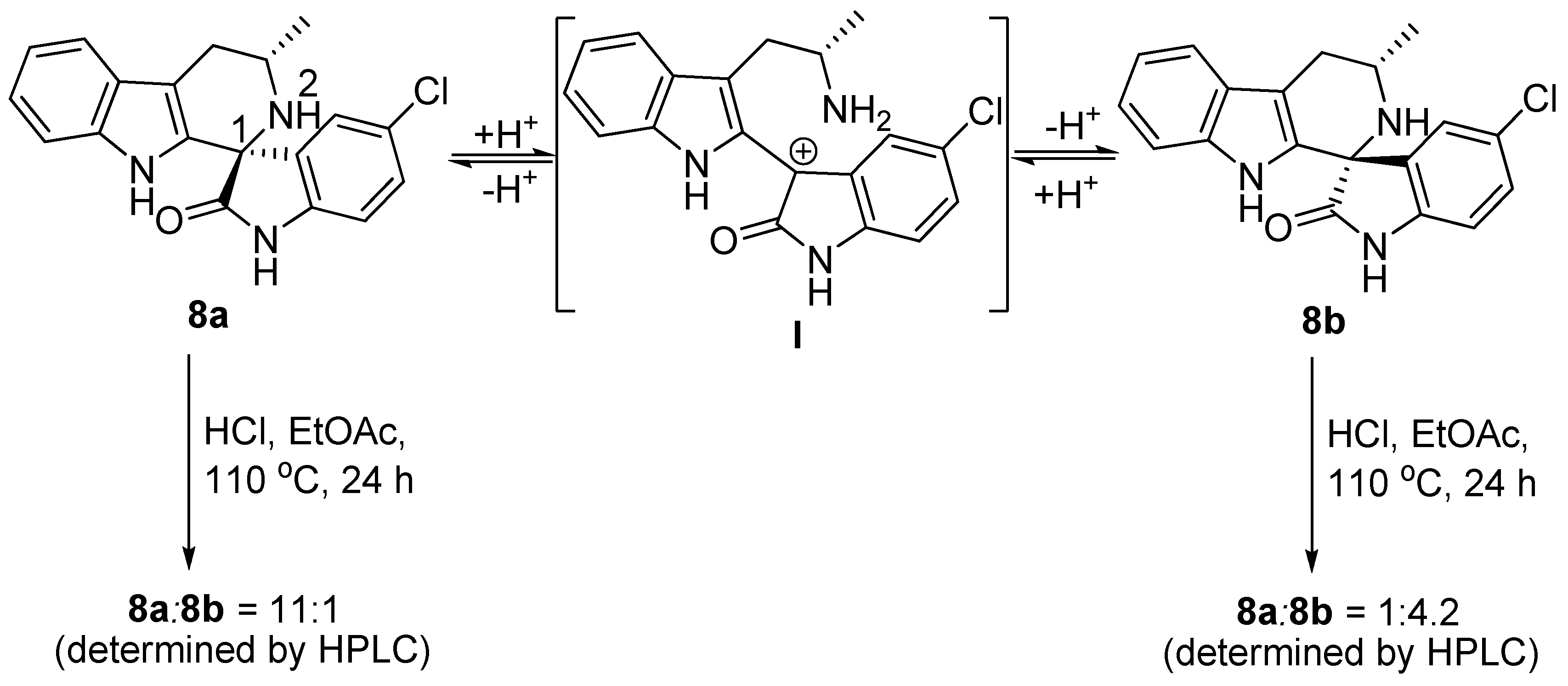
3. Experimental
3.1. Materials and Reagents
3.2. Synthesis of Imines 4Z, 4E, and 6Z
3.3. General Procedure for Cyclization of Imines at Different Temperatures
4. Conclusions
Supplementary Materials
Acknowledgments
References and Notes
- World Health Organization (WHO). World Malarial Report 2011. Available online: http://www.who.int/malaria/world_malaria_report_2011/en/index.html (accessed on 14 August 2012).
- Noedl, H.; Se, Y.; Schaecher, K.; Smith, B.L.; Socheat, D.; Fukuda, M.M. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 2008, 359, 2619–2620. [Google Scholar]
- Dondorp, A.M.; Yeung, S.; White, L.; Nguon, C.; Day, N.P.; Socheat, D.; von Seidlein, L. Artemisinin resistance: Current status and scenarios for containment. Nat. Rev. Microbiol. 2010, 8, 272–280. [Google Scholar]
- World Health Organization (WHO). Update on artemisinin resistance-September 2011. Available online: http://www.who.int/malaria/diagnosis_treatment/resistance/updateonartemsininresistancesept2011.pdf (accessed on 14 August 2012).
- Rottmann, M.; McNamara, C.; Yeung, B.K.S.; Lee, M.C.S.; Zou, B.; Russell, B.; Seitz, P.; Plouffe, D.M.; Dharia, N.V.; Tan, J.; et al. Spiroindolones, a new and potent chemotype for the treatment of malarial. Science 2010, 329, 1175–1180. [Google Scholar] [CrossRef]
- Yeung, B.K.S.; Zou, B.; Rottmann, M.; Lakshminarayana, S.B.; Ang, S.H.; Leong, S.Y.; Tan, J.; Wong, J.; Keller-Maerki, S.; Fischli, C.; et al. Spirotetrahydroβ-carbolines (Spiroindolones): A new class of potent and orally efficacious compounds for the treatment of malaria. J. Med. Chem. 2010, 53, 5155–5164. [Google Scholar]
- Cox, E.D.; Cook, J.M. The Pictet-Spengler condensation: A new direction for an old reaction. Chem. Rev. 1995, 95, 1797–1842. [Google Scholar] [CrossRef]
- Cox, E.D.; Hamaker, L.K.; Li, M.; Yu, P.; Czerwinksi, K.M.; Deng, L.; Bennett, D.W.; Cook, J.M. Enantiospecific formation of Trans 1,3-disubstituted tetrahydro-β-carbolines by the Pictet-Spengler reaction and conversion of cis diastereomers into their trans counterparts by scission of the C-1/N-2 bond. J. Org. Chem. 1997, 62, 44–61. [Google Scholar] [CrossRef]
- Lorenz, M.; Van Linn, M.L.; Cook, J.M. The asymmetric Pictet-Spengler reaction. Curr. Org. Synth. 2010, 7, 189–223. [Google Scholar] [CrossRef]
- Fang, H.; Wu, X.; Nie, L.; Dai, X.; Chen, J.; Cao, W.; Zhao, G. Diastereoselective syntheses of indoloquinolizidines by a Pictet-Spengler/Lactamization cascade. Org. Lett. 2010, 12, 5366–5369. [Google Scholar] [CrossRef]
- Cheng, D.; Wu, H.; Tian, S. Catalytic asymmetric Pictet-Spengler-Type reaction for the Synthesis of optically active indolo[3,4-cd][1]benzazepines. Org. Lett. 2011, 13, 5636–5639. [Google Scholar]
- Rashid, N.; Alam, S.; Hasan, M.; Khan, N.; Khan, K.M.; Duddeck, H.; Pescitelli, G.; Kenéz, A.; Antus, S.; Kurtán, T. Cis-diastereoselectivity in Pictet-Spengler reactions of L-tryptophan and electronic circular dichrosim studies. Chirality 2012. [Google Scholar]
- Schönherr, H.; Leighton, J.L. Direct and highly enantioselectiveIso-Pictet-Spengler reactions with α-ketoamides: Access to underexplored indole core structures. Org. Lett. 2012, 14, 2610–2613. [Google Scholar]
- Duce, S.; Pesciaioli, F.; Gramigna, L.; Bernardi, L.; Mazzanti, A.; Ricci, A.; Bartoli, G.; Bencivenni, G. An easy entry to optically active spiroindolinones: ChiralBrønsted acid-catalysedPictet-Spengler reactions of isatins. Adv. Synth. Catal. 2011, 353, 860–864. [Google Scholar] [CrossRef]
- Badillo, J.J.; Silva-García, A.; Shupe, B.H.; Fettinger, J.C.; Franz, A.K. EnantioselectivePictet-Spengler reactions of isatins for the synthesis of spiroindolones. TetrahetronLett. 2011, 52, 5550–5553. [Google Scholar] [CrossRef]
- Semonov, B.B.; Novikov, K.A.; Azev, V.N.; Kachala, V.V. Diastereoselective synthesis of 1,1-disubstituted 4-phenyl-2,3,4,9-tetrahydrospiro-β-carbolines from β-phenyltryptamine and isatin derivatives. Russ. Chem Bull. 2005, 54, 988–991. [Google Scholar] [CrossRef]
- Pogosyan, S.A.; Grigoryan, N.P.; Paronikyan, R.G. Synthesis and anticonvulsant activity of dihydrochlorides of indoline-3′spiro-1-(1,2,3,4-tetrahydro-β-carboline derivatives. Pharm. Chem. J. 2007, 31, 527–528. [Google Scholar]
- Grigoryan, N.P.; Pogosyan, S.A.; Paronikyan, R.G. Synthesis and anti-apasmodic activity of hydrochlorids 2′-oxo-(5′-bromindolin)-3′-spiro-1-(1.2.3.4-tetrahydro)-β-carbolines and indoloasepins. Armen. Chem. J. 2005, 58, 100–104. [Google Scholar]
- Bailey, P.D.; Beard, M.A.; Phillips, T.R. Unexpected cis selectivity in the Pictet-Spengler reaction. Tetrahedron Lett. 2009, 50, 3645–3647. [Google Scholar] [CrossRef]
- Bailey, P.D.; Hollinshead, S.P.; McLay, N.R.; Morgan, K.; Palmer, S.J.; Prince, S.N.; Reynolds, C.D.; Wood, S.D. Diastereo- and enantio-selectivity in the Pictet-Spengler reaction. J. Chem. Soc. Perkin Trans. 1 1993, 431–439. [Google Scholar]
- Bailey, P.D. Direct proof of the involvement of a spiro intermediate in the Pictet-Spengler reaction. J. Chem. Res. (S) 1987, 6, 202–203. [Google Scholar]
- Zou, H.; Han, D.; Lao, X.; Cook, J.M. First regiospecific, enantiospecific total synthesis of gardnerine and gargnutine. Tetrahedron Lett. 2005, 46, 4219–4224. [Google Scholar] [CrossRef]
- Kumpati, H.J.; van Linn, M.L.; Kabir, M.S.; Försterling, F.H.; Deschamps, J.R.; Cook, J.M. Study of the cis to trans isomerization of 1-phenyl-2,3-disubstituted tetrahydro-beta-carbolines: Evidence for the carbocation-mediated mechanism. J. Org. Chem. 2009, 74, 2771–2779. [Google Scholar] [CrossRef]
- Van Linn, M.L.; Cook, J.M. Mechanistic studies on the cis to trans epimerization of trisubstituted 1,2,3,4-tetrahydro-beta-carbolines. J. Org. Chem. 2010, 75, 3587–3599. [Google Scholar] [CrossRef]
- Kuo, F.M.; Tseng, M.C.; Yen, Y.H.; Chu, Y.H. Microwave accelerated Pictet-Spengler reactions of tryptophan with ketones directed toward the preparation of 1,1-disubstituted alkaloids. Tetrahedron 2004, 60, 12075–12084. [Google Scholar]
- Mangion, I.K.; Northrup, A.B.; MacMillan, D.W.C. The importance of iminium geometry control in enamine catalysis: Identification of a new catalyst architecture for aldehyde-aldehyde couplings. Angew. Chem. Int. Ed. 2004, 43, 6722–6724. [Google Scholar]
- Guo, Q.; Liu, Y.; Li, X.; Zhong, L.; Peng, Y. Enantioselective and solvent-controlled diastereoselectiveMannich reaction of isatin imines with hydroxyacetone: Synthesis of 3-substituted 3-aminooxindoles. J. Org. Chem. 2012, 77, 3589–3594. [Google Scholar] [CrossRef]
- Parasuk, W.; Parasuk, V. Theoretical investigations on the stereoselectivity of the proline catalyzed Mannich reaction in DMSO. J. Org. Chem. 2008, 73, 9388–9392. [Google Scholar] [CrossRef]
- Abadi, A.H.; Abou-Seri, S.M.; Abdel-Rahman, D.E.; Klein, C.; Lozach, O.; Meijer, L. Synthesis of 3-substituted-2-oxoindole analogues and their evaluation as kinase inhibitors, anticancer and antiangiogenic agents. Eur. J. Med. Chem. 2006, 41, 296–305. [Google Scholar]
- Under standard HPLC conditions both 4E and 4Z decomposed rapidly back to starting materials.
- Sample Availability: Samples of the compounds 4Z, 4E, 6Z, 3a, 3b, 7a and 7b are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zou, B.; Yap, P.; Sonntag, L.-S.; Leong, S.Y.; Yeung, B.K.S.; Keller, T.H. Mechanistic Study of the Spiroindolones: A New Class of Antimalarials. Molecules 2012, 17, 10131-10141. https://doi.org/10.3390/molecules170910131
Zou B, Yap P, Sonntag L-S, Leong SY, Yeung BKS, Keller TH. Mechanistic Study of the Spiroindolones: A New Class of Antimalarials. Molecules. 2012; 17(9):10131-10141. https://doi.org/10.3390/molecules170910131
Chicago/Turabian StyleZou, Bin, Peiling Yap, Louis-Sebastian Sonntag, Seh Yong Leong, Bryan K. S. Yeung, and Thomas H. Keller. 2012. "Mechanistic Study of the Spiroindolones: A New Class of Antimalarials" Molecules 17, no. 9: 10131-10141. https://doi.org/10.3390/molecules170910131
APA StyleZou, B., Yap, P., Sonntag, L.-S., Leong, S. Y., Yeung, B. K. S., & Keller, T. H. (2012). Mechanistic Study of the Spiroindolones: A New Class of Antimalarials. Molecules, 17(9), 10131-10141. https://doi.org/10.3390/molecules170910131