Synthesis and Characterization of New Thiazolidinones Containing Coumarin Moieties and Their Antibacterial and Antioxidant Activities
Abstract
:1. Introduction
2. Results and Discussion
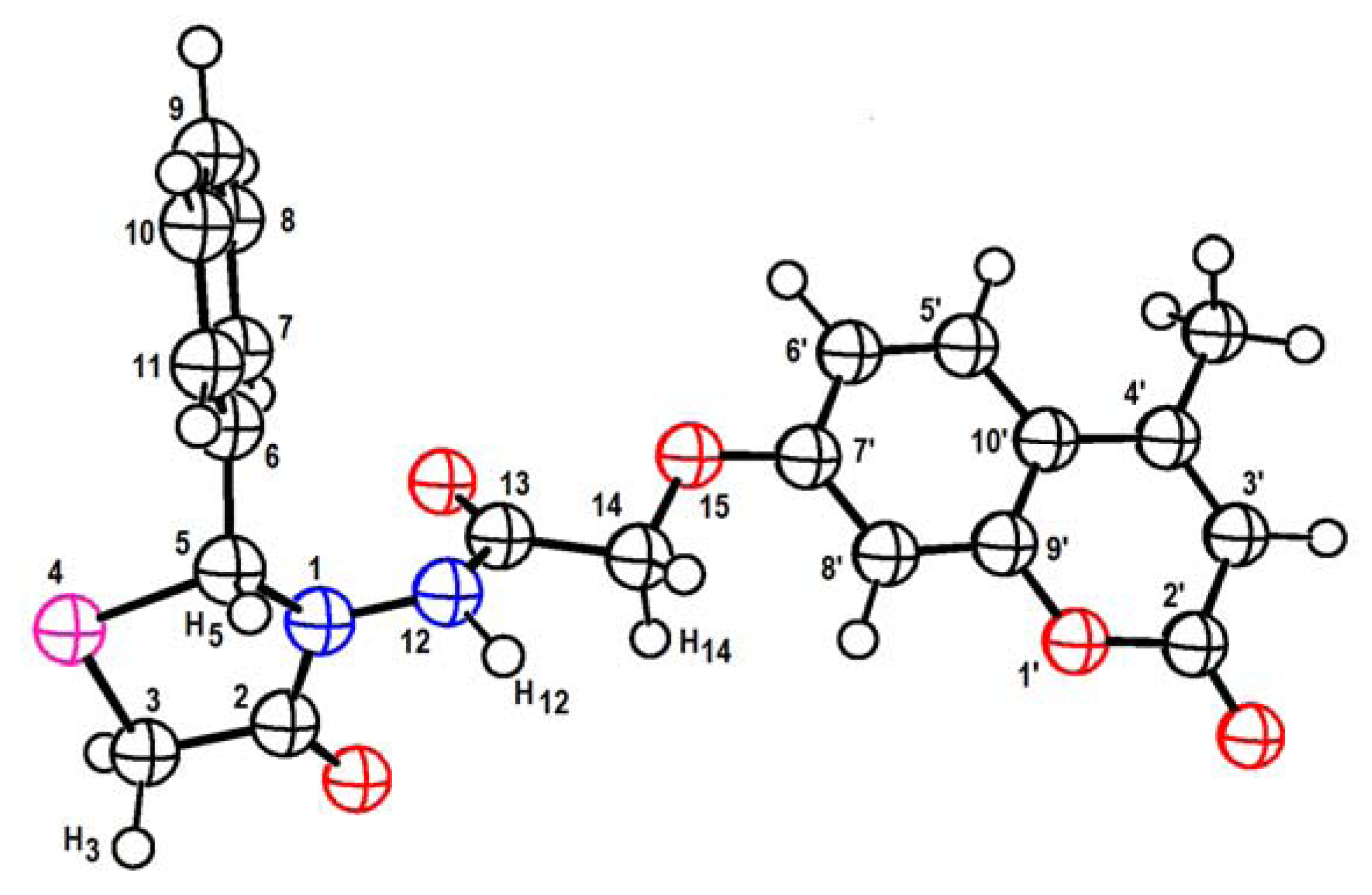
| Compounds | 5a | 5b | 5c | 5d |
|---|---|---|---|---|
| 1-2 | 1,385 | 1.384 | 1.383 | 1.386 |
| 1-12 | 1.374 | 1.374 | 1.372 | 1.374 |
| 1-5 | 1.463 | 1.465 | 1.462 | 1.462 |
| 2-3 | 1.520 | 1.521 | 1.520 | 1.520 |
| 3-4 | 1.829 | 1.829 | 1.826 | 1.829 |
| 4-5 | 1.863 | 1.864 | 1.866 | 1.863 |
| 5-6 | 1.510 | 1.506 | 1.509 | 1.510 |
| 12-13 | 1.394 | 1.393 | 1.389 | 1.394 |
| 13-14 | 1,530 | 1.530 | 1.531 | 1.529 |
| 14-15 | 1.401 | 1.401 | 1.402 | 1.401 |
| 15-7′ | 1.352 | 1.352 | 1.352 | 1.353 |
| 1′-2′ | 1.396 | 1.396 | 1.396 | 1.397 |
| 1′-9′ | 1.354 | 1.354 | 1.354 | 1.354 |
| 2′-3′ | 1.457 | 1.457 | 1.457 | 1.457 |
| 3′-4′ | 1.362 | 1.362 | 1.362 | 1.362 |
| 4′-10′ | 1.454 | 1.454 | 1.454 | 1.454 |
| 5-H5 | 1.105 | 1.106 | 1.101 | 1.105 |
| 12-H12 | 1.020 | 1.020 | 1.020 | 1.020 |
| 14-H14 | 1.107 | 1.108 | 1.108 | 1.108 |
| 1-2-3 | 111.1 | 111.0 | 110.7 | 111.1 |
| 2-3-4 | 107.8 | 107.8 | 107.6 | 107.8 |
| 3-4-5 | 92.9 | 92.8 | 93.0 | 92.9 |
| 4-5-1 | 103.4 | 103.2 | 103.4 | 103.5 |
| 1-5-6 | 115.5 | 115.6 | 115.6 | 115.5 |
| 1-5-H5 | 109.5 | 109.5 | 108.4 | 109.6 |
| 2-1-12 | 118.1 | 118.2 | 119.5 | 118.1 |
| 1-12-H12 | 114.9 | 114.9 | 114.7 | 114.8 |
| 1-12-13 | 119,0 | 118.9 | 121.2 | 119.0 |
| 12-13-14 | 111.9 | 111.9 | 111.2 | 112.0 |
| 13-14-15 | 108.3 | 108.3 | 108.9 | 108.4 |
| 1′-2′-3′ | 115.4 | 115.4 | 115.4 | 115.4 |
| 2′-3′-4′ | 123.3 | 123.3 | 123.3 | 123.3 |
| 3′-4′-10′ | 118.6 | 118.6 | 118.5 | 118.5 |
| 4′-10′-9′ | 118.0 | 118.0 | 117.9 | 118.0 |
| 1-2-3-4 | 6.1 | 7.5 | −13.9 | 6.5 |
| 2-3-4-5 | −15.4 | −17.0 | 18.6 | −15.8 |
| 3-4-5-1 | 19.9 | 21.1 | −17.9 | 20.0 |
| H5-5-6-11 | −12.6 | −11.8 | −0.7 | −12.8 |
| 3-4-5-6 | 144.3 | 145.8 | 109.4 | 144.5 |
| 1-5-6-7 | −136.1 | −135.1 | −122.1 | −136.4 |
| 2-3-5-12 | 1.0 | 1.1 | −0.9 | −11.6 |
| 2-5-7′-6′ | 113.0 | 114.1 | 109.1 | 118.2 |
| 12-13-14-15 | −158.1 | −157.1 | −161.7 | −155.2 |
| 13-14-15-7′ | −175.5 | −174.8 | −177.7 | −174.9 |
| 14-15-7′-6′ | 178.5 | 178.2 | 176.8 | 179.2 |
| 7′-8′-9′-10′ | 0.0 | 0.0 | 0.4 | 0.1 |
| 10′-9′-1′-2′ | 0.0 | 0.1 | 0.0 | 0.0 |
| 1′-2′-3′-4′ | 0.1 | 0.2 | 0.6 | 0.1 |
| Experimental | Theoretical | |||
|---|---|---|---|---|
| Functional group | Frequencies | Intensities | Frequencies | Intensities |
| CO thiaz. | 1666 | strong | 1831 | 328 |
| CO lactone | 1682 | strong very | 1862 | 652 |
| CO amide | 1712 | mean | 1871 | 130 |
| NH | 3313 | weak | 3513 | 23 |
| Hi | Charge |
|---|---|
| H3 | 0.25 |
| H5 | 0.22 |
| H12 | 0.39 |
| H14 | 0.22 |
2.1. Antioxidant Activities
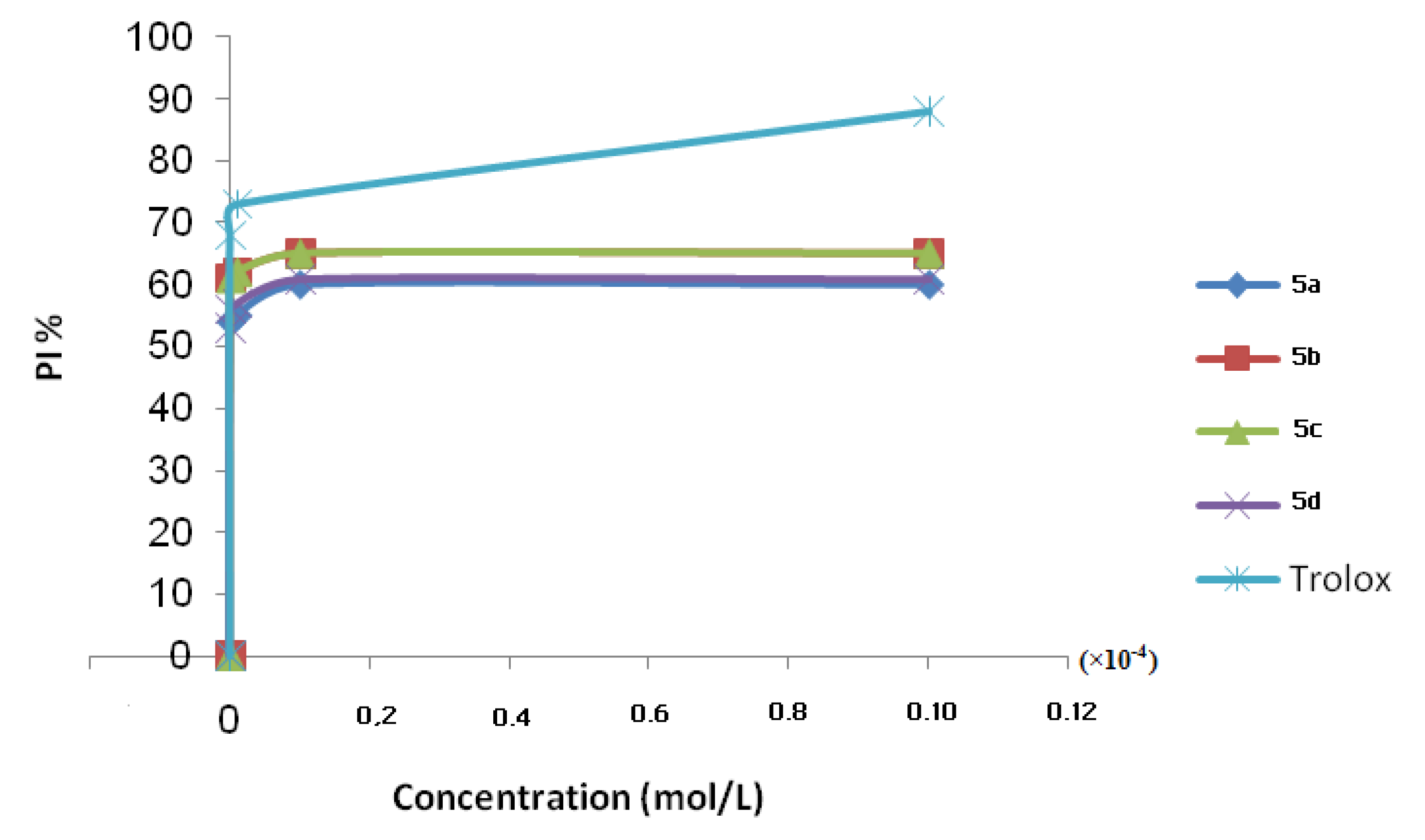
| Compounds 5 | IC50 (10−9 mol/L) |
|---|---|
| 5a | 92.60 |
| 5b | 82.00 |
| 5c | 8.62 |
| 5d | 9.43 |
| Trolox | 7.35 |
2.2. ABTS Radical Cation Decolourization Assay
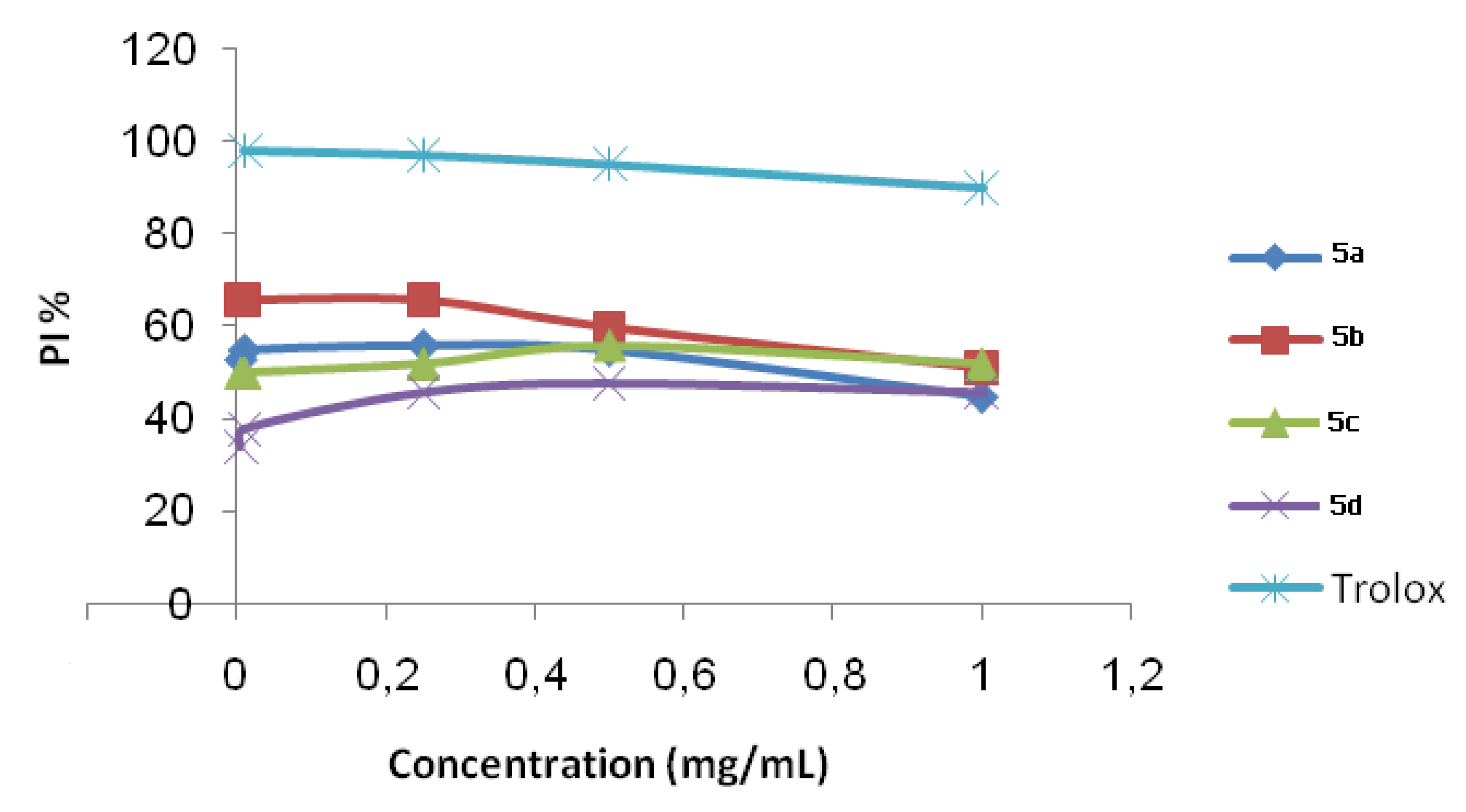
| Compounds 5 | IC50 (10−9 mol/L) |
|---|---|
| 5a | 92.60 |
| 5b | 82.00 |
| 5c | 8.62 |
| 5d | 9.43 |
| Trolox | 7.35 |
2.3. Antibacterial Activity
| Organism | Compounds | Standard-drug | |||
|---|---|---|---|---|---|
| 5a | 5b | 5c | 5d | ampicillin | |
| E. coli ATCC1225 | 19 | 19 | 19 | 19 | 16 |
| P. vulgaris | 20 | 20 | 20 | 20 | 22 |
| B. megaterium | 17 | 17 | 17 | 17 | 23 |
| S. aureus ATCC2353 | 18 | 18 | 18 | 18 | 25 |
3. Experimental
3.1. General
3.2. DPPH Test
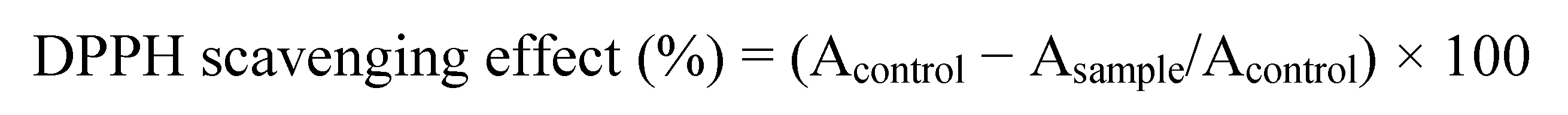
3.3. ABTS Radical Cation Decolourization Assay
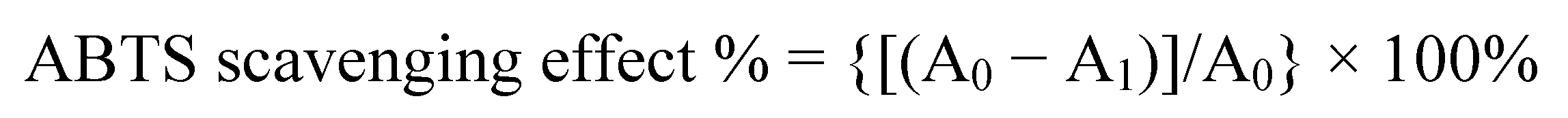
3.4. Antibacterial Activity
4. Conclusions
Acknowledgements
References
- Geronikaki, A.; Hadjiparlon-Litina, D.; Chatzioponlos, C.; Soloupis, G. Synthesis and biological evolution of new 4, 5-disubstituted-thiazoylamides derivatives of 4-hydroxy-Piperidine or 4-N-methyl-Piperadine. Molecules 2003, 8, 472–479. [Google Scholar] [CrossRef]
- Sup, R.C.; Sup, R.Y.; Bang, C.W. Synthesis and antiinflammatory activity of [2-(benzothiazol-2-ylimino)-4-oxo-3-pheylthiazolidin-5-yl]-acetic acid derivatives. J. Korean Chem. Soc. 1995, 93, 237–240. [Google Scholar]
- Sonwane, S.K.; Srivastava, S.D. Synthesis and biological significance of 2-amino-4-phenyl-1, 3-thiazole derivatives. Proc. Natl. Acad. Sci. India 2008, 78, 129–136. [Google Scholar]
- Capan, G.; Ulusoy, N.; Ergenc, N.; Kiraz, M. New 6-phenylimidazo[2,1-b]thiazole derivatives: Synthesis and antifungal activity. Monatsh. Chem. 1999, 130, 1399–1407. [Google Scholar]
- Vigorita, M.; Ottana, R.; Monforte, F.; Maccari, R.; Trovato, A.; Monforte, M.T.; Ftaviano, M. Synthesis and antiinflammatory, analgesic activity of 3,3′-(1,2-Ethanediyl)-bis[2-aryl-4-thiazolidinone] chiral compounds Part 10. Bioorg. Med. Chem. Lett. 2001, 11, 2791–2794. [Google Scholar]
- Kavitha, C.; Basappa, S.; Nanjunda, S.; Mantelingu, K.; Doreswamy, S.; Sridhar, M.A.; Sprasad, J.; Rangappa, K. Synthesis of new bioactive venlafaxine analogs: Novel thiazolidin-4-ones as antimicrobials. Bioorg. Med. Chem. 2006, 14, 2290–2299. [Google Scholar] [CrossRef]
- Ottana, R.; Maccari, R.; Barreca, M.; Rotondo, G.; Rossi, A.; Chiricosta, A.; Paola, G.; Sautebin, R.; Cuzzocrea, L.; Vigorita, S. Arylidene-2-imino-4-thiazolidinones: Design and synthesis of novel anti-inflammatory agents. Bioorg. Med. Chem. 2005, 13, 4243–4252. [Google Scholar]
- Kucukguzel, G.; Kocatepe, A.; Clercq, E.; Sahin, F.; Gulluce, M. Synthesis and biological activity of 4-thiazolidinones, thiosemicarbazides derived from diflunisal hydrazide. Eur. J. Med. Chem. 2006, 41, 353–359. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Musa, A.Y.; Kadhum, A.H.; Mohamad, A. The use of umbelliferone in the synthesis of new heterocyclic compounds. Molecules 2011, 16, 6833–6843. [Google Scholar] [CrossRef]
- Al-Amiery, A.; Kadhum, A.; Mohamad, A. Antifungal activities of new coumarins. Molecules 2012, 17, 5713–5723. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Musa, A.Y.; Kadhum, A.H.; Mohamad, A.B. The antioxidant activity of new coumarin derivatives. Int. J. Mol. Sci. 2011, 12, 5757–5761. [Google Scholar]
- Ocal, N.; Yolacan, C.; Kaban, S.; Leonor, Y.; Vargas, M.; Kouznetsov, V.J. Transformations of schiff bases derived from quinoline-8-carbaldehyde. Synthesis of C-8 substituted quinolines. J. Heterocycl. Chem. 2001, 38, 233–236. [Google Scholar]
- Mendez, L.; Kouznetsov, V.; Poveda, J.; Yolaçan, C.; Ocal, N.; Aydogan, F. Transformations of 4-N-arylamino-4-(8-quinolinyl)-1-butenes and 3-aryl-2-(8-quinolinyl)-4-thiazolidinones. Heterocycl. Commun. 2001, 7, 129–134. [Google Scholar]
- Aydogan, F.; Ocal, N.; Turgut, Z.; Yolacan, C. Synthesis of thiazolidino-fused compounds. Bull. Korean Chem. Soc. 2001, 22, 476–480. [Google Scholar]
- Shashikant, R.; Prajact, K.; Nachiket, S.; Sunil, A.; Deepak, S.; Smita, K.; Daithankar, A. Synthesis and biological evaluation of some 1, 3, 4-thiadiazoles. J. Chem. Pharm. Res. 2009, 1, 1191–1198. [Google Scholar]
- Srivastava, S.D. Synthesis and Antimicrobial Activity of Some 2-[(4-Substituted-Phenyl-3-Chloro- Azetidin-2-One)-5-(2′-Methylamino-4-Phenyl-1′,3′-Thiazolyl-]-1,3,4-Thiadiazoles. J. Sci. Islam. Repub. Iran 2009, 20, 227–232. [Google Scholar]
- Sharma, S.C. Synthesis of new fungicides. 2-(4′-arylthiazolyl-2′-imino)-3-aryl-4-thiazolidones. Bull. Chem. Soc. Jpn. 1967, 40, 2422–2424. [Google Scholar]
- Akerblom, E.B. Synthesis and structureactivity relations of a series of antibacterially active 5-(5-nitro-2-furfurylidene)thiazolones, 5-(5-nitro-2-urylpropenylidene)thiazolones, and 6-(5-nitro-2-furyl)-4H-1,3-thiazinones. J. Med. Chem. 1974, 17, 609–615. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 series programs-Revision A.1; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R. Synthesis and studying the antitumor activity of novel 5-(2-methylbenzimidazol-5-yl)-1,3,4-oxadiazole-2(3H)-thiones. Phys. Rev. B 1988, 37, 785–803. [Google Scholar]
- Weigend, F.; Ahlrichs, R. Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3304. [Google Scholar] [CrossRef]
- Şahin, F.; Karaman, I.; Güllüce, M.; Öğütçü, H.; Şengül, M.; Adigüzel, A.; Öztürk, S.; Kotan, R. Evaluation of antimicrobial activities of Satureja hortensis L. J. Ethnopharmacol 2003, 85, 231–238. [Google Scholar] [CrossRef]
- Soares, J.; Dins, T.; Cunha, A.; Ameida, L. Antioxidant activities of some extracts of thymus zygis. Free Radical Res. 1997, 26, 469–476. [Google Scholar] [CrossRef]
- Güven, K.; Yücel, E.; Çetintaş, F. Antimicrobial activities of fruits of crataegus and pyrus species. Pharm. Biol. 2006, 44, 79–86. [Google Scholar] [CrossRef]
- Tepe, B.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem. 2005, 90, 333–339. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 2-5 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hamdi, N.; Al-Ayed, A.S.; Ben Said, R.; Fabienne, A. Synthesis and Characterization of New Thiazolidinones Containing Coumarin Moieties and Their Antibacterial and Antioxidant Activities. Molecules 2012, 17, 9321-9334. https://doi.org/10.3390/molecules17089321
Hamdi N, Al-Ayed AS, Ben Said R, Fabienne A. Synthesis and Characterization of New Thiazolidinones Containing Coumarin Moieties and Their Antibacterial and Antioxidant Activities. Molecules. 2012; 17(8):9321-9334. https://doi.org/10.3390/molecules17089321
Chicago/Turabian StyleHamdi, Naceur, Abdullah Sulaiman Al-Ayed, Ridha Ben Said, and Alary Fabienne. 2012. "Synthesis and Characterization of New Thiazolidinones Containing Coumarin Moieties and Their Antibacterial and Antioxidant Activities" Molecules 17, no. 8: 9321-9334. https://doi.org/10.3390/molecules17089321
APA StyleHamdi, N., Al-Ayed, A. S., Ben Said, R., & Fabienne, A. (2012). Synthesis and Characterization of New Thiazolidinones Containing Coumarin Moieties and Their Antibacterial and Antioxidant Activities. Molecules, 17(8), 9321-9334. https://doi.org/10.3390/molecules17089321





